Key Points
This work is the first identification of a neutrophil surface thiol isomerase regulating adhesive function of αMβ2 integrin.
PDI is required for neutrophil recruitment during vascular inflammation and its isomerase activity is critical for the regulatory effect.
Abstract
β2 integrins play a crucial role during neutrophil recruitment into the site of vascular inflammation. However, it remains unknown how ligand-binding activity of the integrin is regulated. Using fluorescence intravital microscopy in mice generated by crossing protein disulfide isomerase (PDI) floxed mice with lysozyme-Cre transgenic mice, we demonstrate that neutrophil PDI is required for neutrophil adhesion and crawling during tumor necrosis factor-α–induced vascular inflammation in vivo. Rescue experiments show that the isomerase activity of extracellular PDI is critical for its regulatory effect on neutrophil recruitment. Studies with blocking anti-PDI antibodies and αLβ2 or αMβ2 null mice suggest that extracellular PDI regulates αMβ2 integrin-mediated adhesive function of neutrophils during vascular inflammation. Consistently, we show that neutrophil surface PDI is important for αMβ2 integrin-mediated adhesion of human neutrophils under shear and static conditions and for binding of soluble fibrinogen to activated αMβ2 integrin. Confocal microscopy and biochemical studies reveal that neutrophil surface PDI interacts with αMβ2 integrin in lipid rafts of stimulated neutrophils and regulates αMβ2 integrin clustering, presumably by changing the redox state of the integrin. Thus, our results provide the first evidence that extracellular PDI could be a novel therapeutic target for preventing and treating inappropriate neutrophil sequestration.
Introduction
Protein disulfide isomerase (PDI), a prototypic thiol isomerase, catalyzes disulfide bond modification during protein synthesis in the endoplasmic reticulum (ER).1 Studies of PDI gene deletion in yeast demonstrate that PDI is essential for cell viability,2 probably because of its critical function during protein folding. The catalytic activity of PDI requires the integrity of 2 vicinal dithiol (CGHC) active motifs.1 Although PDI contains an ER retention sequence, it also has a distinct localization site on the cell surface; however, the function of cell-surface PDI remains enigmatic. Previous studies showed that inhibition of PDI with blocking anti-PDI antibodies disrupts platelet adhesion to collagen-coated surfaces and agonist-induced platelet aggregation.3,4 Further, fluorescence intravital microscopic studies have demonstrated that extracellular PDI regulates platelet accumulation at the site of arteriolar injury in live mice.5 A recent study shows that extracellular PDI interacts with platelet and endothelial cell β3 integrins during thrombus formation, thereby regulating integrin function.6
Neutrophils are essential for the innate immune response during vascular inflammation and tissue injury. Neutrophil recruitment into the site of vascular injury is a multistep process, consisting of initial rolling, firm adhesion, crawling, and transmigration. Interaction of selectins with their ligands plays a critical role in neutrophil rolling over the activated endothelium, thereby subsequently activating integrins.7 Activated αMβ2 and αLβ2 integrins interact with their ligands such as intercellular adhesion molecule-1 (ICAM-1) and induce stable adhesion and crawling of neutrophils on the activated endothelium.8 However, the regulatory mechanism of β2 integrin function is poorly understood. Reducing agents such as dithiothreitol are known to promote αMβ2- and αLβ2-mediated leukocyte adhesion to ICAM-1.9,10 Modification of disulfide bonds in the I domain of αL and αM subunits by introducing pairs of Cys residues alters the affinity of ligand binding,11,12 implicating that thiol exchange in integrins regulates interaction of β2 integrins with their ligands. Although it is reported that PDI is localized on the neutrophil surface,13 the role of neutrophil surface PDI during vascular inflammation remains unknown.
Using intravital microscopy in myeloid-specific PDI conditional knockout (CKO) mice, we first demonstrate that neutrophil PDI regulates αMβ2 integrin-mediated neutrophil recruitment during vascular inflammation in a manner dependent on its isomerase activity. Microscopic and biochemical studies suggest that neutrophil surface PDI interacts with activated αMβ2 integrin and regulates the integrin clustering on fMLF-stimulated neutrophils. Using surface plasmon resonance, we show that PDI directly binds to αMβ2 integrin. Studies with surface-labeling probes reveal that sulfhydryl exposure in the αM subunit could be regulated by neutrophil surface PDI during cell activation. Thus, we provide the first evidence for the critical role of neutrophil surface PDI in regulating αMβ2 integrin-mediated adhesive function of neutrophils during vascular inflammation.
Materials and methods
Mice
Five- to 6-week-old wild-type (WT), lysozyme-Cre (lys-Cre), and integrin null mice were purchased from The Jackson Laboratory. Generation of PDI CKO mice is described in the supplemental Methods (available on the Blood website).
Expression and purification of recombinant PDI
Complementary DNA for His-tagged rat wtPDI and double-mutant PDI (dmPDI) was generated as described previously.14
Isolation of human and mouse neutrophils
Human neutrophils were isolated by Percoll gradient of citrate-treated blood.15 Approval to collect blood samples was obtained from the University of Illinois-Chicago review board in accordance with the Declaration of Helsinki. Bone marrow neutrophils isolated from the femur of killed mice were isolated by Ficoll gradient.16 Human and mouse neutrophils were stimulated with 0.5 and 10 μM fMLF, respectively, for 10 minutes at 37°C, unless otherwise stated. Isolation of mouse platelets, lymphocytes, and monocytes is described in the supplemental Methods.
Flow cytometry
To detect surface molecules, unstimulated and fMLF-stimulated mouse neutrophils were incubated with fluorescently labeled control immunoglobulin G (IgG) or antibodies. For the assay of soluble fibrinogen (FG) binding, neutrophils pretreated with blocking antibodies were incubated with Alexa Fluor 488–labeled FG in the absence or presence of fMLF. In some experiments, mouse neutrophils preincubated with 50 μg/mL wtPDI or dmPDI were stimulated with fMLF and then washed with RPMI 1640 medium or carbonate buffer (0.1 M Na2CO3, pH 9.0) 2 times. After incubation with phycoerythrin (PE)–conjugated anti-poly His antibodies, cells were analyzed by flow cytometry (Cyan ADP; Beckman Coulter).
Adhesion assay
Flow chamber assays were performed as described previously,17 and the detailed methods are presented in the supplemental Methods.
Isolation of lipid rafts
Lipid rafts/nonlipid raft fractions were prepared as described previously,18 and the detailed methods are presented in the supplemental Methods.
Pull-down and immunoprecipitation assay, surface plasmon resonance, and confocal microscopy
The detailed methods are described in the supplemental Methods.
Intravital microscopy in vivo
Male mice (6-8 weeks old) were used for intravital microscopy as described previously.19 Briefly, control and PDI CKO mice were intrascrotally injected with murine tumor necrosis factor-α (TNF-α; 0.5 μg). Three hours after TNF-α injection, the mouse was anesthetized by intraperitoneal injection of ketamine and xylazine. The cremaster muscle was exteriorized, and neutrophils were monitored by infusion of an Alexa Fluor 647–conjugated anti–Gr-1 antibody (0.05 μg/g body weight [BW]) through a jugular cannulus. Rolling and adherent neutrophils were monitored in an area of 0.02 mm2 over 5 minutes in the top half of inflamed cremaster venules and were recorded using an Olympus BX61W microscope with a 60×/1.0 NA water-immersion objective and a high-speed camera (Hamamatsu C9300) through an intensifier (Video Scope International). Captured images were analyzed using Slidebook (version 5.0; Intelligent Imaging Innovations). Six to 8 different venules were monitored in 1 mouse. Then, recombinant wtPDI or dmPDI, 100 μg, was infused, and neutrophil recruitment was subsequently monitored in another 6 to 7 different venules in the same mouse.
In some experiments, blocking antibodies were injected into WT or KO mice through a tail vein, followed by intrascrotal injection of TNF-α. Three hours after TNF-α injection, the same dose of the antibody was infused through a jugular cannulus. Eight to 10 different venules were monitored in 1 mouse. The rolling influx and velocity of neutrophils and the percentage of crawling neutrophils were determined over 5 minutes in each venule. Neutrophils with a displacement of >10 μm during 1 minute were considered crawling. The number of adherent neutrophils that were stationary for >30 seconds and crawled but did not roll over were counted.
Statistics
Statistical significance was assessed by ANOVA and the Dunnett test for comparison of multiple groups or Student t test for comparison of 2 groups (GraphPad Prism4). A P value < .05 was considered significant.
Results
PDI is required for neutrophil recruitment during TNF-α–induced vascular inflammation and the isomerase activity of extracellular PDI is critical for its regulatory effect
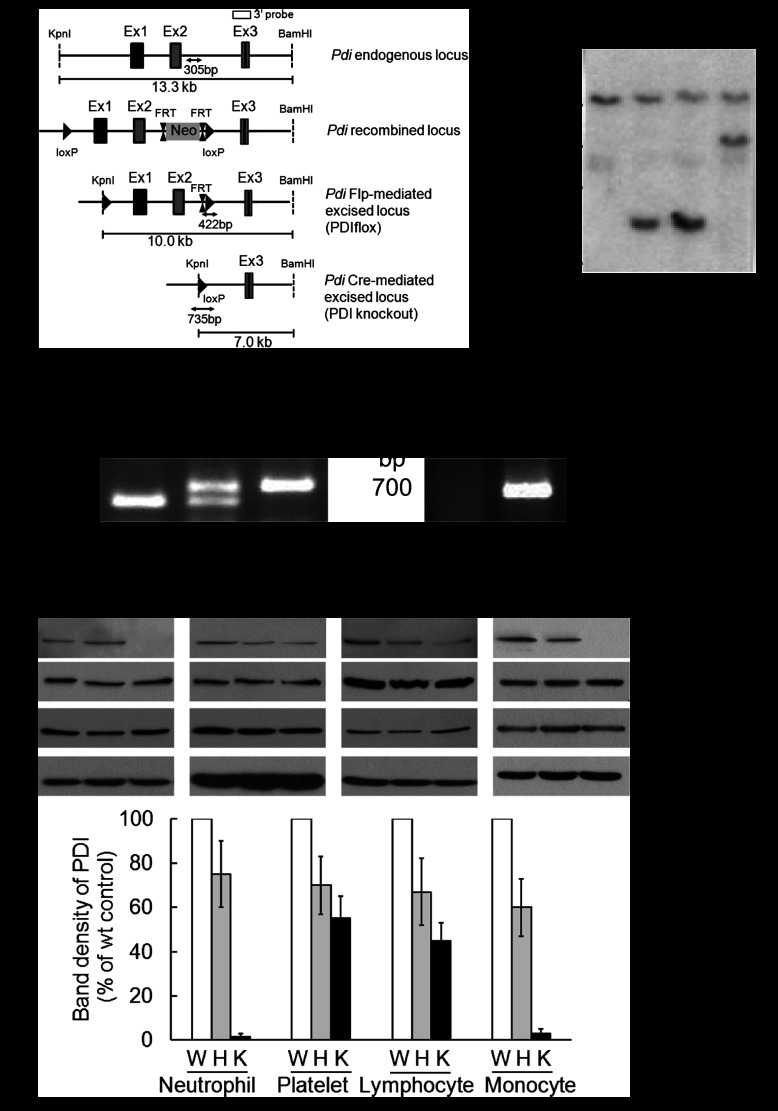
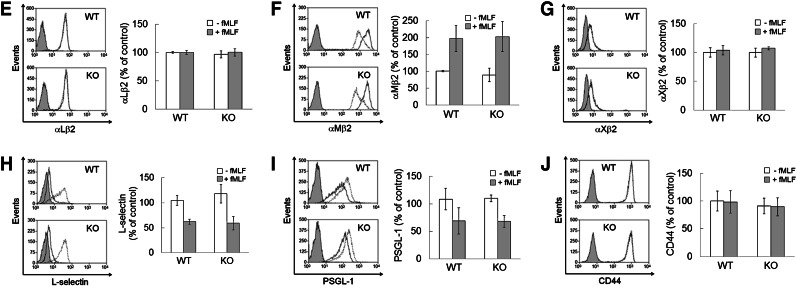
To explore the role of PDI in neutrophil recruitment during vascular inflammation, we generated myeloid-specific PDI CKO mice using a Cre-LoxP strategy. Southern blotting of genomic DNA from mouse tails confirmed the correct site-specific targeting (Figure 1A-B). Littermate WT (PDIflox/+), heterozygous (PDIflox/+;Lys-Cre or PDIflox/−), and CKO (PDIflox/−;Lys-Cre) were confirmed by polymerase chain reaction analysis (Figure 1C). Compared with WT mice, PDI CKO mice showed complete deletion (>97%) of the PDI protein in neutrophils and monocytes, and partial reduction in platelets and even lymphocytes (Figure 1D). Disruption of the PDI gene did not alter the expression of ERp72, ERp57, and actin. Furthermore, the surface expression of αMβ2, αLβ2, αXβ2, L-selectin, P-selectin glycoprotein ligand-1, and CD44 was comparable between WT and PDI null neutrophils upon fMLF stimulation (Figure 1E-J). The number of circulating blood cells was not changed in CKO mice (Table 1).
Figure 1.
Generation of myeloid-specific PDI CKO mice. (A) Targeting construct and homologous recombination. (B) Southern blotting analysis of genomic DNA isolated from mouse tails. (C) Polymerase chain reaction analysis of WT, heterozygous, and CKO mice with primers for floxed PDI and Lys-Cre. (D) Immunoblotting with lysates of cells isolated from WT (W), heterozygous (H), and homozygous PDI CKO (K) mice. The band density of PDI represents mean ± SD (n = 5-6 mice per group). (E-J) Flow cytometric analysis shows the expression of β2 integrins, L-selectin, PSGL-1, and CD44 on unstimulated (dot line) and fMLF-stimulated (black line) WT (WT) and PDI CKO (KO) neutrophils. The gray histogram represents the fluorescence intensity of control IgG on stimulated neutrophils. The mean fluorescence intensity of antibodies was normalized to that of control IgG, and data are shown as 100% (mean ± SD, n = 3-6 mice per group). PSGL-1, P-selectin glycoprotein ligand-1.
Table 1.
Analysis of PDI CKO mouse blood
| PDI | WBC, 103/μL | NE, 103/μL | LY, 103/μL | MO, 103/μL | RBC, 106/μL | PLT, 103/μL |
|---|---|---|---|---|---|---|
| WT | 4.3 ± 1.2 | 0.7 ± 0.2 | 3.1 ± 1.0 | 0.3 ± 0.1 | 8.2 ± 0.4 | 696.1 ± 97.2 |
| HT | 4.6 ± 0.4 | 1.0 ± 0.4 | 2.8 ± 0.4 | 0.2 ± 0.1 | 7.9 ± 0.4 | 764.5 ± 55.7 |
| KO | 3.0 ± 0.8 | 0.7 ± 0.3 | 2.1 ± 0.6 | 0.2 ± 0.1 | 8.2 ± 0.4 | 624.8 ± 55.8 |
Blood cells in littermate WT control (WT), heterozygous (HT), and CKO (KO) mice were counted using HEMAVET 950 (Drew Scientific). Data represent mean ± SD (n = 6 mice per group).
LY, lymphocyte; MO, monocyte; NE, neutrophil; PLT, platelet; RBC, red blood cell; WBC, white blood cell.
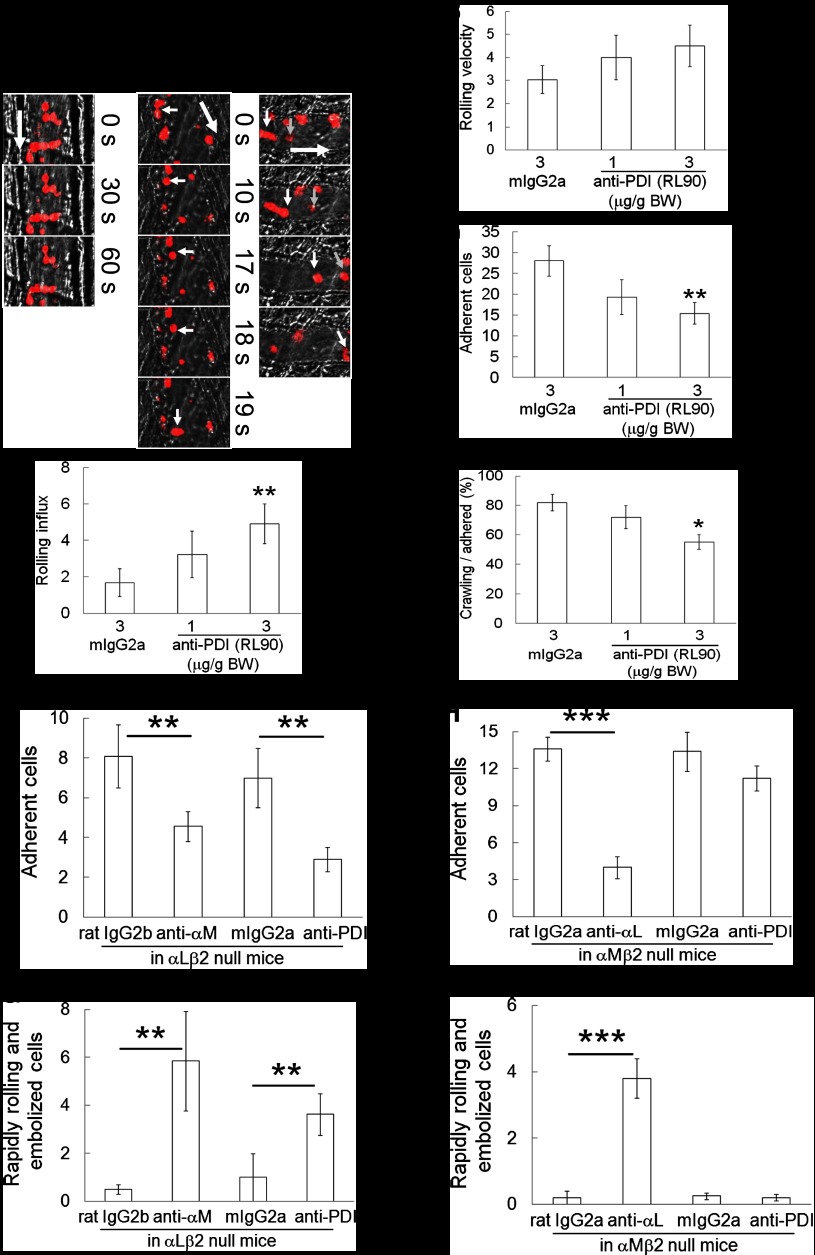
Using intravital microscopy in PDI CKO mice, we examined whether neutrophil PDI is important for neutrophil recruitment during TNF-α–induced cremaster venular inflammation. Compared with WT mice, PDI CKO mice increased the rolling influx and reduced the number of adherent neutrophils to the inflamed endothelium (Figure 2A-B,D, supplemental Videos 1-2). Notably, infusion of wtPDI, 100 μg, into PDI CKO mice significantly reduced the rolling influx of neutrophils and increased the number of adherent neutrophils during vascular inflammation (supplemental Video 3). In contrast, dmPDI did not show any rescue effects (supplemental Video 4). The rolling velocity of neutrophils was similar in all groups and within a range of 3.5 to 5.1 μm/s (Figure 2C). The percentage of crawling cells among adherent neutrophils was significantly reduced in PDI CKO mice, compared with WT mice (Figure 2E). Infusion of wtPDI but not dmPDI into the PDI CKO mice restored the crawling number of PDI null neutrophils to the WT level. These results indicate that extracellular PDI regulates neutrophil adhesion and crawling during TNF-α–induced vascular inflammation and that the isomerase activity of PDI is required for the regulatory effect.
Figure 2.
PDI is required for neutrophil recruitment during TNF-α–induced vascular inflammation. Intravital microscopy was performed as described in “Materials and methods.” Neutrophils were visualized by infusion of an Alexa Fluor 647–conjugated anti–Gr-1 antibody. Six to 8 different inflamed venules were monitored in WT and PDI CKO mice. Then, wtPDI or dmPDI, 100 μg, was infused into PDI CKO mice and neutrophil recruitment was further monitored. (A) Representative images. Small (white and gray) and large arrows show rolling neutrophils and blood flow, respectively. (B-D) The rolling influx (rolling cells per minute) and velocity (micrometers per second) of neutrophils and the number of adherent neutrophils (number per field per 5 minutes) are shown. Data represent mean ± SEM (n = 17-24 venules in 3-4 mice per group). (E) The crawling population of adherent neutrophils was monitored over 5 minutes (n = 80-100 neutrophils in 3-4 mice per group). *P < .05, **P < .01, and ***P < .001 vs WT mice; #P < .05 and ###P < .001 vs PDI CKO mice with or without wtPDI or dmPDI after ANOVA and the Dunnett test. The diameter of microvenules observed was in the range of 33.3 to 45.5 μm and the wall-shear rate in the TNF-α–inflamed cremaster venules was approximately 400 to 650 s−1 as described previously.19 (F-G) WT and PDI KO neutrophils were stimulated with fMLF in the presence of 50 μg/mL His-tagged wtPDI or dmPDI. Bound PDI was washed with RPMI media or carbonate buffer. Binding of recombinant PDI was analyzed by flow cytometry using a PE-conjugated anti-poly His antibody. The gray histogram represents the fluorescent signal of the anti-poly His antibody on PDI-untreated, stimulated neutrophils. (G) PDI binding is shown as a fold increase by the ratio of the geometric mean intensity value of a PE-conjugated anti-His antibody on PDI-treated vs untreated neutrophils (mean ± SD, n = 3). *P < .05 vs unstimulated neutrophils after the Student t test. (H-I) wtPDI or dmPDI, 100 μg, was infused into PDI CKO mice. Neutrophils, β2 integrin, and PDI were visualized by infusion of Alexa Fluor 647–conjugated anti-Gr-1 (red), Alexa Fluor 488–conjugated anti-β2 (green), and PE-conjugated anti-His antibodies (blue), respectively, into PDI CKO mice. Without recombinant PDI, no fluorescence signal was observed by the PE-conjugated anti-poly His antibody in PDI KO mice (data not shown). White arrows and arrowheads show blood flow and rolling neutrophils, respectively. Representative fluorescence images are shown at different time points following recording (n = 17-18 venules in 3 PDI CKO mice). Neutrophils and β2 (yellow); β2 and PDI (turquoise); neutrophils and PDI (magenta); neutrophils, β2, and PDI (white). (I) Fluorescence intensity of PE-conjugated anti-His antibodies was quantified over 2 minutes.
To determine whether exogenously added PDI directly binds to neutrophils, flow cytometric analysis was performed using anti-poly His antibodies. His-tagged wtPDI and dmPDI equally bound to PDI KO neutrophils, and their binding to the neutrophil surface was significantly enhanced following fMLF stimulation (Figure 2F-G). PDI bound to the neutrophil surface was completely dissociated by washing with carbonate buffer, suggesting a charge-dependent interaction of PDI with surface molecules.20 To further investigate whether exogenously added PDI binds to PDI KO neutrophils in vivo, His-tagged wtPDI or dmPDI was infused into the PDI CKO mice 3 hours after intrascrotal injection of TNF-α. As visualized by a PE-conjugated anti-poly His antibody, exogenous wtPDI and dmPDI were detected on adherent neutrophils and seemed to colocalize with β2 integrins (Figure 2H, supplemental Videos 5-6). In addition, recombinant PDI bound to the inflamed endothelial cells to which neutrophils adhered. As normalized with the number of adherent neutrophils, there was no significant difference of the fluorescence signal between wtPDI and dmPDI (Figure 2I). Taken together, our results suggest that the rescue effect of exogenous PDI results from direct association between PDI and its binding molecule(s) on activated neutrophils and endothelial cells during vascular inflammation.
Extracellular PDI regulates αMβ2 integrin-mediated neutrophil adhesion and crawling to the inflamed endothelium during TNF-α–induced vascular inflammation
To further investigate whether extracellular PDI regulates neutrophil recruitment during vascular inflammation, we used a function-blocking anti-PDI antibody (RL90).5 Compared with control IgG, infusion of the anti-PDI antibody into WT mice dose-dependently increased the rolling influx of neutrophils but had no effect on the rolling velocity (Figure 3A-C, supplemental Videos 7-8). The antibody at 3 μg/g BW significantly inhibited neutrophil adhesion and crawling on the inflamed endothelium, compared with control IgG (Figure 3D-E). These results indicate that extracellular PDI regulates neutrophil adhesion and crawling during vascular inflammation in vivo.
Figure 3.
Extracellular PDI regulates αMβ2 integrin-mediated adhesion and crawling of neutrophils during vascular inflammation. Control IgG (mIgG2a) or anti-PDI antibody (RL90, 1 or 3 μg/g BW) was infused into WT mice. (A-E) Rolling, adherent, and crawling neutrophils were monitored and analyzed as described in the Figure 2 legend. (A) Representative images. Small (white, gray, and black) and large arrows show rolling neutrophils and blood flow, respectively. Data represent mean ± SEM (n = 24-28 venules in 3-5 mice per group). *P < .05 and **P < .01 vs control IgG after ANOVA and the Dunnett test. (F-I) Control IgG (rat IgG2a, rat IgG2b, or mouse IgG2a) or a blocking antibody against αM, αL, or PDI, 2 μg/g BW, was infused into αLβ2 or αMβ2 null mice. Adherent neutrophils and rapidly rolling (>10 μm/s) and embolized cells were counted. Data represent mean ± SEM (n = 18-22 venules in 3 mice per group). **P < .01 or ***P < .005 vs control IgG after the Student t test.
Because both αLβ2 and αMβ2 integrins play crucial roles in neutrophil recruitment during vascular inflammation,21 we hypothesized that extracellular PDI regulates β2 integrin-mediated neutrophil recruitment. Infusion of an anti-αM or anti-PDI antibody into αLβ2 null mice before and 3 hours after TNF-α injection additionally reduced the number of adherent neutrophils, compared with control IgG (Figure 3F). Both antibodies significantly increased the number of rapidly rolling (>10 μm/s) and embolized neutrophils in αLβ2 null mice (Figure 3G). In contrast, an anti-αL but not an anti-PDI antibody significantly inhibited the number of adherent neutrophils and increased the number of rapidly rolling and embolized neutrophils in αMβ2 null mice, compared with control IgG (Figure 3H-I). These results suggest that extracellular PDI could regulate αMβ2-mediated adhesive function of neutrophils during vascular inflammation.
Neutrophil surface PDI regulates αMβ2 integrin-mediated adhesion under shear and static conditions
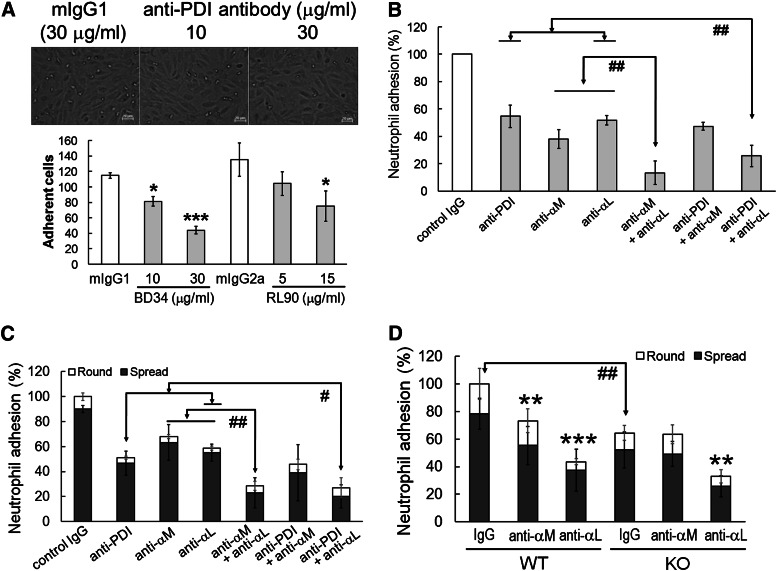
Next, we examined whether neutrophil surface PDI regulates β2 integrin–mediated adhesion of human neutrophils to TNF-α–stimulated human umbilical vein endothelial cells (HUVECs) under venous shear.22 We observed that blocking anti-PDI antibodies dose-dependently inhibited neutrophil adhesion to activated HUVECs under shear (Figure 4A). A combination of anti-αM and anti-αL antibodies inhibited neutrophil adhesion greater than each antibody alone (Figure 4B). Interestingly, the inhibitory effect of an anti-PDI antibody on neutrophil adhesion was enhanced by a combination with anti-αL but not with anti-αM antibodies. These data strongly suggest that neutrophil surface PDI regulates αMβ2 integrin-mediated neutrophil adhesion to the activated endothelium under shear.
Figure 4.
Surface PDI regulates αMβ2-mediated neutrophil adhesion under shear and static conditions. (A-B) Confluent HUVECs on FG-coated glass coverslips were stimulated with TNF-α (20 ng/mL) and placed into a flow chamber. Human neutrophils, 3 × 106, were pretreated with blocking antibodies and stimulated with fMLF. (B) Anti-PDI (BD34, 30 μg/mL) and either anti-αM (15 μg/mL) or anti-αL (50 μg/mL) antibodies were coincubated. Neutrophils were perfused for 10 minutes over activated HUVECs under venous shear of 1 dyne/cm2. Then, the medium was perfused for 5 minutes to wash out weakly bound cells. Adherent neutrophils were monitored in a field of 0.15 mm2 and counted in 5 to 7 separate fields. (C) Human neutrophils were incubated with anti-PDI (30 μg/mL), anti-αM (10 μg/mL), and anti-αL antibodies (30 μg/mL). Cells were plated onto immobilized ICAM-1 in the presence of fMLF. (D) WT or PDI KO mouse neutrophils were treated with antibodies and plated onto immobilized mouse ICAM-1 in the presence of fMLF. (B-D) The number of adherent neutrophils treated with an inhibitor was normalized to that of adherent neutrophils treated with control IgG (100%). Data represent mean ± SD (n = 3-4 for human neutrophils and n = 6 WT and 6 PDI CKO mice). *P < .05, **P < .01, or ***P < .001 vs control IgG after ANOVA and the Dunnett test. #P < .05 and ##P < .01 vs either anti-PDI + anti-αL or anti-αL + anti-αM antibodies after ANOVA and the Dunnett test (for human neutrophils) or vs WT control after the Student t test (for mouse neutrophils). Round and spread neutrophils are shown as open and filled bars, respectively. Statistical significance was determined by comparison of the number of total adherent (round and spread) cells.
We further investigated the role of surface PDI in neutrophil adhesion to immobilized ICAM-1 under static conditions. The total number of adherent (round and spread) human neutrophils was significantly inhibited by an anti-PDI antibody (Figure 4C). When the anti-PDI antibody was combined with an anti-αL but not anti-αM antibody, neutrophil adhesion was further reduced, compared with each antibody alone. Consistently, the total number of adherent WT neutrophils decreased by an anti-αM or anti-αL antibody, compared with control IgG (Figure 4D). Compared with WT neutrophils, PDI KO neutrophils displayed reduced adhesion to ICAM-1–coated surfaces. We found that PDI KO neutrophil adhesion is further inhibited by the anti-αL but not anti-αM antibody. These results indicate that neutrophil PDI regulates αMβ2 integrin–mediated neutrophil adhesion.
Neutrophil surface PDI interacts with activated αMβ2 integrin and such interaction likely occurs in lipid rafts
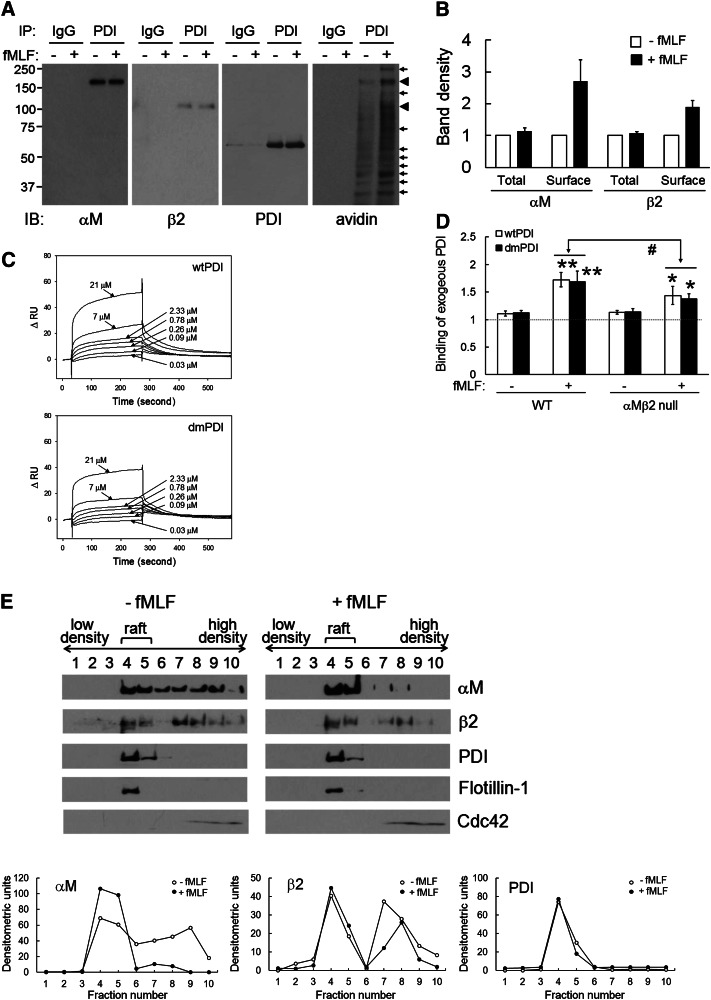
To investigate whether PDI interacts with αMβ2 integrins on the neutrophil surface, we performed immunoprecipitation assays using lysates of human neutrophils labeled with a membrane-impermeable biotin derivative which reacts with primary amines, sulfo-NHS-biotin (SSB). We found that αMβ2 coimmunoprecipitates with PDI (Figure 5A). The band density of total (inside and cell surface) αM at 170 kDa and β2 at 100 kDa was not changed following fMLF stimulation. However, when the blot was reprobed with peroxidase-conjugated avidin, the band density at 170 and 100 kDa increased by 2.7- and 1.9-fold, respectively, upon fMLF stimulation (Figure 5A-B). In addition to 170- and 100-kDa bands, the density of several other bands was also enhanced, suggesting that neutrophil surface PDI could interact with other surface molecules. Conversely, PDI coimmunoprecipitated with αM, and, as reprobed with avidin, the density of the 60-kDa band increased following fMLF stimulation (supplemental Figure 1A). Although PDI also immunoprecipitated with αL, αL-associated PDI was not from the cell surface because the 60-kDa band was not detected with avidin (supplemental Figure 1B). These results implicate that surface PDI interacts with αMβ2 integrin on stimulated neutrophils.
Figure 5.
Surface PDI-αMβ2 interaction is enhanced on stimulated neutrophils. (A-B) Human neutrophils were incubated without or with fMLF. After SSB labeling, lysates were immunoprecipitated with anti-PDI antibodies and immunoblotted with indicated antibodies (total interaction). The blots were reprobed with peroxidase-conjugated avidin (surface interaction). (B) The band density was quantitated by densitometry (mean ± SD, n = 4-5). (C) Surface plasmon resonance assay was performed as described in “Materials and methods.” The extracellular domain of recombinant αMβ2 (25 μg/mL in 10 mM acetate buffer, pH 5.0) was immobilized on the surface of a CM5 chip. Recombinant PDI (0.03-21 μM) or purified FG (0.02-5 μM, data not shown) in running buffer (10 mM HEPES, pH 7.5, 150 mM NaCl, 0.005% P20 with 2 mM MnCl2) were infused over the reference and αMβ2-immobilized surfaces at a flow rate of 5 μL/min for 240 seconds, followed by a dissociation phase of 300 seconds. The representative graph is shown from triplicate. The dissociation constant, Kd, was calculated based on the Kon and Koff value. (D) WT and αMβ2 null neutrophils were stimulated with fMLF in the presence of 50 μg/mL His-tagged wtPDI or dmPDI. Binding of recombinant PDI was analyzed by flow cytometry as described in the panel G section of the Figure 2 legend. Data represent mean ± SD (n = 3-4). *P < .05 and **P < .01 vs unstimulated neutrophils, and #P < .05 vs PDI binding to stimulated WT neutrophils after the Student t test. (E) Using lysates of unstimulated and fMLF-stimulated neutrophils, fractions containing lipid rafts and nonrafts were collected and immunoblotted. Representative blots and densitometric analysis are shown (n = 3).
To determine direct interaction between PDI and αMβ2, we performed surface plasmon resonance assays using recombinant proteins. The extracellular domain of recombinant αMβ2 integrin was confirmed by protein staining, immunoblotting, and ligand binding (supplemental Figure 2). In the presence of Mn2+, wtPDI and dmPDI bound to αMβ2 with a dissociation constant (Kd) of 0.35 ± 0.16 μM and 0.59 ± 0.24 μM, respectively (Figure 5C and Table 2). We also observed that FG binds to αMβ2 with a Kd of 2.8 ± 0.91 μM, which was similar to a previous result showing interaction between the αM I domain and FG (Kd = 3.98 ± 0.86 μM).23 Therefore, our results clearly demonstrate that PDI binds to αMβ2 integrin. We further determined whether exogenous PDI binds to αMβ2 null neutrophils in flow cytometric analysis using anti-poly His antibodies. Binding of wtPDI and dmPDI to neutrophils was enhanced following fMLF stimulation. However, PDI binding to αMβ2 null neutrophils significantly decreased, compared with WT neutrophils (Figure 5D), suggesting that exogenous PDI binds to αMβ2 integrin and other surface molecules.
Table 2.
Determination of equilibrium dissociation constant (Kd, μM) by surface plasmon resonance
| Analyte | Immobilized ligand αMβ2, mean ± SD, n = 3 |
|---|---|
| wtPDI | 0.35 ± 0.16 |
| dmPDI | 0.59 ± 0.24 |
| Fibrinogen | 2.80 ± 0.91 |
It is reported that αMβ2 integrin translocates to lipid rafts during neutrophil activation.24,25 Although most proteins were detected in nonraft fractions, we found that the majority of PDI is localized in lipid rafts irrespective of fMLF stimulation (Figure 5E, supplemental Figure 3A-B). Using lipid raft (flotilin-1) and nonraft (cdc42) markers, we confirmed that αMβ2 integrin translocates to lipid rafts upon fMLF stimulation. Inhibition of neutrophil surface PDI did not affect the translocation of PDI and αMβ2 integrin to lipid rafts (supplemental Figure 4A-B). Although it remains to be determined how PDI localizes to lipid rafts, these results implicate that the PDI-αMβ2 interaction likely occurs in lipid rafts of stimulated neutrophils.
Neutrophil surface PDI regulates soluble FG binding to activated αMβ2 integrin and clustering of the integrin
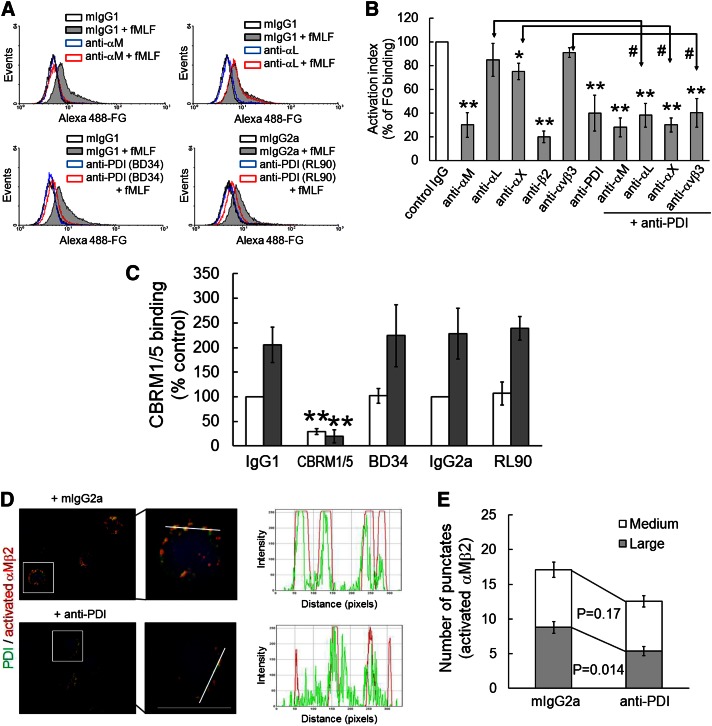
Because soluble FG binds to αMβ2 but not αLβ2 integrin on activated neutrophils,26 we examined whether neutrophil surface PDI regulates FG binding to activated neutrophils. Treatment of human neutrophils with a blocking anti-αM, anti-β2, or anti-PDI antibody inhibited soluble FG binding to fMLF-stimulated neutrophils by 65% to 80% of the control IgG. Although a blocking anti-αX antibody partially diminished FG binding, an anti-αL or anti-αVβ3 antibody did not show any inhibitory effect on FG binding (Figure 6A-B). Compared with the anti-αL, anti-αX, or anti-αVβ3 antibody, a combination of each antibody with the anti-PDI antibody resulted in an increased inhibitory effect. In contrast, a combination of anti-PDI and anti-αM antibodies did not potentiate the inhibitory effect, compared with each antibody alone. These results strongly suggest that PDI regulates the interaction of FG and activated αMβ2 integrin. To further determine whether neutrophil surface PDI regulates αMβ2 integrin activation, flow cytometry analysis was performed using an antibody against activated human αMβ2 integrin (CBRM1/5). We observed that unlabeled CBRM1/5, but not anti-PDI antibodies, inhibited binding of the fluorescently labeled CBRM1/5 to neutrophils (Figure 6C), suggesting that neutrophil surface PDI does not regulate the conformational change of αMβ2 integrin.
Figure 6.
Surface PDI regulates ligand-binding activity and clustering of αMβ2 integrin without affecting its conformational change. (A-B) Human neutrophils were pretreated with blocking antibodies (10 μg/mL), followed by incubation with fMLF and Alexa Fluor 488–conjugated FG. The fluorescence intensity of FG on inhibitor-treated neutrophils was normalized to that on control cells (100%, white bar). Data represent mean ± SD (n = 3-6). *P < .05 or **P < .01 vs control IgG after ANOVA and the Dunnett test. #P < .05 vs each antibody alone after the Student t test. (C) Human neutrophils were pretreated with mouse IgG1 or IgG2a (IgG1 or IgG2a), or a blocking antibody against PDI (RL90 and BD34) or activated αM (CBRM1/5), 15 μg/mL, and then incubated with or without fMLF. Binding of the PE-conjugated CBRM1/5 was analyzed by flow cytometry. CBRM1/5 binding to neutrophils treated with control IgG was normalized as 100% (white bar). Data represent mean ± SD (n = 3-4). **P < .01 vs control IgG after the Student t test. (D-E) Confocal microscopy was performed as described in “Materials and methods.” Neutrophils were stimulated with fMLF in the presence of control IgG or a blocking anti-PDI antibody and plated onto ICAM-1 surfaces. Adherent cells were stained with PE-conjugated anti-activated αM (CBRM1/5) (red) and Alexa Fluor 488–conjugated anti-PDI antibodies (green). Representative images are shown as low (left panel) and high (right panel) magnifications (n = 4). Little signal was detected by isotype control IgG (data not shown). Colocalization histograms show the intensity of surface PDI and activated αMβ2 integrin along the white line. Bar = 10 μm. (E) The number of medium- (10-100 pixels) and large-sized (>100 pixels) punctates of activated αMβ2 integrin was quantified in confocal images (n = 25-30 cells in 4 independent experiments). P value was obtained from the Mann-Whitney test.
In addition to its conformational change, adhesive strength and membrane redistribution of the integrin after ligand-dependent adhesion could regulate the ligand-binding activity of αMβ2 integrin.27,28 Thus, we determined whether neutrophil surface PDI regulates clustering of activated αMβ2 integrin using confocal microscopy. When nonpermeabilized human neutrophils adherent to ICAM-1–coated surfaces were stained with fluorescently labeled CBRM1/5, activated αMβ2 integrin was detected as punctate structures (Figure 6D). Surface PDI was also found as punctate structures that colocalized well with activated αMβ2 integrin but not with αLβ2 integrin (supplemental Figure 1C). Notably, pretreatment with the anti-PDI antibody significantly reduced colocalization of PDI with activated αMβ2 integrin on neutrophils (Figure 6D). To quantify the number and size of punctates of activated αMβ2 integrin as an indicator of integrin clustering,29 the punctates with a range of 10 to 5000 pixels were counted. Compared with control IgG, the anti-PDI antibody significantly decreased the number of large-sized (>100 pixels), but not medium-sized (10-100 pixels), punctates of activated αMβ2 integrin on stimulated neutrophils (Figure 6E). These results suggest that the activity of neutrophil surface PDI is important for surface interaction of PDI with activated αMβ2 integrin and for the integrin clustering.
PDI regulates thiol exchange on αMβ2 integrin during neutrophil activation
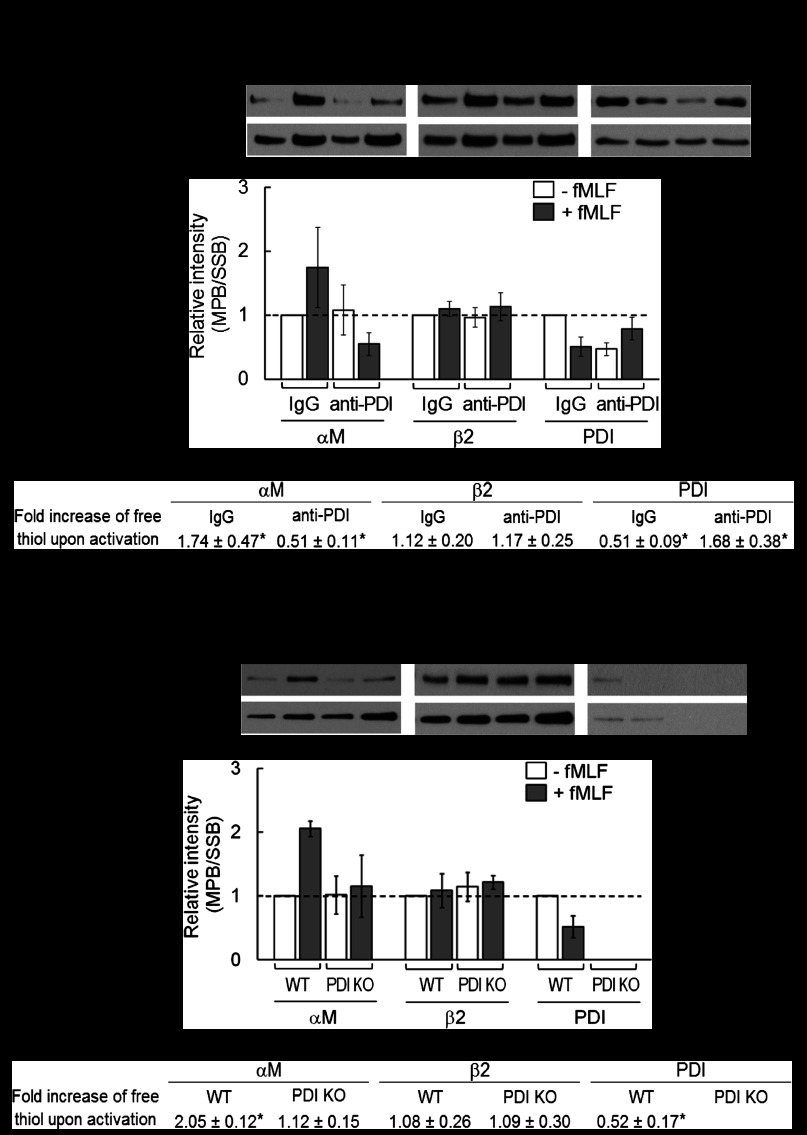
A previous report showed that thiol exchange may promote αMβ2-mediated leukocyte adhesion to ICAM-1.10 Using surface-labeling studies, we investigated whether sulfhydryl exposure on αMβ2 integrin is regulated by neutrophil surface PDI. Because neutrophil activation changes the expression level of numerous surface proteins including αMβ2 integrin, we carefully examined both the surface protein expression and the corresponding sulfhydryl exposure. Neutrophils were labeled with either SSB or membrane-impermeable biotin derivatives which react with free sulfhydryls (N-maleimido-propionyl) biocytin (MPB).30 SSB- and MPB-labeled surface proteins were then pulled down by avidin agarose beads. After immunoblotting, the relative band density was quantitated. Exposure of the free thiol groups on surface αM, β2, and PDI was determined by normalizing the band density of MPB labeling (sulfhydryl exposure of surface proteins) to that of SSB labeling (quantitative expression of surface proteins). When human neutrophils were pretreated with control IgG, surface αM, β2, and PDI thiols showed a 1.7-, 1.1-, and 0.5-fold change, respectively, during neutrophil activation (Figure 7A-C). These results suggest that sulfhydryl exposure in the αM subunit increases, whereas in PDI it reciprocally decreases during neutrophil activation. When the isomerase activity of surface PDI was inhibited by an anti-PDI antibody, the sulfhydryl exposure in the αM subunit and PDI was strikingly reversed (0.5- and 1.7-fold change, respectively) but the β2 subunit had no change of the sulfhydryl exposure (1.2-fold increase). Because the blocking antibody itself affected exposure of free thiols on PDI in unstimulated neutrophils, we tested PDI KO neutrophils in the same assay. As seen in human neutrophils, control neutrophils showed a similar pattern of sulfhydryl exposure on the surface αM, β2, and PDI with a 2.1-, 1.1-, and 0.5-fold change, respectively, following fMLF stimulation (Figure 7D-F). In contrast, sulfhydryl groups in the αM subunit of PDI KO neutrophils were not exposed during cell activation (1.1-fold change). These results suggest that the activity of neutrophil PDI regulates sulfhydryl exchange of αMβ2 integrin, on the αM subunit, during neutrophil activation.
Figure 7.
PDI regulates sulfhydryl exposure of αMβ2 integrin during neutrophil activation. (A-C) Human neutrophils pretreated without or with an anti-PDI antibody were stimulated with fMLF, followed by labeling with SSB or MPB. Lysates were pulled down with avidin agarose beads and immunoblotted. (D-F) WT and PDI KO neutrophils were stimulated with fMLF and used in the same pulldown assay. (B,E) The free thiol contents of surface αM, β2, and PDI (band density of MPB labeling) were normalized to the surface expression of each protein (band density of SSB labeling). Quantitative graphs are shown as mean ± SD (5-6 independent experiments for human neutrophils and 3 independent experiments for mouse neutrophils [5 WT and 5 KO mice per experiment in each group]). (C,F) The fold change of free thiol levels during cell activation was obtained by dividing the normalized value in stimulated cells by that in unstimulated cells in each group (mean ± SD). The number greater or lower than 1 implicates that sulfhydryl exposure per exposed surface protein increases or decreases following fMLF stimulation. *P < .05 vs unstimulated and IgG- or vehicle-treated control (MPB/SSB = 1).
Discussion
We demonstrate here that extracellular PDI plays a critical role in regulating αMβ2-mediated adhesive function of neutrophils during vascular inflammation. Further, our studies reveal that neutrophil surface PDI is associated with activated αMβ2 integrin within lipid rafts and that the isomerase activity of neutrophil surface PDI regulates αMβ2 integrin clustering. Thus, we provide the first identification of a neutrophil surface thiol isomerase regulating ligand-binding activity of αMβ2 integrin and neutrophil recruitment under inflammatory conditions.
The mouse model of TNF-α–induced cremaster venular inflammation has been used to study the molecular mechanisms of neutrophil recruitment into the site of inflammation.31,32 Using intravital microscopy of myeloid-specific PDI CKO mice, we demonstrate that neutrophil PDI is required for neutrophil adhesion and crawling during vascular inflammation. It is controversial whether the Lys-Cre system induces specific gene deletion in myeloid cells,33,34 and we observed that PDI CKO mice partially reduced the expression of PDI in lymphocytes. However, the rolling and adherent cells on the TNF-α–inflamed endothelium are mainly neutrophils as confirmed by Gr-1 labeling.32 Furthermore, we confirmed that adherent monocytes are only 5% of the total adherent cells during vascular inflammation and that neutrophil adhesion is not affected under thrombocytopenic conditions (supplemental Figure 5). Thus, these results suggest that neutrophil PDI, but not other blood cell PDI, is critical for neutrophil recruitment during vascular inflammation. We are aware that the genetic approach cannot differentiate between the role of extracellular and intracellular PDI in regulating integrin function. Nevertheless, our finding that the expression of other proteins including thiol isomerases, β2 integrins, and selectin ligands is not altered in PDI KO neutrophils suggests that the targeted deletion of the PDI gene does not influence protein synthesis in the ER. Therefore, the regulatory effect of PDI gene deletion on neutrophil recruitment is likely to be derived from a defect of αMβ2 integrin function rather than a defect in integrin synthesis.
Both αMβ2 and αLβ2 integrins play important roles in neutrophil recruitment into the activated endothelium with distinct and overlapping functions.31,35 Although αM and αL subunits have approximately 34% homology and share the same β2 subunit, our in vitro results pointed out the importance of neutrophil surface PDI for regulating adhesive function of αMβ2 integrin. Nevertheless, our findings that exogenous PDI still binds to αMβ2 null neutrophils (Figure 5D) suggest that the regulatory role of PDI in integrin function may not be limited to αMβ2 integrin. Moreover, our results and others have shown that PDI directly binds to αMβ2, αIIbβ3, and αVβ3 integrins,6,36 implicating that PDI could bind to other neutrophil integrins. In our studies, we could not prove that extracellular PDI regulates adhesive function of αLβ2 integrin. It is reported that the activation state of αLβ2 integrin is very transient following fMLF stimulation and only activated αLβ2 integrin is localized in the lipid rafts, which may result in rapid translocation of the integrin out of lipid rafts.37,38 In contrast, αMβ2 integrin is maintained in the activated state after fMLF stimulation, which would allow surface PDI to interact with αMβ2 integrin constantly in the rafts and to regulate its function. Therefore, different kinetics of αMβ2 and αLβ2 integrin activation may account for the specific regulation of αMβ2 integrin by PDI. Alternatively, the regulatory effect of PDI on αMβ2 integrin function may result from the dominant and increased surface expression of αMβ2 integrin on activated neutrophils.39 Consistent with a previous report,40 we found that neutrophil adhesion is not inhibited when a blocking anti-αM antibody is infused into αL KO mice 3 hours after TNF-α injection (data not shown). However, when the KO mice were treated with the anti-αM or anti-PDI antibodies both before and 3 hours after TNF-α injection, αLβ2 null neutrophil adhesion was further diminished (Figure 3F-G). Dunne et al reported that αMβ2 integrin regulates leukocyte adhesion to TNF-α–inflamed cremaster venules with a low shear rate (200-500 s−1).31 Therefore, our findings that αMβ2 integrin plays an important role in both stable adhesion and crawling of neutrophils could result from the different treatment of the blocking antibody and the shear conditions (400-650 s−1) in our studies.
The regulatory effect of PDI on thiol exchange on αMβ2 integrin could result from the direct interaction through disulfide bond(s). However, we could not detect the PDI-αM or PDI-β2 complex under nonreduced conditions in immunoprecipitation assays (data not shown). Instead, flow cytometric analysis demonstrates that binding of exogenous PDI to surface molecules is mediated by electrostatic interactions. A recent report showed that oxidation of the active site of PDI induces its conformational change, thereby exposing the buried substrate-binding sites and even activating its chaperone activity.41 Because exposure of free thiols on surface PDI decreases in fMLF-stimulated human and mouse neutrophils, our results suggest that the αMβ2-binding site on PDI may be favorably exposed during neutrophil activation, which allows cell-surface PDI to initially interact with αMβ2 integrin in a charge-dependent manner and then regulate thiol exchange on αMβ2 integrin and its adhesive activity.
It has long been speculated that thiol exchange on β2 integrins regulates their adhesive function. Modification of disulfide bonds in the I domain of αL and αM subunits alters the affinity of ligand binding.11,12 Further, dithiothreitol promotes αMβ2- and αLβ2-mediated leukocyte adhesion to ICAM-1.9,10 Our studies with cell-impermeable probes demonstrate that free sulfhydryl groups are exposed on αM subunit following neutrophil activation. It is of interest to note that the increased sulfhydryl exposure in the αM subunit is significantly abolished by inhibition of PDI or its gene deletion. Although we cannot eliminate a possibility that PDI also regulates thiol exchange on the β2 subunit without affecting the net change of surface thiol exposure, our results show that PDI would interact with the αM subunit, thereby regulating integrin function. Furthermore, our results implicate that thiol modifications of surface proteins could be different during neutrophil activation: some proteins like PDI are oxidized (decreasing sulfhydryl groups), whereas others like αM are isomerized or reduced (thereby increasing exposure of their sulfhydryl groups). Therefore, our findings in part support a long-standing hypothesis that thiol exchange on αMβ2 integrin, which could be regulated by neutrophil surface PDI, would be a regulatory mechanism for its adhesive function. Future studies are required to investigate whether PDI has a binding specificity to integrin subunits and which Cys residues on the integrin are modified by PDI.
Taken together, our studies suggest that neutrophil surface PDI interacts with activated αMβ2 integrin within lipid rafts presumably via electrostatic interactions and catalyzes thiol exchange on the integrin, thereby regulating the clustering and ligand-binding activity of αMβ2 integrin during neutrophil activation. Therefore, our results provide the first evidence that extracellular PDI could be a novel therapeutic target for preventing inappropriate neutrophil sequestration.
Supplementary Material
Acknowledgments
The authors thank Drs Deane Mosher and Asrar Malik for their constructive criticisms.
This work was supported in part by grants from the National Institutes of Health (P30HL101302 and R01HL109439; J.C.) and the American Heart Association (SDG 5270005; J.C.).
Footnotes
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: E.H. designed and performed research, collected and analyzed data, and wrote the manuscript; J.L. and K.K. performed research and analyzed data; S.H. performed research; S.R. provided important reagents; and J.C. initiated, designed, and performed research, collected and analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jaehyung Cho, Department of Pharmacology and Anesthesiology, University of Illinois College of Medicine, Chicago, IL 60612; e-mail: thromres@uic.edu.
References
- 1.Ellgaard L, Ruddock LW. The human protein disulphide isomerase family: substrate interactions and functional properties. EMBO Rep. 2005;6(1):28–32. doi: 10.1038/sj.embor.7400311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LaMantia M, Miura T, Tachikawa H, Kaplan HA, Lennarz WJ, Mizunaga T. Glycosylation site binding protein and protein disulfide isomerase are identical and essential for cell viability in yeast. Proc Natl Acad Sci U S A. 1991;88(10):4453–4457. doi: 10.1073/pnas.88.10.4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lahav J, Wijnen EM, Hess O, et al. Enzymatically catalyzed disulfide exchange is required for platelet adhesion to collagen via integrin alpha2beta1. Blood. 2003;102(6):2085–2092. doi: 10.1182/blood-2002-06-1646. [DOI] [PubMed] [Google Scholar]
- 4.Lahav J, Jurk K, Hess O, et al. Sustained integrin ligation involves extracellular free sulfhydryls and enzymatically catalyzed disulfide exchange. Blood. 2002;100(7):2472–2478. doi: 10.1182/blood-2001-12-0339. [DOI] [PubMed] [Google Scholar]
- 5.Cho J, Furie BC, Coughlin SR, Furie B. A critical role for extracellular protein disulfide isomerase during thrombus formation in mice. J Clin Invest. 2008;118(3):1123–1131. doi: 10.1172/JCI34134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho J, Kennedy DR, Lin L, et al. Protein disulfide isomerase capture during thrombus formation in vivo depends on the presence of β3 integrins. Blood. 2012;120(3):647–655. doi: 10.1182/blood-2011-08-372532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kansas GS. Selectins and their ligands: current concepts and controversies. Blood. 1996;88(9):3259–3287. [PubMed] [Google Scholar]
- 8.Zarbock A, Ley K. Neutrophil adhesion and activation under flow. Microcirculation. 2009;16(1):31–42. doi: 10.1080/10739680802350104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edwards BS, Southon EA, Curry MS, et al. Oxidant inhibition of alphaLbeta2 integrin adhesion: evidence for coordinate effects on conformation and cytoskeleton linkage. J Leukoc Biol. 1998;63(2):190–202. doi: 10.1002/jlb.63.2.190. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz BR, Harlan JM. Sulfhydryl reducing agents promote neutrophil adherence without increasing surface expression of CD11b/CD18 (Mac-1, Mo1). Biochem Biophys Res Commun. 1989;165(1):51–57. doi: 10.1016/0006-291x(89)91032-2. [DOI] [PubMed] [Google Scholar]
- 11.Shimaoka M, Lu C, Palframan RT, et al. Reversibly locking a protein fold in an active conformation with a disulfide bond: integrin alphaL I domains with high affinity and antagonist activity in vivo. Proc Natl Acad Sci U S A. 2001;98(11):6009–6014. doi: 10.1073/pnas.101130498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimaoka M, Lu C, Salas A, Xiao T, Takagi J, Springer TA. Stabilizing the integrin alpha M inserted domain in alternative conformations with a range of engineered disulfide bonds. Proc Natl Acad Sci U S A. 2002;99(26):16737–16741. doi: 10.1073/pnas.252633099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennett TA, Edwards BS, Sklar LA, Rogelj S. Sulfhydryl regulation of L-selectin shedding: phenylarsine oxide promotes activation-independent L-selectin shedding from leukocytes. J Immunol. 2000;164(8):4120–4129. doi: 10.4049/jimmunol.164.8.4120. [DOI] [PubMed] [Google Scholar]
- 14.Uehara T, Nakamura T, Yao D, et al. S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature. 2006;441(7092):513–517. doi: 10.1038/nature04782. [DOI] [PubMed] [Google Scholar]
- 15.Yan P, Nanamori M, Sun M, et al. The immunosuppressant cyclosporin A antagonizes human formyl peptide receptor through inhibition of cognate ligand binding. J Immunol. 2006;177(10):7050–7058. doi: 10.4049/jimmunol.177.10.7050. [DOI] [PubMed] [Google Scholar]
- 16.Zarbock A, Schmolke M, Spieker T, Jurk K, Van Aken H, Singbartl K. Acute uremia but not renal inflammation attenuates aseptic acute lung injury: a critical role for uremic neutrophils. J Am Soc Nephrol. 2006;17(11):3124–3131. doi: 10.1681/ASN.2006040358. [DOI] [PubMed] [Google Scholar]
- 17.Gopalan PK, Burns AR, Simon SI, Sparks S, McIntire LV, Smith CW. Preferential sites for stationary adhesion of neutrophils to cytokine-stimulated HUVEC under flow conditions. J Leukoc Biol. 2000;68(1):47–57. [PubMed] [Google Scholar]
- 18.Oshikawa J, Kim SJ, Furuta E, et al. Novel role of p66Shc in ROS-dependent VEGF signaling and angiogenesis in endothelial cells. Am J Physiol Heart Circ Physiol. 2012;302(3):H724–H732. doi: 10.1152/ajpheart.00739.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barthel SR, Wiese GK, Cho J, et al. Alpha 1,3 fucosyltransferases are master regulators of prostate cancer cell trafficking. Proc Natl Acad Sci U S A. 2009;106(46):19491–19496. doi: 10.1073/pnas.0906074106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terada K, Manchikalapudi P, Noiva R, Jauregui HO, Stockert RJ, Schilsky ML. Secretion, surface localization, turnover, and steady state expression of protein disulfide isomerase in rat hepatocytes. J Biol Chem. 1995;270(35):20410–20416. doi: 10.1074/jbc.270.35.20410. [DOI] [PubMed] [Google Scholar]
- 21.Alon R, Ley K. Cells on the run: shear-regulated integrin activation in leukocyte rolling and arrest on endothelial cells. Curr Opin Cell Biol. 2008;20(5):525–532. doi: 10.1016/j.ceb.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang L, Froio RM, Sciuto TE, Dvorak AM, Alon R, Luscinskas FW. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-alpha-activated vascular endothelium under flow. Blood. 2005;106(2):584–592. doi: 10.1182/blood-2004-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCleverty CJ, Liddington RC. Engineered allosteric mutants of the integrin alphaMbeta2 I domain: structural and functional studies. Biochem J. 2003;372(Pt 1):121–127. doi: 10.1042/BJ20021273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solomkin JS, Robinson CT, Cave CM, Ehmer B, Lentsch AB. Alterations in membrane cholesterol cause mobilization of lipid rafts from specific granules and prime human neutrophils for enhanced adherence-dependent oxidant production. Shock. 2007;28(3):334–338. doi: 10.1097/shk.0b013e318047b893. [DOI] [PubMed] [Google Scholar]
- 25.Jerke U, Rolle S, Dittmar G, et al. Complement receptor Mac-1 is an adaptor for NB1 (CD177)-mediated PR3-ANCA neutrophil activation. J Biol Chem. 2011;286(9):7070–7081. doi: 10.1074/jbc.M110.171256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pluskota E, Woody NM, Szpak D, et al. Expression, activation, and function of integrin alphaMbeta2 (Mac-1) on neutrophil-derived microparticles. Blood. 2008;112(6):2327–2335. doi: 10.1182/blood-2007-12-127183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bazzoni G, Hemler ME. Are changes in integrin affinity and conformation overemphasized? Trends Biochem Sci. 1998;23(1):30–34. doi: 10.1016/s0968-0004(97)01141-9. [DOI] [PubMed] [Google Scholar]
- 28.Carman CV, Springer TA. Integrin avidity regulation: are changes in affinity and conformation underemphasized? Curr Opin Cell Biol. 2003;15(5):547–556. doi: 10.1016/j.ceb.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Cluzel C, Saltel F, Lussi J, Paulhe F, Imhof BA, Wehrle-Haller B. The mechanisms and dynamics of (alpha)v(beta)3 integrin clustering in living cells. J Cell Biol. 2005;171(2):383–392. doi: 10.1083/jcb.200503017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bayer EA, Zalis MG, Wilchek M. 3-(N-Maleimido-propionyl)biocytin: a versatile thiol-specific biotinylating reagent. Anal Biochem. 1985;149(2):529–536. doi: 10.1016/0003-2697(85)90609-8. [DOI] [PubMed] [Google Scholar]
- 31.Dunne JL, Ballantyne CM, Beaudet AL, Ley K. Control of leukocyte rolling velocity in TNF-alpha-induced inflammation by LFA-1 and Mac-1. Blood. 2002;99(1):336–341. doi: 10.1182/blood.v99.1.336. [DOI] [PubMed] [Google Scholar]
- 32.Hidalgo A, Chang J, Jang JE, Peired AJ, Chiang EY, Frenette PS. Heterotypic interactions enabled by polarized neutrophil microdomains mediate thromboinflammatory injury. Nat Med. 2009;15(4):384–391. doi: 10.1038/nm.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Förster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8(4):265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 34.Ye M, Iwasaki H, Laiosa CV, et al. Hematopoietic stem cells expressing the myeloid lysozyme gene retain long-term, multilineage repopulation potential. Immunity. 2003;19(5):689–699. doi: 10.1016/s1074-7613(03)00299-1. [DOI] [PubMed] [Google Scholar]
- 35.Ding ZM, Babensee JE, Simon SI, et al. Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J Immunol. 1999;163(9):5029–5038. [PubMed] [Google Scholar]
- 36.Swiatkowska M, Szymański J, Padula G, Cierniewski CS. Interaction and functional association of protein disulfide isomerase with alphaVbeta3 integrin on endothelial cells. FEBS J. 2008;275(8):1813–1823. doi: 10.1111/j.1742-4658.2008.06339.x. [DOI] [PubMed] [Google Scholar]
- 37.Leitinger B, Hogg N. The involvement of lipid rafts in the regulation of integrin function. J Cell Sci. 2002;115(Pt 5):963–972. doi: 10.1242/jcs.115.5.963. [DOI] [PubMed] [Google Scholar]
- 38.Eniola-Adefeso O, Huang RB, Smith CW. Kinetics of LFA-1 mediated adhesion of human neutrophils to ICAM-1—role of E-selectin signaling post-activation. Ann Biomed Eng. 2009;37(4):737–748. doi: 10.1007/s10439-009-9647-8. [DOI] [PubMed] [Google Scholar]
- 39.Wright SD, Weitz JI, Huang AJ, Levin SM, Silverstein SC, Loike JD. Complement receptor type three (CD11b/CD18) of human polymorphonuclear leukocytes recognizes fibrinogen. Proc Natl Acad Sci U S A. 1988;85(20):7734–7738. doi: 10.1073/pnas.85.20.7734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sumagin R, Prizant H, Lomakina E, Waugh RE, Sarelius IH. LFA-1 and Mac-1 define characteristically different intralumenal crawling and emigration patterns for monocytes and neutrophils in situ. J Immunol. 2010;185(11):7057–7066. doi: 10.4049/jimmunol.1001638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang C, Yu J, Huo L, Wang L, Feng W, Wang CC. Human protein-disulfide isomerase is a redox-regulated chaperone activated by oxidation of domain a’. J Biol Chem. 2012;287(2):1139–1149. doi: 10.1074/jbc.M111.303149. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.