Abstract
The impact of 17β-oestradiol (E2) exposure on autonomic control of orthostasis in young women is unclear. We tested the hypothesis that autonomic cardiovascular regulation is more sensitive to E2 exposure in women with low orthostatic tolerance. Women underwent an initial maximal lower body negative pressure (LBNP) test to place them into a low (LT, n= 7, 22 ± 1 years old, body mass index 22 ± 1 kg m−2) or a high orthostatic tolerance group (HT, n= 7, 22 ± 1 years old, body mass index 24 ± 1 kg m−2). We then suppressed endogenous reproductive hormone production using a gonadotrophin-releasing hormone antagonist (GnRHant) for 10 days, with E2 administration during the last 7 days of GnRHant. We measured R–R interval and beat-by-beat blood pressure during the modified Oxford protocol, and changes in heart rate, blood pressure and forearm vascular resistance (FVR) during submaximal LBNP. During submaximal LBNP, FVR increased in HT (ANOVA P < 0.05) but not in LT (ANOVA P > 0.05), and stroke volume was lower in LT relative to HT at all levels of LBNP (P < 0.05). Compared with GnRHant, E2 administration shifted FVR lower in LT (ANOVA P < 0.05), with no effect in HT. Administration of E2 increased baroreflex control of heart rate (derived from the modified Oxford protocol) in LT (GnRHant 10.7 ± 2.5 ms mmHg−1 vs. E2 16.1 ± 2.4 ms mmHg−1, P < 0.05) but not in HT (GnRHant 13.4 ± 1.9 ms mmHg−1 vs. E2 15.3 ± 2.4 ms mmHg−1, n.s.). In conclusion, blunted peripheral vasoconstriction and lower stroke volume contribute to compromised orthostatic tolerance in women; this inability to vasoconstrict is further exacerbated by exposure to E2. Furthermore, E2 administration increases baroreflex-mediated heart rate responses to orthostasis in low orthostatic tolerant women, which is likely to be a compensatory mechanism for the blunted peripheral vascular resistance and lower central volume.
Key points
The maintenance of blood pressure upon standing is accomplished through an integration of physiological systems. The inability to maintain blood pressure upon standing is called orthostatic intolerance and occurs more frequently in women than in men.
Given that ovarian hormones fluctuate throughout the menstrual cycle, it is difficult to isolate the effects of oestradiol on cardiovascular control systems in humans.
We utilize a novel study design in which we suppress endogenous ovarian hormones, then add back oestradiol to isolate its effects on blood pressure-regulating systems.
We show that women with low orthostatic tolerance have a lower vasoconstrictor response to gravitational stress and lower stroke volume in comparison to women with normal/high orthostatic tolerance. Oestradiol further suppresses the vasoconstrictor response to gravitational stress in women with low orthostatic tolerance; heart rate increases more to compensate for this impairment.
These results help us to better understand why women are more susceptible to orthostatic intolerance and how oestradiol affects the regulation of blood pressure.
Introduction
Women generally have a lower orthostatic tolerance than men (White et al. 1996; Convertino, 1998) and are more likely to experience symptoms such as lightheadedness and syncope upon standing. Forty per cent of young women experience at least one syncopal episode in their lifetime and there is over a 60% chance of reccurrence (Ganzeboom et al. 2006). Women with low orthostatic tolerance routinely experience syncope when remaining upright even for short periods of time or when changing positions from supine or seated to standing. Despite no evidence of cardiovascular disease, syncope and the symptoms associated with orthostatic intolerance can be debilitating.
Cardiovascular responses to orthostatic stress are regulated by the autonomic nervous system via the baroreflex, and individuals with orthostatic intolerance often have impaired baroreflex function (Farquhar et al. 2000). When assuming an upright posture, there is a shift in blood volume (∼700 ml) to the lower limbs. This translocation of blood volume is sensed by the baroreceptors and results in a reflex response of increases in both heart rate (HR) and peripheral vasoconstriction to maintain blood pressure and support blood flow to the heart and brain (Rowell, 1993). Thus, impairments of the end-organ responses of increased heart rate and peripheral vasoconstriction during baroreflex unloading contribute to lower orthostatic tolerance and, in some cases, frequent orthostatic intolerance. Autonomic function is modulated by ovarian hormones such as oestrogens and progesterone; we propose that the influence of these hormones on blood pressure control systems may play a role in the greater prevalence of low orthostatic tolerance in women.
Evidence in animal models and in humans indicates that ovarian hormones modulate baroreflex-mediated changes in heart rate and peripheral vasoconstriction. In rodents, baroreflex control of heart rate is reduced after ovariectomy (el-Mas & Abdel-Rahman, 1998), whereas administration of oestradiol after ovariectomy enhances baroreflex control of heart rate (el-Mas & Abdel-Rahman, 1998; Pamidimukkala et al. 2003). In women, however, it has been difficult to isolate the effects of oestradiol on baroreflex-mediated changes in heart rate and peripheral vasoconstriction because oestrogens, progestins and gonadotrophins fluctuate during the menstrual cycle and vary widely with age and among individuals. Thus, it is not surprising that the findings regarding the effects of ovarian hormones on baroreflex function are conflicting (Minson et al. 2000a; Cooke et al. 2002; Tanaka et al. 2003; Carter et al. 2009; Fu et al. 2009). Furthermore, although previous studies have examined baroreflex function during orthostatic challenges in women (Carter et al. 2009; Fu et al. 2009), none has considered individual differences in baseline orthostatic tolerance or has specifically targeted women with low orthostatic tolerance. We previously demonstrated that women with low orthostatic tolerance were insensitive to the vasoconstrictor effects of progesterone observed in women with high orthostatic tolerance (Wenner et al. 2011), indicating that sensitivity of ovarian hormones on cardiovascular control systems may play a role in compromised orthostatic tolerance in women.
The purpose of this study was to examine the interaction of orthostatic tolerance and exposure to oestradiol on the baroreflex-mediated changes in heart rate and peripheral vasoconstriction in women. To control and therefore isolate reproductive hormone exposure, we first suppressed endogenous hormone production with a gonadotrophin-releasing hormone antagonist (GnRHant), then administered oestradiol at a level similar to endogenous oestradiol exposures seen throughout a natural menstrual cycle. We hypothesized that oestradiol administration improves baroreflex-mediated changes in heart rate in women with low but not high orthostatic tolerance.
Methods
Fourteen non-smoking, healthy young women completed the study. All women had regular menstrual cycles (26–32 days), were not engaged in any regular exercise and reported no evidence of cardiovascular or gynaecological disease on a medical history questionnaire. All women gave written informed consent to participate in the study, which conformed to the guidelines contained in the Declaration of Helsinki and had prior approval by the Human Investigation Committee of Yale School of Medicine.
Determination of orthostatic tolerance
Each woman completed a maximal lower body negative pressure (LBNP) test to determine her level of orthostatic tolerance. Experiments were conducted in a temperature-controlled room (27°C, <30% relative humidity) in the morning after an overnight fast. Women were instructed to avoid alcohol and heavy exercise for 24 h prior to the study visit and to avoid caffeine for at least 12 h prior. Hydration status was confirmed by measuring urine specific gravity (<1.020). All subjects lay in the supine position with their legs inside the LBNP box, which was sealed at the level of the iliac crest. An intravenous catheter was placed in the left arm for blood sampling. Subjects were instrumented for measurements of heart rate (single-lead ECG), beat-to beat blood pressure (BP; Finometer; Finipres, Amsterdam, The Netherlands) and respiration (Pneumotrace II model 1132; UFI, Morro Bay, CA, USA). An automated upper arm blood pressure cuff was also used for standard brachial blood pressure measurements (Colin Medical Instruments, Kyoto, Japan) during the LBNP test as a back-up for subject safety, but the Finometer data were used in all analyses. After a 30 min supine rest period, a blood sample was taken and 5 min of baseline measurements commenced. The LBNP test started with application of negative pressure at −15 mmHg for 3 min, followed by −20 mmHg for 3 min. Each subsequent stage decreased in pressure by 10 mmHg (−30, −40, −50 mmHg, etc.) in 3 min intervals until presyncope (Fu et al. 2004b, 2005). Test termination was determined using any one of the following criteria: a decrease in systolic blood pressure (SBP) to <80 mmHg; a decrease in SBP to <90 mmHg associated with symptoms of lightheadedness, nausea, sweating or diaphoresis; or progressive symptoms of presyncope accompanied by a request from the subject to terminate the test. A second blood sample was taken at test termination. Blood samples were analysed for haemoglobin, haematocrit, noradrenaline (NA), adrenaline (Adr) and plasma renin activity (PRA). A cumulative stress index (CSI) was calculated for each woman by summing the product of the negative pressure (in millimetres of mercury) and the time (in minutes) spent at that stage (Fu et al. 2004b, 2005). A more negative CSI indicated a higher negative pressure attained prior to presyncopal symptoms and thus higher orthostatic tolerance. Use of CSI as an index of orthostatic tolerance is reproducible within an individual (Lightfoot et al. 1989, 1991; Howden et al. 2001). The women were divided into low (LT) and normal/high (HT) orthostatic tolerance groups. Low orthostatic tolerance was defined a priori as CSI ≥−600 mmHg min based on previous data (Sather et al. 1986; Fu et al. 2004a, 2005). Normal/high orthostatic tolerance was defined as CSI <−600 mmHg min. Given that maximal orthostatic tolerance level does not change across the different phases of the menstrual cycle (Meendering et al. 2005; Claydon et al. 2006; Fu et al. 2009), the maximal LBNP test was not performed at a specific time point of the menstrual cycle.
Hormonal intervention
Ganirelix acetate (Organon, Roseland, NJ, USA) is a synthetic decapeptide with high antagonistic activity against naturally occurring gonadotrophin-releasing hormone (GnRH). Ganirelix acetate is derived from native GnRH with substitutions at positions 1, 2, 3, 6, 8 and 10. When ganirelix acetate is given in therapeutic doses, it acts by competitively blocking the GnRH receptors on the pituitary gonadotroph and the subsequent transduction pathway. It induces a rapid, reversible suppression of gonadotrophin secretion (Oberye et al. 1999a,b). In young women with regular menstrual cycles, continued administration of ganirelix acetate leads to suppression of oestrogens and progesterone to postmenopausal levels. These decreases occur after 36–48 h of administration, and the suppression of the hypothalamic–pituitary–ovarian axis is reversed upon cessation of drug therapy (Oberye et al. 1999a,b).
Women began using the GnRH antagonist (250 μg in 0.5 ml normal saline) on days 25–28 of their menstrual cycle. At this point of the menstrual cycle, the corpus luteum is almost completely involuted and the endometrium is normally shed. We begin the GnRHant at this point to allow normal menstrual bleeding at the time of expected menses. Women self-administered (by subcutaneous injection) the antagonist daily for 10 days to suppress the normally variable production of endogenous reproductive hormones. 17β-Oestradiol (E2; 0.2 mg day−1 patch; Vivelle; CIBA Pharmaceuticals, Summit, NJ, USA) was administered on days 4–10 of the GnRHant administration to obtain a fixed and consistent E2 serum concentration. Women using hormonal oral contraceptive pills (n= 6; two HT and four LT) stopped taking their pills and began taking the injections on what would have been the final day of the pill cycle; they were not tested until a full 3 days after stopping their contraceptive pills.
Baroreflex protocol
All women completed a baroreflex assessment protocol in the following two experimental conditions: (i) GnRHant; and (ii) GnRHant + E2. The experimental protocols were separated by 1 week. During each visit, we assessed cardiovagal baroreflex function (baroreflex control of heart rate) during pharmacological perturbation of blood pressure (modified Oxford protocol; see ‘Baroreflex function assessment’ below) and integrated baroreflex function using LBNP (submaximal protocol; see ‘Baroreflex function assessment’ below). All studies were conducted in a temperature-controlled room (27°C, <30% relative humidity) in the morning after an overnight fast. As with the maximal LBNP studies, the women were instructed to avoid alcohol and heavy exercise for 24 h prior to the study visit and to avoid caffeine for at least 12 h prior. Hydration status was confirmed by measuring urine specific gravity (<1.020).
Baroreflex function assessment
Women lay in the supine position and were instrumented for measures of heart rate and blood pressure. Heart rate was measured by a single-lead ECG (Spacelabs, Inc., Redmond, WA, USA). Beat-by-beat blood pressure was measured by the Finometer. An intravenous catheter was inserted in an antecubetal vein, and blood was drawn for the analysis of E2 and progesterone (P4). After 30 min of supine rest, baseline heart rate and blood pressure were recorded for 10 min.
Cardiovagal baroreflex function was assessed with the modified Oxford procedure (Ebert & Cowley, 1992; Farquhar et al. 2000). Through the intravenous catheter, a bolus infusion of sodium nitroprusside (SNP; 100 μg) was given, followed 60 s later by a bolus of phenylephrine (PE; 150 μg). Following SNP administration, blood pressure falls initially, whereas PE causes blood pressure to rise, so that the total change in blood pressure is ∼15–20 mmHg. Two trials were performed, with a minimum of 15 min between each trial to allow HR and BP to return to baseline.
Integrated baroreflex function was assessed using LBNP. After completing the modified Oxford trials, the women moved to the LBNP box, lay supine and were sealed in the box at the level of the iliac crest with a neoprene skirt. During a 30 min rest period, women were instrumented for measures of heart rate (ECG), blood pressure (Finometer), stroke volume (Modelflow; derived from Finometer) and forearm blood flow with venous occlusion plethysmography (Hokanson EC6 Plethysmograph, Bellevue, WA, USA). Measurement of haemodynamic variables, such as stroke volume, using the Modelflow technique has been validated during various cardiovascular stressors, such as orthostasis (Harms et al. 1999; Matsukawa et al. 2004). Following baseline measures, the protocol consisted of the following stages, each lasting 4 min: a 5 deg head-down tilt; neutral (supine); and LBNP at −10, −20, −30 and −40 mmHg. Heart rate, blood pressure, stroke volume and forearm vascular resistance [FVR; mean arterial pressure (MAP)/forearm blood flow] were assessed during the last 2 min of each stage. While low-level LBNP has been used to isolate cardiopulmonary baroreceptor input, arterial baroreceptors appear to be involved even at very mild levels (under −20 mmHg) of LBNP (Lacolley et al. 1992; Taylor et al. 1995). We therefore use the term ‘integrated’ to acknowledge the limitations of distinguishing cardiopulmonary vs. arterial baroreceptor functions (Stachenfeld et al. 1998; Farquhar et al. 2000), which was not a focus of this investigation. We collected blood samples immediately prior to and following the LBNP study.
Blood analysis
An aliquot of whole blood was transferred into a tube without anticoagulant for the determination of serum 17β-oestradiol (s[E2]), progesterone (s[P4]) and aldosterone concentrations (s[Aldo]). A second aliquot was transferred into prechilled K+-EDTA tubes for the determination of PRA, angiotensin II ([Ang II]) and atrial natiuretic peptide ([ANP]). A final aliquot was placed into a prechilled K+-EDTA tube containing EGTA and glutathione for the determination of catecholamines {plasma noradrenaline ([pNA]) and adrenaline concentrations (p[Adr])}. The samples were centrifuged, frozen immediately and stored at −80°C until analysis.
Serum concentrations of E2, P4, Aldo, Ang II, ANP and PRA were measured using competitive binding radioimmunoassay methods. The intra-assay coefficient of variation for the mid-range standard for s[E2][176 (13) pg ml−1] was 2.4% (Siemens Healthcare Diagnostics, Los Angeles, CA, USA), and for s[P4][3.4 (0.2) ng ml−1] was 3.0% (Siemens Healthcare Diagnostics). Intra- and interassay coefficients of variation for PRA (standards range 4.5–8.3 ng Ang I ml−1 h−1) were 2.8 and 3.7%, respectively (Diasorin, Stillwater, MN, USA). Intra- and interassay coefficients of variation for Aldo [standard 155 (15.5) pg ml−1] were 1.7 and 1.6%, respectively (Siemens Healthcare Diagnostics). Intra- and interassay coefficients of variation for Ang II (standards range 14.3–72.2 pmol l−1) were 6.0 and 7.2%, respectively (IBL America, Minneapolis, MN, USA). Intra- and interassay coefficients of variation for ANP (standards range 6.2–18.8 pg ml−1) were 13.7 and 11.8%, respectively (ALPCO, Windham, NH, USA).
Catecholamines were analysed using high-performance liquid chromatography with electrochemical detection (Colorchem Detector, ESA Corp., Acton, MA, USA) with intra-assay and interassay coefficients of variation of 1 and 10%, respectively, for noradrenaline and adrenaline.
Data analysis and statistics
Baroreflex control of heart rate was assessed by measuring changes in R–R interval and systolic blood pressure during the modified Oxford procedure (Ebert & Cowley, 1992; Farquhar et al. 2000). The R–R interval data were binned in 2 mmHg blood pressure bins (Minson et al. 2000a,b). We used linear regression to determine the R–R interval and systolic blood pressure relationship and used the slope as an index of cardiovagal baroreflex sensitivity (Minson et al. 2000a,b). All regressions attained were r2≥ 0.80. Integrated baroreflex function was assessed during submaximal LBNP by determining changes in FVR as a function of LBNP, and the R–R interval–LBNP slope was assessed to determine baroreflex sensitivity. All data were recorded at 1000 Hz using LabChart 7 (ADInstruments, Bella Vista, NSW, Australia). We used Student's unpaired t tests to compare baseline characteristics and Student's paired t tests to compare the effects of oestradiol on cardiovagal baroreflex sensitivity in women with low and high orthostatic tolerance. A two-way repeated-measures ANOVA (SPSS 19; IBM Corporation, Armonk, NY, USA) was used to compare integrated baroreflex function (group by LBNP level). All data are presented as means ± SEM in graphs and means (SD) in tables.
Sample size calculations
Data from a similar group of healthy women demonstrated a change in cardiovagal baroreflex sensitivity of 2.5 (1.5) ms mmHg−1 (effect size 1.6) with administration of hormonal contraceptive (Minson et al. 2000b). Thus, with an α-level of 0.05, seven subjects per group allows >90% power to detect significant changes in baroreflex function due to E2 administration (Erdfelder et al. 1996; Faul et al. 2007, 2009).
Results
Seven women were classified as LT (CSI −438 ± 52, range −216 to −600 mmHg min) and seven women as HT (CSI −840 ± 54, range −645 to −1011 mmHg min). Haemodynamic and hormonal responses to the maximal LBNP test were similar in both groups (Table 1), and all women reached their maximal orthostatic tolerance by the previously described criteria. Women in the high and low orthostatic tolerance groups were also similar with respect to age (22 ± 1 vs. 22 ± 1 years) and body mass index (24 ± 1 vs. 22 ± 1 kg m−2).
Table 1.
Haemodynamic and hormonal responses to maximal lower body negative pressure (LBNP)
| Maximal LBNP test | ||||
|---|---|---|---|---|
| HT | LT | |||
| Baseline | Maximum | Baseline | Maximum | |
| SBP (mmHg) | 122 (7) | 83 (11)* | 124 (12) | 90 (27)* |
| DBP (mmHg) | 58 (7) | 46 (12)* | 61 (4) | 47 (16) |
| MAP (mmHg) | 79 (5) | 58 (11)* | 80 (6) | 60 (18)* |
| HR (beats min-1) | 63 (8) | 93 (12)* | 66 (6) | 93 (13)* |
| p[NA] (pg ml−1) | 103 (50) | 208 (120)* | 144 (45) | 227 (60)* |
| p[Adr] (pg ml−1) | 16 (16) | 38 (25) | 14 (12) | 50 (30)* |
| PRA (ng angiotensin I ml−1 h−1) | 1.7 (1.6) | 3.4 (2.3)* | 1.2 (0.9) | 3.0 (2.3)* |
| Haematocrit (%) | 35.9 (3.6) | 38.4 (2.7)* | 34.9 (3.6) | 36.6 (3.7)* |
| Haemoglobin (mg dl−1) | 11.9 (1.5) | 12.4 (1.2) | 11.0 (2.1) | 11.3 (2.2) |
Data are presented as means (SD). Abbreviations: DBP, diastolic blood pressure; HR, heart rate; HT, high orthostatic tolerant women; LT, low orthostatic tolerant women; MAP, mean arterial pressure; p[Adr], plasma adrenaline concentration; p[NA], plasma noradrenaline concentration; PRA, plasma renin activity; and SBP, systolic blood pressure.
P < 0.05 compared with baseline within group.
Administration of GnRH antagonist suppressed s[E2] and s[P4] in both groups, and s[E2] increased in both groups when E2 was administered (Table 2; P < 0.05). Resting supine BP was similar between HT and LT during hormone suppression with the GnRHant (Table 2). Although the mean resting SBP was 10 mmHg lower in LT compared with HT, this difference was due to one subject with SBP of 103 mmHg, accounting both for the lower mean and the large SD in LT. This woman was not an outlier (i.e. not greater than two standard deviations from the mean in BP) and was similar to the other LT subjects in the other physiological parameters, so was retained in the study. Administration of E2 decreased supine SBP, diastolic blood pressure and MAP in HT but not LT (Table 2). Resting p[Ang II] was higher, but resting p[ANP] was lower in LT compared with HT during GnRHant, but these group differences were not apparent during E2 administration (Table 3). Resting p[NA] was similar between LT and HT and was not altered by E2 administration (Table 3).
Table 2.
Reproductive hormones and cardiovascular variables during hormone intervention
| HT | LT | |||
|---|---|---|---|---|
| Parameter | GnRHant | E2 | GnRHant | E2 |
| s[E2] (pg ml−1) | 28.4 (19.4) | 155.8 (79.3)* | 18.3 (17) | 190.6 (105.9)* |
| s[P4] (ng ml−1) | 0.8 (0.5) | 1.0 (0.6) | 0.7 (0.3) | 0.7 (0.3) |
| SBP (mmHg) | 125 (5) | 114 (8)* | 115 (11) | 110 (9) |
| DBP (mmHg) | 58 (8) | 54 (4)* | 58 (13) | 57 (10) |
| MAP (mmHg) | 79 (5) | 74 (4)* | 75 (13) | 75 (9) |
| HR (beats min-1) | 64 (10) | 67 (8) | 65 (8) | 68 (9)* |
Data are presented as means (SD). Abbreviations: DBP, diastolic blood pressure; E2, oestradiol; GnRHant, gonadotrophin-releasing hormone antagonist; HR, heart rate; HT, high orthostatic tolerant women; LT, low orthostatic tolerant women; MAP, mean arterial pressure; SBP, systolic blood pressure; s[E2], serum oestradiol concentration; and s[P4], serum progesterone concentration.
P < 0.05 compared with GnRHant.
Table 3.
Hormonal responses to submaximal LBNP
| HT | LT | |||||||
|---|---|---|---|---|---|---|---|---|
| GnRHant | E2 | GnRHant | E2 | |||||
| Parameter | Baseline | Final | Baseline | Final | Baseline | Final | Baseline | Final |
| PRA (ng angiotensin | 0.3 (0.2) | 0.8 (0.7)* | 0.6 (0.4) | 1.5 (1.2)* | 0.5 (0.4) | 1.4 (1.1)* | 0.8 (0.9) | 1.8 (1.7) |
| I ml−1 h−1) | ||||||||
| Ang II (pmol l−1) | 6.1 (1.3) | 9.0 (2.6)* | 7.5 (2.1) | 10.7 (5.1)* | 9.6 (2.6)‡ | 12.7 (3.0)*‡ | 9.5 (4.6) | 14.9 (9.0) |
| Aldo (pg ml−1) | 24.5 (13.9) | 32.7 (19.9) | 45.9 (24.5) | 50.0 (30.9) | 50.6 (32.1) | 42.8 (43.5) | 55.3 (43.4) | 65.6 (63.4) |
| p[NA] (pg ml−1) | 128 (51) | 160 (96) | 130 (40) | 181 (77)* | 139 (22) | 182 (44) | 122 (29) | 181 (34)* |
| p[Adr] (pg ml−1) | 8.6 (5.8) | 17.3 (9.9)* | 11.0 (5.1) | 28.1 (8.6)*† | 9.8 (5.0) | 12.5 (7.3) | 7.7 (4.8) | 21.7 (17.0) |
| ANP (pg ml−1) | 80.0 (23.1) | 68.7 (16.3) | 80.3 (28.4) | 60.6 (17.0)* | 51.6 (18.6)‡ | 37.2 (17.5)‡* | 73.9 (28.5) | 62.1 (23.1) |
Data are presented as means (SD). Abbreviations: Aldo, aldosterone; Ang II, angiotensin II; ANP, atrial naturetic peptide; E2, oestradiol; GnRHant, gonadotrophin-releasing hormone antagonist; HT, high orthostatic tolerant women; LT, low orthostatic tolerant women; p[Adr], plasma adrenaline; p[NA], plasma noradrenaline; and PRA, plasma renin activity.
P≤ 0.05 compared with baseline.
P < 0.05 compared with GnRHant.
P < 0.05 compared with HT.
During submaximal LBNP, FVR increased in HT women to a similar extent during both GnRHant and E2 conditions (Fig. 1). Within HT, the slope of the relationship between LBNP and FVR increased during E2 administration (GnRHant slope 0.158 ± 0.025 units mmHg−1 vs. E2 slope 0.263 ± 0.036 units mmHg−1, P= 0.04), but this slope was unaffected by E2 administration in LT (GnRHant slope 0.140 ± 0.034 units mmHg−1 vs. E2 slope 0.128 ± 0.044 units mmHg−1, P= 0.84). In contrast, FVR did not increase during submaximal LBNP in LT women during either hormonal condition. However, FVR was lower during E2 exposure in LT women (Fig. 1; P < 0.05). Stroke volume decreased to a similar extent during submaximal LBNP in all women in both GnRHant and E2 conditions (Fig. 2), although stroke volume was lower in LT at baseline and throughout LBNP (P < 0.05). Cardiovagal baroreflex sensitivity was similar between HT and LT during administration of GnRHant alone, but additional administration of E2 increased baroreflex sensitivity only in LT (Fig. 3). During LBNP, the R–R interval shortened progressively in both groups (GnRHant: HT from 1006 ± 53 to 786 ± 44 ms and LT from 967 ± 41 to 711 ± 26 ms, from rest to −40 mmHg LBNP), with no effect of E2 administration on the R–R interval in either group (E2: HT from 951 ± 58 to 721 ± 40 ms and LT from 974 ± 32 to 697 ± 34 ms, from rest to −40 mmHg LBNP). The slope of the relationship between the R–R interval and LBNP was similar between HT and LT regardless of hormone condition (GnRHant slope, HT −4.60 ± 0.76 and LT −5.37 ± 0.73 ms mmHg−1; and E2 slope, HT −4.72 ± 0.50 and LT −5.97 ± 1.02 ms mmHg−1). Plasma concentrations of Ang II and PRA increased in HT during submaximal LBNP in both hormone conditions, but increased during GnRHant only in LT (Table 3). Plasma concentrations of Adr increased in HT during submaximal LBNP in both hormone conditions, but did not increase in LT (Table 3). Plasma concentrations of NA increased to a similar extent in HT and LT during submaximal LBNP, but only during E2 administration (Table 3).
Figure 1.
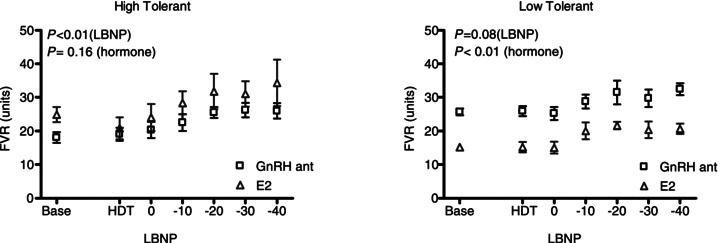
Forearm vascular resistance (FVR) in women with high (left panel) and low orthostatic tolerance (right panel) during gonadotrophin-releasing hormone antagonist (GnRHant) and 17β-oestradiol (E2) administration as a function of lower body negative pressure (LBNP) preceded by head-down tilt (HDT)
Figure 2.
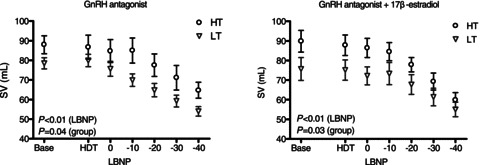
Stroke volume (SV) changes during GnRHant (left panel) and E2 administration (right panel) in women with high (HT) and low orthostatic tolerance (LT) as a function of LBNP preceded by head-down tilt (HDT)
Figure 3. Cardiovagal baroreflex sensitivity (CV BRS) in women with high (left panel) and low orthostatic tolerance (right panel) during GnRHant and E2 administration.
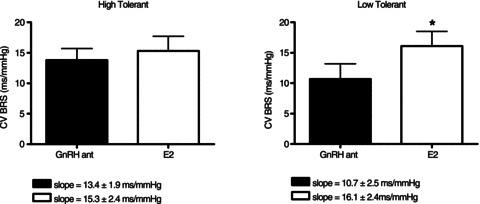
*P < 0.05 compared with GnRHant alone.
Discussion
Ours is the first study to isolate the effects of oestradiol on baroreflex function specifically in young women with low orthostatic tolerance. Orthostatic intolerance remains a debilitating medical issue for many young women, but the mechanisms contributing to their low orthostatic tolerance are unclear. The primary findings of the present study are as follows: (i) peripheral vasoconstriction during baroreflex unloading is blunted in women with low compared with high orthostatic tolerance; (ii) oestradiol administration reduces peripheral vasoconstriction during baroreflex unloading in women with low orthostatic tolerance; (iii) stroke volume is lower across all levels of LBNP in women with low compared with high orthostatic tolerance; and (iv) oestradiol exposure increases baroreflex control of heart rate in women with low but not high orthostatic tolerance. Taking these findings together, it is clear that a greater sensitivity to oestradiol contributes to low orthostatic tolerance in young, otherwise healthy women. Thus, we propose that women with low orthostatic tolerance have less vasoconstrictor reserve, which accounts for their greater risk of syncope when exposed to a vasodilator substance, such as oestradiol. Our data also demonstrate that women with low orthostatic tolerance compensate for this blunted peripheral vasoconstrictor ability and reduced stroke volume through baroreflex-mediated increases in heart rate to maintain posture. These findings are consistent with previous data suggesting that factors such as stroke volume and cardiac output are as important as neural mechanisms to explain low orthostatic tolerance in women (Fu et al. 2004a,b, 2005, 2009).
Oestradiol and progesterone exposure alter peripheral vascular function (Freedman & Girgis, 2000; Wenner et al. 2011). During the menstrual cycle, brachial artery flow-mediated vasodilatation increases during the late follicular phase immediately prior to ovulation, when oestrogen exposure peaks (Adkisson et al. 2010). Furthermore, oestrogen exposure attenuates the vasoconstrictor effects of noradrenaline (Sudhir et al. 1996). Oestrogen receptors are located on both the endothelium and vascular smooth muscle. Oestradiol exposure increases NO bioavailability, and it is well accepted that oestrogen-associated vasodilatation occurs through an NO-mediated mechanism (Sudhir et al. 1996). In the present investigation, we demonstrate that the increase in FVR during mild LBNP is blunted in women with low orthostatic tolerance by as much as 20% at −40 mmHg of LBNP compared with women having high orthostatic tolerance (Fig. 1). We interpret these findings to indicate that women with low orthostatic tolerance are ∼20% less effective at vasoconstriction in the support of blood pressure during orthostatic stress. Oestradiol administration shifts this FVR response lower in women with low orthostatic tolerance, such that oestradiol exposure is associated with an attenuation of peripheral vasoconstriction. Given the similar p[NA] responses in the women with either high or low orthostatic tolerance, the lower vasoconstrictor response in women with low orthostatic tolerance during LBNP reveals reduced vascular responsiveness to sympathetic nervous system activation. This conclusion is consistent with the findings of little sympathetic support for blood pressure in young women (Hart et al. 2009), which may be a function of greater β-adrenergic vasodilatation in women (Hart et al. 2011). Whether this attenuated vasoconstrictor response in women with low orthostatic tolerance is due to lower sympathetic transduction or whether differences in adrenergic receptor-mediated control of peripheral blood flow contribute to low orthostatic tolerance is an interesting area for future investigation.
Several studies have investigated the contribution of ovarian hormones to cardiovascular regulatory control by examining changes in autonomic function either during different phases of the menstrual cycle or during oral contraceptive administration and have yielded conflicting data (Minson et al. 2000a,b; Carter et al. 2009, 2010; Fu et al. 2009). For example, Minson et al. (2000a) demonstrated greater sympathetic baroreflex sensitivity (modified Oxford technique) during the midluteal compared with the early follicular phase of the menstrual cycle, and no change in cardiovagal baroreflex sensitivity, which was interpreted to mean that elevations in both oestrogens and progesterone enhance sympathetic baroreflex function. However, subsequent studies comparing the follicular and luteal phases of the menstrual cycle during orthostatic challenges using tilt-table testing (Fu et al. 2009) or LBNP (Carter et al. 2009) did not find changes the in sympathetic and cardiovagal baroreflex sensitivity as a function of reproductive hormone status. Moreover, oral contraceptives containing both oestradiol and progesterone attenuated sympathetic and cardiovagal baroreflex sensitivity compared with the contraceptive placebo phase during modified Oxford studies (Minson et al. 2000b), while oral contraceptive exposure did not impact baroreflex function during an LBNP protocol (Carter et al. 2010). We suspect that differences in hormone exposure between the menstrual phase studies and the studies using oral contraceptive contribute to the different findings with regard to baroreflex sensitivity across previous studies. Oestrogen and progestin exposures are much higher with oral contraceptives relative to menstrual phases, and the placebo phase has limitations as a ‘low hormone’ period (van Heusden & Fauser, 1999).
In the present study, we classified women based on orthostatic tolerance level and controlled ovarian hormone exposure to isolate the effects of oestradiol on baroreflex function with our hormone suppression–add back model. We demonstrated that oestradiol exposure increases baroreflex control of heart rate in women with low orthostatic tolerance. Interestingly, we saw no differences related to orthostatic tolerance level in the HR response during LBNP in our subjects. This finding is in contrast to studies in men demonstrating a greater increase in HR in men with high tolerance, indicating a higher HR reserve (Convertino et al. 2012). We propose that the differences between studies are related to sex differences in cardiovascular regulatory control in high and low tolerant men and women and warrant further investigation. Additionally, future studies may also examine maximal orthostatic tolerance changes with oestradiol exposure, either using oral contraceptives or our hormone suppression–add back model.
Women have consistently lower stroke volume and greater heart rate responses to LBNP than men (with similar peripheral vasoconstriction), indicating that their smaller cardiac volume contributes to the lower orthostatic tolerance in women than men (Fu et al. 2004a, 2005). The data in this present investigation extend these findings (Fu et al. 2004a, 2005) to demonstrate that low stroke volume is also a contributor to low orthostatic tolerance within women. Thus, our data suggest that lower cardiac volume is not only important with regard to sex differences in orthostatic tolerance, but is also an important predictor of orthostatic tolerance within women. Based on the findings of the present study and previous data from our laboratory (Wenner et al. 2011), ovarian hormone sensitivity also contributes to orthostatic tolerance within women. Taken together with the present findings, women with low orthostatic tolerance appear to be more sensitive to oestradiol exposure, whereas women with high orthostatic tolerance are more sensitive to progesterone exposure. Serum oestradiol levels measured during the initial maximal LBNP test were comparable between groups (LT 55.4 ± 20.9 vs. HT 58.4 ± 22.0 pg/mL). Although there is some slight variability because of absorption and metabolism, serum oestradiol was similar between groups during oestradiol administration (P= 0.51; Table 2), yet baroreflex-mediated changes in forearm vascular resistance and heart rate were altered in low tolerant women only, suggesting that women with low orthostatic tolerance are more sensitive to oestradiol exposure.
Our findings of greater p[Ang II] but lower p[ANP] in women with low orthostatic tolerance merit comment. These hormones can vary at rest and across the menstrual cycle (Stachenfeld et al. 1999) and are within normal clinical limits for both groups. It is, however, somewhat unexpected that the powerful vasoconstrictor (Ang II) is elevated in women with low orthostatic tolerance. These findings merit further study, but may be compensatory for impaired vasoconstrictor function. Finally, oestradiol activates ANP in rat hearts (Jankowski et al. 2001) and hormone therapy increases p[ANP] in postmenopausal women (Maffei et al. 2001), indicating that this hormone is sensitive to alterations in oestradiol. The interaction of these cardiovascular and fluid regulatory hormones with sex hormones and the role they play in orthostatic tolerance in women is an exciting area for future research.
In the present investigation, we relied on p[NA] as our index of sympathetic nervous system activity because we were not able consistently to obtain resting nerve activity in women with low orthostatic tolerance in order to make meaningful inferences regarding differences in muscle sympathetic nerve activity between the groups. Interestingly, challenges attaining consistent nerve activity in low orthostatic tolerance were recently reported for this same group during LBNP (Convertino et al. 2012). Thus, there are technical challenges to performing microneurography in both men and women, but in our hands, these challenges were exaggerated in women with low orthostatic tolerance, who often had lower resting nerve activity. Therefore, other measures, such as p[NA] or NA spillover, may be more reliable indicators of sympathetic nervous system activity in women with low orthostatic tolerance. Importantly, our data demonstrate different peripheral vasoconstrictor responses between high and low orthostatic tolerance despite similar increases in p[NA], and that women with low orthostatic tolerance are more sensitive to oestradiol effects in the peripheral vasculature. Finally, because muscle sympathetic nerve activity is not a good predictor of total peripheral resistance in young women (Hart et al. 2009), future studies can focus on adrenergic receptor function in women with high and low orthostatic tolerance to determine whether β-adrenergic vasodilatation (Hart et al. 2011) is greater in women with low orthostatic tolerance.
We recognize that FVR as an index of peripheral vasoconstriction is limited to the measure of one regional vascular bed. However, FVR reflects overall peripheral circulatory responses during LBNP (Tripathi & Nadel, 1986; Tripathi et al. 1989; Mack et al. 1991; Convertino et al. 1994; Convertino, 1998). A measure of total peripheral resistance derived from the Modelflow method would be of interest, but we did not directly capture this variable, and its calculation introduced considerable variability.
Perspectives
Orthostatic intolerance is the most common blood pressure disorder in young women, affecting approximately 500,000 Americans (Robertson, 1999), 10% of whom are otherwise healthy young women. Our data indicate that peripheral vascular responses in women with lower orthostatic tolerance are more sensitive to oestradiol exposure compared with those of women who have normal or high orthostatic tolerance. Taken together with our previous findings indicating that women with low orthostatic tolerance are less sensitive to effects of progesterone on peripheral vasoconstriction (Wenner et al. 2011), we have demonstrated that cardiovascular sensitivity to changes in ovarian hormone exposure is an important predictor for low orthostatic tolerance. Younger and older women using oestradiol either for contraception or for menopausal hormone therapy may need to consider alternative therapies if they experience episodes of orthostatic intolerance. Finally, these findings demonstrate the importance of not only controlling for ovarian hormones when studying orthostatic tolerance in women, but also considering preparticipation orthostatic tolerance level when testing autonomic function in women.
Acknowledgments
We gratefully acknowledge Cheryl Leone MA, Andy Grabarek BS and Justus Verhagen PhD for technical assistance, Osama Abdelghany PharmD, BCOP for modified Oxford drug preparations and the subjects for their time. This research was supported by NIH grant R01 HL071159 awarded to N.S.S.
Glossary
- Adr
adrenaline
- Aldo
aldosterone
- Ang II
angiotensin II
- BP
blood pressure
- CSI
cumulative stress index
- CV BRS
cardiovagal baroreflex sensitivity
- DBP
diastolic blood pressure
- E2
17β-oestradiol
- FVR
forearm vascular resistance
- GnRH
gonadotrophin-releasing hormone
- GnRHant
gonadotrophin-releasing hormone antagonist
- HDT
head-down tilt
- HR
heart rate
- HT
high tolerant
- LBNP
lower body negative pressure
- LT
low tolerant
- MAP
mean arterial pressure
- NA
noradrenaline
- NO
nitric oxide
- p[Adr]
plasma adrenaline concentration
- PE
phenylephrine
- [pNA]
plasma noradrenaline concentration
- PRA
plasma renin activity
- P4
progesterone
- s[Aldo]
serum aldosterone concentration
- SBP
systolic blood pressure
- s[E2]
serum 17-β oestradiol concentration
- SNP
sodium nitroprusside
- s[P4]
serum progesterone concentration
- SV
stroke volume
Author contributions
M.M.W. participated in the concepts underlying this work, data collection, data analysis and the writing of the manuscript. A.S.H. provided medical supervision and participated in the concepts underlying this work, data collection and the writing of the manuscript. H.S.T. provided medical oversight and participated in the concepts underlying this work and the writing of the manuscript. N.S.S. participated in the concepts underlying this work, supervision, data collection, data analysis and the writing of the manuscript. All authors approved the final version of the manuscript. All experiments were performed at The John B. Pierce Laboratory.
References
- Adkisson EJ, Casey DP, Beck DT, Gurovich AN, Martin JS, Braith RW. Central, peripheral and resistance arterial reactivity: fluctuates during the phases of the menstrual cycle. Exp Biol Med (Maywood) 2010;235:111–118. doi: 10.1258/ebm.2009.009186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JR, Klein JC, Schwartz CE. Effects of oral contraceptives on sympathetic nerve activity during orthostatic stress in young, healthy women. Am J Physiol Regul Integr Comp Physiol. 2010;298:R9–R14. doi: 10.1152/ajpregu.00554.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JR, Lawrence JE, Klein JC. Menstrual cycle alters sympathetic neural responses to orthostatic stress in young, eumenorrheic women. Am J Physiol Endocrinol Metab. 2009;297:E85–E91. doi: 10.1152/ajpendo.00019.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claydon VE, Younis NR, Hainsworth R. Phase of the menstrual cycle does not affect orthostatic tolerance in healthy women. Clin Auton Res. 2006;16:98–104. doi: 10.1007/s10286-006-0330-y. [DOI] [PubMed] [Google Scholar]
- Convertino VA. Gender differences in autonomic functions associated with blood pressure regulation. Am J Physiol Regul Integr Com Physiol. 1998;275:R1909–R1920. doi: 10.1152/ajpregu.1998.275.6.R1909. [DOI] [PubMed] [Google Scholar]
- Convertino VA, Doerr DF, Ludwig DA, Vernikos J. Effect of simulated microgravity on cardiopulmonary baroreflex control of forearm vascular resistance. Am J Physiol Regul Integr Comp Physiol. 1994;266:R1962–R1969. doi: 10.1152/ajpregu.1994.266.6.R1962. [DOI] [PubMed] [Google Scholar]
- Convertino VA, Rickards CA, Ryan KL. Autonomic mechanisms associated with heart rate and vasoconstrictor reserves. Clin Auton Res. 2012;22:123–130. doi: 10.1007/s10286-011-0151-5. [DOI] [PubMed] [Google Scholar]
- Cooke WH, Ludwig DA, Hogg PS, Eckberg DL, Convertino VA. Does the menstrual cycle influence the sensitivity of vagally mediated baroreflexes. Clin Sci (Lond) 2002;102:639–644. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- Ebert TJ, Cowley AW., Jr Baroreflex modulation of sympathetic outflow during physiological increases of vasopressin in humans. Am J Physiol Heart Circ Physiol. 1992;262:H1372–H1378. doi: 10.1152/ajpheart.1992.262.5.H1372. [DOI] [PubMed] [Google Scholar]
- el-Mas MM, Abdel-Rahman AA. Estrogen enhances baroreflex control of heart rate in conscious ovariectomized rats. Can J Physiol Pharmacol. 1998;76:381–386. [PubMed] [Google Scholar]
- Erdfelder E, Faul F, Buchner A. GPOWER: a general power analysis program. Behavior Research Methods, Instruments, & Computers. 1996;28(1):1–11. [Google Scholar]
- Farquhar WB, Taylor JA, Darling SE, Chase KP, Freeman R. Abnormal baroreflex responses in patients with idiopathic orthostatic intolerance. Circulation. 2000;102:3086–3091. doi: 10.1161/01.cir.102.25.3086. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Freedman RR, Girgis R. Effects of menstrual cycle and race on peripheral vascular α-adrenergic responsiveness. Hypertension. 2000;35:795–799. doi: 10.1161/01.hyp.35.3.795. [DOI] [PubMed] [Google Scholar]
- Fu Q, Arbab-Zadeh A, Perhonen MA, Zhang R, Zuckerman JH, Levine BD. Hemodynamics of orthostatic intolerance: implications for gender differences. Am J Physiol Heart Circ Physiol. 2004a;286:H449–H457. doi: 10.1152/ajpheart.00735.2002. [DOI] [PubMed] [Google Scholar]
- Fu Q, Okazaki K, Shibata S, Shook RP, VanGunday TB, Galbreath MM, Reelick MF, Levine BD. Menstrual cycle effects on sympathetic neural responses to upright tilt. J Physiol. 2009;587:2019–2031. doi: 10.1113/jphysiol.2008.168468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q, Witkowski S, Levine BD. Vasoconstrictor reserve and sympathetic neural control of orthostasis. Circulation. 2004b;110:2931–2937. doi: 10.1161/01.CIR.0000146384.91715.B5. [DOI] [PubMed] [Google Scholar]
- Fu Q, Witkowski S, Okazaki K, Levine BD. Effects of gender and hypovolemia on sympathetic neural responses to orthostatic stress. Am J Physiol Regul Integr Comp Physiol. 2005;289:R109–R116. doi: 10.1152/ajpregu.00013.2005. [DOI] [PubMed] [Google Scholar]
- Ganzeboom KS, Mairuhu G, Reitsma JB, Linzer M, Wieling W, van Dijk N. Lifetime cumulative incidence of syncope in the general population: a study of 549 Dutch subjects aged 35–60 years. J Cardiovasc Electrophysiol. 2006;17:1172–1176. doi: 10.1111/j.1540-8167.2006.00595.x. [DOI] [PubMed] [Google Scholar]
- Harms MP, Wesseling KH, Pott F, Jenstrup M, Van Goudoever J, Secher NH, Van Lieshout JJ. Continuous stroke volume monitoring by modelling flow from non-invasive measurement of arterial pressure in humans under orthostatic stress. Clin Sci (Lond) 1999;97:291–301. [PubMed] [Google Scholar]
- Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH, Joyner MJ. Sex differences in sympathetic neural-hemodynamic balance: implications for human blood pressure regulation. Hypertension. 2009;53:571–576. doi: 10.1161/HYPERTENSIONAHA.108.126391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach J, Joyner MJ. Sex and ageing differences in resting arterial pressure regulation: the role of the β-adrenergic receptors. J Physiol. 2011;589:5285–5297. doi: 10.1113/jphysiol.2011.212753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howden R, Tranfield PA, Lightfoot JT, Brown SJ, Swaine IL. The reproducibility of tolerance to lower-body negative pressure and its quantification. Eur J Appl Physiol. 2001;84:462–468. doi: 10.1007/s004210100398. [DOI] [PubMed] [Google Scholar]
- Jankowski M, Rachelska G, Donghao W, McCann SM, Gutkowska J. Estrogen receptors activate atrial natriuretic peptide in the rat heart. Proc Natl Acad Sci U S A. 2001;98:11765–11770. doi: 10.1073/pnas.201394198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacolley PJ, Pannier BM, Slama MA, Cuche JL, Hoeks AP, Laurent S, London GM, Safar ME. Carotid arterial haemodynamics after mild degrees of lower-body negative pressure in man. Clin Sci (Lond) 1992;83:535–540. doi: 10.1042/cs0830535. [DOI] [PubMed] [Google Scholar]
- Lightfoot JT, Febles S, Fortney SM. Adaptation to repeated presyncopal lower body negative pressure exposures. Aviat Space Environ Med. 1989;60:17–22. [PubMed] [Google Scholar]
- Lightfoot JT, Hilton F, Jr, Fortney SM. Repeatability and protocol comparability of presyncopal symptom limited lower body negative pressure exposures. Aviat Space Environ Med. 1991;62:19–25. [PubMed] [Google Scholar]
- Mack GW, Thompson CA, Doerr DF, Nadel ER, Convertino VA. Diminished baroreflex control of forearm vascular resistance following training. Med Sci Sports Exerc. 1991;23:1367–1374. [PubMed] [Google Scholar]
- Maffei S, Del Ry S, Prontera C, Clerico A. Increase in circulating levels of cardiac natriuretic peptides after hormone replacement therapy in postmenopausal women. Clin Sci (Lond) 2001;101:447–453. [PubMed] [Google Scholar]
- Matsukawa K, Kobayashi T, Nakamoto T, Murata J, Komine H, Noso M. Noninvasive evaluation of cardiac output during postural change and exercise in humans: comparison between the modelflow and pulse dye-densitometry. Jpn J Physiol. 2004;54:153–160. doi: 10.2170/jjphysiol.54.153. [DOI] [PubMed] [Google Scholar]
- Meendering JR, Torgrimson BN, Houghton BL, Halliwill JR, Minson CT. Menstrual cycle and sex affect hemodynamic responses to combined orthostatic and heat stress. Am J Physiol Heart Circ Physiol. 2005;289:H631–H642. doi: 10.1152/ajpheart.00029.2005. [DOI] [PubMed] [Google Scholar]
- Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation. 2000a;101:862–868. doi: 10.1161/01.cir.101.8.862. [DOI] [PubMed] [Google Scholar]
- Minson CT, Halliwill JR, Young TM, Joyner MJ. Sympathetic activity and baroreflex sensitivity in young women taking oral contraceptives. Circulation. 2000b;102:1473–1476. doi: 10.1161/01.cir.102.13.1473. [DOI] [PubMed] [Google Scholar]
- Oberye JJ, Mannaerts BM, Huisman JA, Timmer CJ. Pharmacokinetic and pharmacodynamic characteristics of ganirelix (Antagon/Orgalutran). Part II. Dose-proportionality and gonadotropin suppression after multiple doses of ganirelix in healthy female volunteers. Fertil Steril. 1999a;72:1006–1012. doi: 10.1016/s0015-0282(99)00414-8. [DOI] [PubMed] [Google Scholar]
- Oberye JJ, Mannaerts BM, Kleijn HJ, Timmer CJ. Pharmacokinetic and pharmacodynamic characteristics of ganirelix (Antagon/Orgalutran). Part I. Absolute bioavailability of 0.25 mg of ganirelix after a single subcutaneous injection in healthy female volunteers. Fertil Steril. 1999b;72:1001–1005. doi: 10.1016/s0015-0282(99)00413-6. [DOI] [PubMed] [Google Scholar]
- Pamidimukkala J, Taylor JA, Welshons WV, Lubahn DB, Hay M. Estrogen modulation of baroreflex function in conscious mice. Am J Physiol Regul Integr Comp Physiol. 2003;284:R983–R989. doi: 10.1152/ajpregu.00761.2001. [DOI] [PubMed] [Google Scholar]
- Robertson D. The epidemic of orthostatic tachycardia and orthostatic intolerance. Am J Med Sci. 1999;317:75–77. doi: 10.1097/00000441-199902000-00001. [DOI] [PubMed] [Google Scholar]
- Rowell L. Human Cardiovascular Control. New York: Oxford University Press, Inc; 1993. [Google Scholar]
- Sather TM, Goldwater DJ, Montgomery LD, Convertino VA. Cardiovascular dynamics associated with tolerance to lower body negative pressure. Aviat Space Environ Med. 1986;57:413–419. [PubMed] [Google Scholar]
- Stachenfeld NS, DiPietro L, Kokoszka CA, Silva C, Keefe DL, Nadel ER. Physiological variability of fluid-regulation hormones in young women. J Appl Physiol. 1999;86:1092–1096. doi: 10.1152/jappl.1999.86.3.1092. [DOI] [PubMed] [Google Scholar]
- Stachenfeld NS, Mack GW, DiPietro L, Morocco TS, Jozsi AC, Nadel ER. Regulation of blood volume during training in post-menopausal women. Med Sci Sports Exerc. 1998;30:92–98. doi: 10.1097/00005768-199801000-00013. [DOI] [PubMed] [Google Scholar]
- Sudhir K, Jennings GL, Funder JW, Komesaroff PA. Estrogen enhances basal nitric oxide release in the forearm vasculature in perimenopausal women. Hypertension. 1996;28:330–334. doi: 10.1161/01.hyp.28.3.330. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Sato M, Umehara S, Nishikawa T. Influence of menstrual cycle on baroreflex control of heart rate: comparison with male volunteers. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1091–R1097. doi: 10.1152/ajpregu.00162.2003. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Halliwill JR, Brown TE, Hayano J, Eckberg DL. ‘Non-hypotensive’ hypovolaemia reduces ascending aortic dimensions in humans. J Physiol. 1995;483:289–298. doi: 10.1113/jphysiol.1995.sp020585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi A, Mack G, Nadel ER. Peripheral vascular reflexes elicited during lower body negative pressure. Aviat Space Environ Med. 1989;60:1187–1193. [PubMed] [Google Scholar]
- Tripathi AG, Nadel ER. Forearm skin and muscle vasoconstriction during lower body negative pressure. J Appl Physiol. 1986;60:1535–1541. doi: 10.1152/jappl.1986.60.5.1535. [DOI] [PubMed] [Google Scholar]
- van Heusden AM, Fauser BC. Activity of the pituitary-ovarian axis in the pill-free interval during use of low-dose combined oral contraceptives. Contraception. 1999;59:237–243. doi: 10.1016/s0010-7824(99)00025-6. [DOI] [PubMed] [Google Scholar]
- Wenner MM, Taylor HS, Stachenfeld NS. Progesterone enhances adrenergic control of skin blood flow in women with high but not low orthostatic tolerance. J Physiol. 2011;589:975–986. doi: 10.1113/jphysiol.2010.194563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DD, Gotshall RW, Tucker A. Women have lower tolerance to lower body negative pressure than men. J Appl Physiol. 1996;80:1138–1143. doi: 10.1152/jappl.1996.80.4.1138. [DOI] [PubMed] [Google Scholar]


