Abstract
BACKGROUND
Genomic research in cardiovascular disease (CVD) has progressed rapidly over the last five years. However, in most cases these ground-breaking observations have not yet been accompanied by clinically applicable tools for risk prediction, diagnosis, or therapeutic interventions.
CONTENT
We reviewed the English literature for novel methods and promising genomic targets that will permit large-scale screening and follow-up of recent genomic findings for CVD. We anticipate that advances in three key areas will be critical for the success of these projects. First, exome-centered and whole genome next generation sequencing will identify rare and novel genetic variants associated with CVD and its risk factors. Improvements in methods will also greatly advance the field of epigenetics and gene expression in humans. Second, research increasingly acknowledges that static DNA sequence variation explains only a fraction of the inherited phenotype. Therefore we expect that multifold epigenetic and gene expression signatures will be related to CVD in experimental and clinical settings. Leveraging existing large-scale consortia and clinical biobanks combined with electronic health records holds promise to integrate epidemiological and clinical genomics data. Finally, a systems biology approach will be needed to integrate the accumulated multidimensional data.
SUMMARY
Novel methods in sequencing, epigenetics and transcriptomics, and unprecedented large-scale cooperative efforts promise to generate insights into complex CVD. The rapid accumulation and integration of knowledge will shed light onto the considerable proportion of the missing heritability of CVD.
Keywords: genomics, sequencing, epigenetics, transcriptomics
Cardiovascular disease (CVD) morbidity and mortality pose a significant public health burden worldwide (1). Understanding the multifactorial, complex underpinnings of CVD holds promise to have global impact on health promotion. CVD is a heritable condition(2), with heritability estimates of up to 60 percent for coronary heart disease(3). The completion of the Human Genome Project(4) has raised high expectations for substantial insights into the polygenetic architecture of complex human diseases such as CVD. However, despite the rapid identification of a few genetic variations for CVD and its risk factors, the number of findings that have been translated into clinical practice has remained disappointingly low. For this review, we searched the current literature for novel methods and scientific approaches that may follow-up the recent genomic findings for CVD variants and ultimately enable large-scale screening in clinical samples and the general population.
Common complex disease
Unlike Mendelian disorders, there not exist a single genetic variant that is responsible for CVD (Table 1). Rather, CVD phenotypes and other complex diseases result from the sum of multiple polymorphisms, each with relatively small effects on gene expression and disease. Genome-wide association studies (GWAS) have been conducted to dissect the contribution of common polymorphisms and have lead to the identification of more than 20 new loci related to myocardial infarction and other CVD phenotypes(5). Figure 1 summarizes the results of GWAS findings for CVD and its risk factors.
Table 1.
Genetic epidemiology characteristics of samples for rare and common cardiovascular disease
| Rare Mendelian disorders | Common genetic variation | ||
|---|---|---|---|
| Presentation |
|
|
|
| Identification of causal variant |  |
||
| Effect size, absolute risk | Large, high | Small, low | |
| Population attributable risk | Low | High | |
| Public health impact | Low | Possibly high | |
Figure 1.
Genome-wide association study variants for cardiovascular disease and its risk factors (http://www.genome.gov/gwastudies/) summarized in a circos plot (http://circos.ca/). The circle represents the chromosomal and SNP locations. SNPs are indicated by color dots in relation to their cardiovascular phenotypes. We did not identify replicated genome-wide hits for copy number variation for any of the cardiovascular disease phenotypes.
Whereas GWAS has had great success in identifying genetic loci for CVD, many challenges remain. First, these studies typically use only common SNPs which by definition have an allele frequency of over 1%. Thus, rarer SNPs with stronger effects may not be identified by GWAS. Second, as for all association analyses, a clear CVD phenotype is required. For instance, distinct phenotypes of coronary artery disease with different heritability patterns exist(6) that have often been largely ignored in genomic analyses to date. Clinically important variation in manifestation, therapeutic approach and prognosis exist for phenotypes such as left main disease, diffuse versus focal coronary artery disease, ectatic lesions, and degree of coronary calcification. The harmonization of clinically relevant and rigorously defined phenotypes across cohorts is essential to better delineate genetic associations. For continuous and well-standardized phenotypes such as blood lipids highly significant statistical associations have been shown, but they generally have very modest effect sizes(7).
The exact pathophysiological mechanisms behind the vast majority of common genetic polymorphisms from GWAS screening are largely unknown. In many cases the search for the functional variants has been rendered more difficult by the unexpected observation that many genome-wide hits are located in non-coding genomic regions and gene deserts as shown for the 9p21 locus consistently related to CVD(8;9) and the 4q25 locus for atrial fibrillation(10). Finally, the identified genetic variants often explain less than 10 percent of trait or diseasevariability (11). Even the combination of information on an increasing number of common genetic variants promises low discriminatory ability due to small absolute variation in risk between carriers and non-carriers of the alleles (12). Hundreds of frequent genetic variants explain a small proportion of inter-individual common phenotype variability (13;14), and the hypothesis has been raised that some of the missing heritability could be explained by rare/low frequency variants, structural alterations, and epigenetics (15;16).
Despite these challenges, there are a few examples of genetic loci in which the functional importance of a SNP has been elucidated. In a recent elegant investigation of a low-density lipoprotein cholesterol levels GWAS result, a common noncoding polymorphism at the chromosome 1p13 locus was reported to create a transcription factor binding site and alter the expression of the SORT1 gene. In turn, SORT1 affects very low-density lipoprotein secretion in the liver, and potentially may represent a causal pathway from blood lipids to myocardial infarction. Such experiments show the value of whole-genome association study efforts when complemented with follow up functional investigations (17).
Given the challenges posed by the recent GWAS findings and the gaps that remain in translating these findings into clinical practice, in the remainder of the article we will focus on three emerging areas that will be critical to advancing our understanding of CVD phenotypes. Specifically, we will review the application of next generation sequencing, epigenetics, and transcriptomics to CVD phenotypes. We will discuss the importance of utilizing systems biology to integrate data across genomic platforms, and we will consider the potential relevance of genomic findings to the clinical setting.
Next Generation Sequencing
Traditional Sanger sequencing has high-fidelity, but is slow and quite expensive relative to next generation methods. Recent advances in sequence-based technology permit massive parallel sequencing (Table 2). Real-time sequencing replaces natural nucleotides or reversible terminators by detection of continuously added fluorescence labeled nucleotides to the growing DNA strand enhancing the speed and output length of nucleotides(18). The establishment of sequencing libraries and post-sequencing bioinformatics algorithms facilitate the generation, reconstruction, and analysis of sequence reads. Optimizing sequencing accuracy and redundant sequencing reduce sequencing errors. Bioinformatics tools are continuously being refined to store and process the massive amount of sequence data.
Table 2.
Clinically important characteristics of conventional Sanger sequencing and next generation sequencing
| Conventional sequencing | Next generation sequencing | |
|---|---|---|
| Sequencing |
|
|
| Yield | 100,000 basepairs per sequencing run | >100 billion base pairs per sequencing run |
| Computational requirements | Moderate | High |
| Cost per megabase | High | Low |
| Accuracy | High | High |
| Future directions | -- |
|
Several challenges remain with next generation sequencing. Platforms differ by template preparation, sequencing chemistry, imaging, read length and quantity per run. Quality measures are provided by the respective manufacturers, but a uniform quality assessment protocol has not been implemented. Statistical analyses need to account for type I error in the resulting huge data sets and need to develop methods to dissect phenotype relevant variants from commonly shared alleles. Clustering of the total data into conceptually related parts facilitates information digestion(19). Finally, the identification of disease specific genetic variants from bystanders remains a challenge.
Despite these limitations, next generation sequencing has already shown great success and the methods for sequencing have evolved to the point that within the last year sequencing of an entire genome has become considerably less expensive and straightforward. Next generation sequencing has been applied to follow up GWAS loci for CVD phenotypes, to identify rare forms of CVD traits by exome sequencing, and to identify structural variation in the genome.
Evaluation of GWAS loci for CVD phenotypes
The design of GWAS largely has been restricted to genotyping common alleles. However, the accumulating evidence of GWAS suggests that frequent polymorphisms with small odds ratios are unlikely to explain all familial clustering of CVD. Hence, the common disease – common variant rule has to be reconsidered. Instead, many heterogeneous rare or low frequency variants in the population are hypothesized to explain larger proportions of heritability than previously assumed. Re-sequencing or deep sequencing of the candidate genomic region provides a focused approach to assess common as well as less frequent genetic variation at the site of interest as a follow-up of GWAS. Sequencing in larger clinical or even population-based samples requires different prerequisites in methodology compared to Mendelian cardiovascular disorders (Table 1).
Exome sequencing in rare and common forms of CVD
Time and effort can be reduced by focusing on transcribed gene regions in transcriptome or exome sequencing, but leaves out non-coding regions, which may also be of scientific interest, as shown in many GWAS findings. Exome sequencing also will provide a means to shed light on alternative splicing variants in relation to CVD on the way from transcription to translation. In addition, the completion of the 1000 Genomes Project (http://www.1000genomes.org) will give a comprehensive genotype map in HapMap populations that will provide novel variants and may answer many questions without the need of re-sequencing.
Whereas to date, sequencing has mainly been limited to unraveling single or oligogenic CVD causes, the determination of complete DNA sequences of human individuals will bring next generation sequencing technology to the population level. A publicly funded large-scale effort on the application of next generation sequencing to study the protein coding regions of the human genome is the Grand Opportunity Exome Sequencing Project. The study of well-phenotyped cohorts is intended to discover low frequency alleles and novel polymorphisms with large effect sizes as correlates of complex phenotypes and affiliated mechanisms contributing to CVD. The sequencing results and discoveries will be made available to the scientific community in order to improve diagnosis and management of diseases (https://esp.gs.washington.edu/drupal/).
As more experience is accumulated the understanding of the genetic architecture of CVD will deepen. For example, to date, it is largely unknown what role modifier sequence variants play in common CVD. Modifier sequence variants may be important correlates of phenotypes in single gene disorders as they may determine nuances of phenotype besides the primary, causal alleles(20). In common diseases we have been lacking the power to investigate subtle differences that distinguish modifier sequence variants from non-causal variation that is inherited.
Structural variation
The pathophysiological significance of structural variants to CVD is incompletely understood and may range from direct disease association, to quantitatively or qualitatively modifying disease phenotype to no relevant effect. Deletions or insertions affecting one to more than a million base pairs are frequently observed throughout the genome(21). Smaller studies have investigated insertions and deletions in relation to CVD phenotypes such as hypertension and coronary heart disease(22). An insertion/deletion was found in the angiotensin I converting enzyme and has repeatedly been reported in relation to CVD and its risk factors(23).
Among structural variation, copy number variation (CNV) has been an emerging focus of interest. In non-syndromal CVD, an exonic deletion in the BCL2-associated athanogene 3 (BAG3) gene was related to dilated cardiomyopathy in a multigenerational family with autosomal dominant transmission of the disease(24). At the population level, investigations of SNPs and CNVs revealed potential associations with aortic root diameter in hypertensive African Americans(25). In sporadic as well as familial aortic aneurysms and dissections rare CNVs disrupting smooth muscle adhesion or contraction have been identified in inherited forms of aneurysms(26).
To date, no robustly replicated CNVs in relation to CVD have been identified that could have been added to Figure 1. Initial evidence for CNV in early-onset myocardial infarction did not detect differences for genome-wide common and rare CNVs, the number of genes intersected by CNVs, or the total extent of CNVs compared to controls(27). More detailed analyses will be needed to comprehensively assess the implication of CNVs to CVD.
Epigenetics
New sequencing methods also will provide high-resolution maps of epigenetics(28). Epigenetics investigates mechanisms of gene-expression controls independent to genetic variations(29). The most investigated epigenetic modifications are DNA methylation, histone modifications, and nucleosome positioning.
DNA methylation is the covalent methylation of cytosines catalyzed by DNA methyltransferases located in CG dinucleotides (CpGs). Usually, methylated regions are not actively transcribed, and are silenced in fully differentiated tissues (30). In contrast, non-methylated regions mark genes that are either actively expressed or poised to be expressed(31). DNA methylation has been predominantly investigated in regions enriched with CpGs named CpG islands. Whereas CpG islands have been classically considered the main regulatory regions for methylation-related control of gene expression, recent work has suggested that CpG island “shores”, i.e., regions less dense in CpG content located up to 2Kb away from the islands, may be even more enriched in regulatory sequences(32). DNA-methylation can be determined using a candidate-gene approach through bisulfite-pyrosequencing OR MASS-array, or using genome-scale analyses by methylation microarrays or sequencing-based technologies including reduced representation bisulphite sequencing, methylC-sequencing, methylated DNA immunoprecipitation sequencing, or methylated DNA binding domain sequencing(33;34).
The major histones H2A, H2B, H3 and H4 group into globular polymers and form the nucleosomes around which DNA segments are wrapped(35). Histones are not just structural units, but undergo covalent posttranscriptional modifications including methylation, phosphorylation, acetylation, and ubiquitination. Those histone modifications appear to regulate chromosome condensation, gene expression, splicing, and DNA repair mechanisms.
The third major epigenetic regulator of gene transcription is nucleosome positioning. The presence and location of nucleosomes directly interfere with the transcription process. DNA organization in nucleosomes inhibits access of transcription factors and polymerases. Nucleosome-free gene start sites are needed to allow the assembly of the transcription complex and activity of RNA polymerases. The initiation and orchestration of epigenetic mechanisms is little understood. It has been assumed that interaction between epigenetic factors may be central to direction of DNA methylation, histone modification and chromatin remodeling(36).
Epigenetics may be a unifying principle in the etiology of complex diseases(37). In contrast to the static DNA backbone, epigenetic modifications, albeit genetically determined, can also be modified by environmental influences and are dynamic over time(38). Whereas our knowledge on epigenetics and expectations are largely derived from cancer research in which hypomethylation of non-coding DNA and de-novo hypermethylation of tumor suppressor genes has been regularly reported in malignant cells, experience with CVD associations is still much less substantiated.
Epigenetic marks are almost entirely established during embryogenesis and early development and may impact cardiovascular development through stem cell control and differentiation of endothelial and cardiac and vascular muscle cells. During early development DNA methylation is mostly dependent on availability of folic acid and other nutrients in the methyl-donor cycle. Genomic DNA methylation directly correlates with folate status and prenatal folic acid deficiency has been suggested to represent a link to early CVD development and the observation that early atherosclerosis may begin in utero(39). Along these lines, intrauterine exposure to dietary protein restriction can lead to hypertension and endothelial dysfunction in rat offspring, the exact mechanisms being unknown(40).
Endothelial shear stress also has been identified as a trigger of early angiogenic processes and vascular development through by histone deacetylases(41). During early stages of atherosclerosis in apolipoprotein E null mice, global hypomethylation precedes histologically detectable vascular lesions(42). Among proteins that specifically recognize CpG methylated DNA regions is methyl-CpG–binding domain protein 2 which activates histone deacetylases and suppresses gene transcription. Knockdown of the protein was shown to exhibit beneficial effects on endothelial nitric oxide synthase, induce angiogenesis, and to be protective against limb ischemia(43). Observations seemed to be specific to vascular endothelium and promise success in targeted modulation. In fact, endothelial nitric oxide synthase shows striking methylation differences in endothelial cells with highest activity compared to dense methylation in non-endothelial cell types(44).
Hypoxia leads to a global reduction in transcription activity reflected in upregulation of ubiquitous repressive histone methylation(45). Histone modifications seem to be mandatory to maintain constitutive endothelial nitric oxide synthase transcriptional activity. Acetylation of proximal promoter histones under hypoxia results in repression of transcription with deleterious effects on vascular homeostasis(46). Similarly, histone deacetylase 3 has been demonstrated to be critical in endothelial survival and atherosclerosis development in response to disturbed blood flow at vessel bifurcations that are known to be prime locations of atherosclerosis development(47).
Diabetes mellitus is a major risk factor for CVD with clear environmental and genetic influences. Epigenetics modifications have been invoked as potentially causal for diabetes in integrating DNA code and external influences(48). DNA methylation of the insulin promoter region is inversely related to insulin production. De-methylation in mature human insulin-producing cells affects insulin secretion, and pathological methylation patterns in context with environmental influences have been suggested to contribute to development of diabetes(49). At a population level, a differentially methylated CTCF-binding site at 11p15 was discovered in relation to type 2 diabetes(50).
There is exponentially advancing availability of increasingly robust data sets with the fine mapping of the human DNA methylome at base resolution(33), genome-wide nucleosome positioning maps, and the continuing identification of new variants and modifications. Online epigenetics resources have been assembled recently (for review see(51)).
Whereas we have gained most epigenomic insights from basic science, epidemiologic data have remained scarce. We are at the beginning of understanding that epigenetic modifications of the genome may be as important as the DNA information itself in the stable, vertical transmission of gene expression and heritability of phenotypes.
Transcriptomics
A further layer of complexity is added at the transcriptional level. Gene expression is determined by genetic and environmental influences. It can be assumed that qualitative, and, to a larger extent, quantitative differences in gene expression determine an individual’s phenotype(52). Subtle changes in splice site variations, 3’ untranslated regulatory regions, non-coding RNAs, and direct interaction of transcription factors may have significant effects on gene expression patterns and CVD phenotypes that can only be assessed by transcriptome interrogation. Single time point transcriptomics analysis represents a snapshot of cellular and tissue architecture with an enormous amount of information to process, though not reflecting biological variation over time. In particular, the transcriptome in acutely injured tissues, e.g. during myocardial infarction with widespread necrosis, inflammation and oxidative stress and invasion of acute-phase response cells, may have only remote resemblance to diseased or normal tissue under stable conditions. However, acute injury signatures in myocardial infarction may also provide the means to understand the consequences of genetics and epigenetics resulting in the actual phenotype.
More recently, transcriptome assessment has led to the identification of small, single-stranded RNA molecules such as microRNA (miRNA) and short, interfering RNA(53). Small RNAs are short non-coding RNAs that regulate translation of their target messenger RNAs (mRNA) through mRNA degradation or suppression of translation. Advantages of miRNAs are their relative chemical stability, which facilitates the miRNA isolation and analyses from clinical samples. Some miRNAs are actively secreted in exosomes and can be measured in blood specimens rendering miRNAs targets for biomarker discovery(54). Specifically designed short interfering RNAs block miRNA action and may have therapeutic potential(55).
In contrast to the more stable DNA, the protocol for preanalytic steps, extraction and sample preparation for RNA are highly demanding. Source RNA quality is crucial to reliably assess transcription levels(56). In order to analyze the abundance of RNA including non-polyadenylated and non-coding RNA, total RNA measurement has been implemented(57). Advances in RNA sequencing are more reliable than former tiling microarrays(58;59). Conventional microarray systems derived relative image intensities and signal quantification was limited. Low expression transcripts could hardly be detected against transcriptional background noise and non-specific probe binding produced false positive results. Furthermore, probe-hybridization was limited to established genomic sequences. Whole transcriptome sequencing (RNA-seq) allows the detection of transcripts without exact prior knowledge of the genomic sequence. The directly cDNA-derived digital signals show good reproducibility and comparatively few false-positive signals(59), although the challenge of quantification of the level of expression, which is not an on/off phenomenon remains(60). Replication is mandatory in large-scale gene expression studies.
Atherosclerotic CVD originates from the arterial endothelium. Distinct gene expression patterns have been observed for pro-atherosclerotic endothelial phenotypes(61;62), and gene connectivity network analysis in a systems biology approach showed transcript profiles and susceptibility to oxidative stress in coronary arteries(63). Mouse aortic gene expression patterns at different stages of atherosclerotic disease revealed striking similarities to human atherectomy samples(64).
In humans, expression signatures have been related to prognosis in CVD such as first manifestation of heart failure(65). In large human populations the assessment of the transcriptome in CVD is mostly restricted to non-cardiac tissues and cells unless patients undergo risk prone myocardial biopsy. Peripheral blood mononuclear cells and platelets are obvious candidates due to their central role in the atherosclerotic disease process and abundant availability. In fact, transcript levels have been related to atherosclerosis burden of the coronary and carotid arteries(66;67).
Promising is the simultaneous determination of genome-wide gene expression and genetic variability that will unravel direct links between transcriptional regulation and CVD(68;69). First results in peripheral blood thrombocyte transcriptome assessment at the epidemiologic level revealed the heritability of transcripts(70). Inflammatory expression signatures from the nuclear factor-κB pathway were related to body mass index. Genome-wide mononcyte expression interrogation of over 1600 expression traits were related to at least one of the classical cardiovascular risk factors(71). Recently, a novel susceptibility locus for coronary artery disease (LIPA, lipase A) was identified in the general population by combining genome-wide genetic and gene expression data(72).
An emerging field of transcriptomics is miRNA expression profiling. Of the 940 known mature human miRNAs, eighteen have consistently been shown to dominate cardiac miRNA expression(73). They can be detected during physiological cardiac development and in pathological states of CVD(74;75). Endothelial alterations in atherosclerosis are reflected by miRNA expression(76). Cardiac damage leads to detectable release of cardiomyocyte-specific miRNAs into the circulation, whether actively or through cell degradation remains to be investigated(77). Distinct miRNA patterns differentiate between entities of heart failure(75), and the dynamic in miRNA expression indicates therapeutic success in cardiac recompensation(78).
Differential splicing has been related to disease phenotypes such as heart failure. Expression patterns of the same exon in a specific gene may vary largely by alternative RNA generation, although the total amount of messenger RNA for the respective gene may remain constant(79). To better understand the regulation of gene expression, nuclear transcription and RNA stability controlled by miRNA need to be assessed at the same time. New sequencing methods will render differential splicing accessible to large-scale investigation.
First experiences in the clinical setting revealed downregulation of miR-126 and miR-17 cluster usually detected in the endothelium and miR-155 related to inflammation, whereas cardiac muscle–enriched miRNAs were elevated in the blood of patients with coronary artery disease(80). Candidate miRNAs from experimental data showed associations with acute myocardial infarction in human plasma(81). miRNA derived from peripheral blood mononuclear cells showed similarities and differential expression signatures in ischemic versus non-ischemic cardiomyopathy patients and largely differed from control individuals(82). Not only the presence and quantity of miRNA may be relevant to CVD, but initial data suggest that genetic variation in binding sites may be related to CVD, too(83).
Intriguingly, miRNA can be easily and effectively antagonized. Antagomirs targeted at miR-21 reduced remodeling as a response to pressure overload(84), and miR-23a knock-down in mice prevented cardiac hypertrophy(54). Effects of miRNA depletion provided different results after knock-down or biochemical blockade, and systemic application of miRNA mimics may act through effects on remote tissues indicating complex interactions that need to be elucidated. Furthermore, non-coding RNAs are critically involved in epigenetic modifications such as methylation regulation and histone variant replacement in coordinating nucleosome positioning(85). Transcriptomics thus remains a complex field with intensive research work ahead to understand multi-layer interrelationships.
Outlook Systems Biology
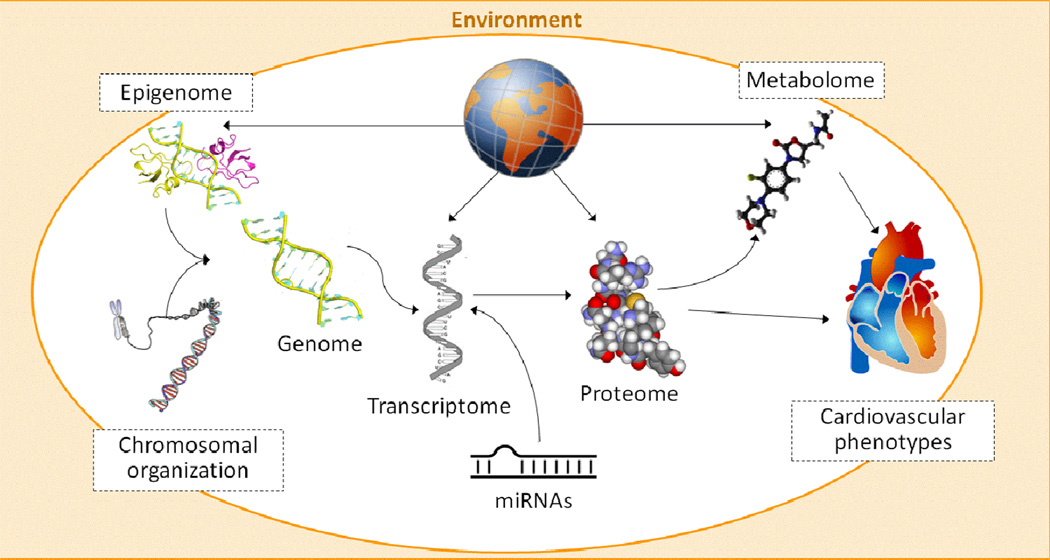
The paradigm of an almost exclusively unidirectional process from DNA to RNA to protein has been challenged. The task for the future remains to integrate knowledge of genomic and nongenomic variation, and accelerate understanding of the multidimensional nature of the genome and transcriptome. Interactions between proteins, regulatory RNA and DNA provide a more comprehensive picture of genetic regulation, transcription and translation into proteins. Further complexity is added at the translational step and posttranslational processing of immature proteins through cleavage, folding, and chemical alterations as well as environmental interactions that finally determine phenotype plasticity of cardiovascular health and disease (Figure 2). We are only beginning to unravel the pathophysiology behind the chromosome 9 finding, one of the most intensively examined genomic loci in relation to coronary heart disease discovered by GWAS. Researchers have applied genetic, epigenetic, and transcriptomic assessment without a conclusive answer to date(86–88).
Figure 2.
Complex relations between the genome, epigenetic and transcriptional regulations and the proteome and metabolome that result in cardiovascular disease phenotypes. Environmental determinants interact at all stages.
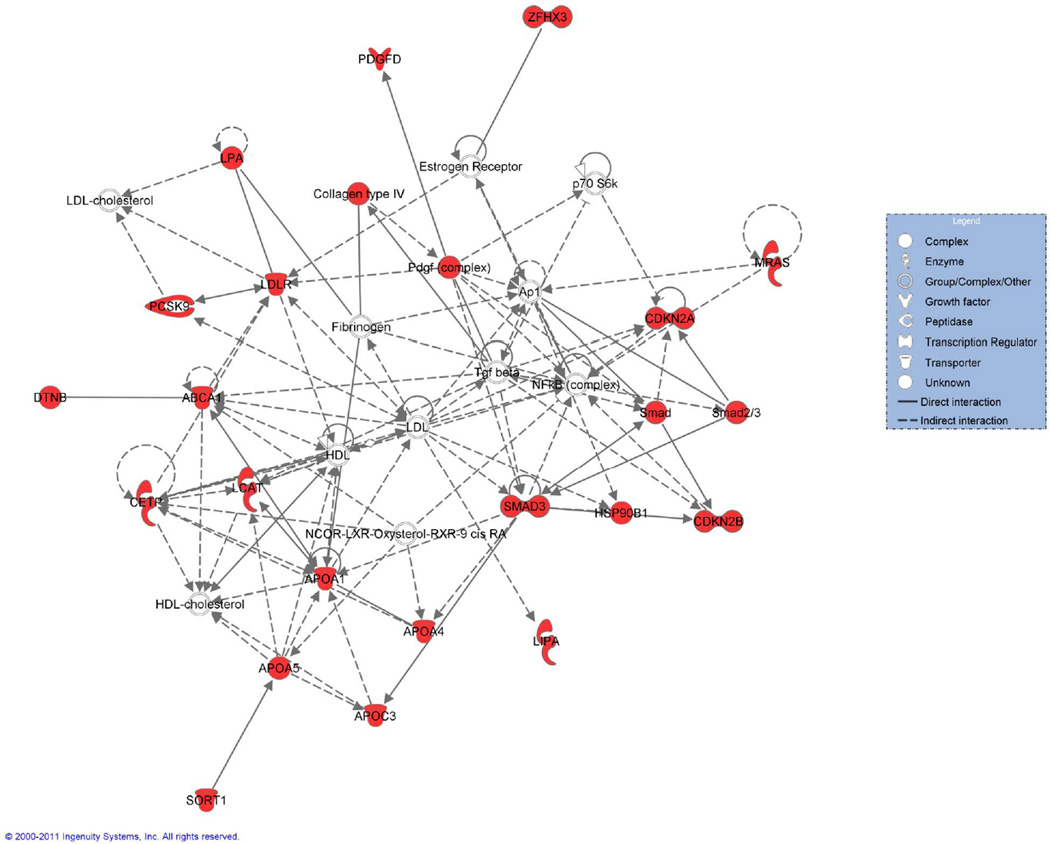
Systems biology tries to integrate interactions between DNA and regulatory elements at the RNA and protein level. We have overcome the notion that complex CVD has a specific molecular basis and we will be able to reduce pathophysiology to singular or few molecular causes. Although most widely applied for ease of use we know that linear models of associations are unlikely to capture the complexity of genome-phenotype determinants. In the past, investigations focused on a single or few intermediate phenotypes, with modest success. In modern systems biology the concept favors modular networks and subnetworks of disease that, ideally, account for time-variation in the nodes and links(89). A simplified gene network enriched for GWAS coronary heart disease loci is depicted in Figure 3. Systems approaches need to appropriately integrate all known (patho-)physiological components to create a dense network including genetics, epigenetics, posttranslational modifications of the proteome and metabolome as well as environmental exposures.
Figure 3.
The network was generated using Ingenuity Pathway Analysis (Ingenuity Systems, www.ingenuity.com). A data set containing 80 genes associated with coronary heart disease in GWAS was uploaded and overlaid onto the molecular networks developed from information contained in the Ingenuity Knowledge Base. Networks of Network Eligible Molecules were then algorithmically generated based on their connectivity. The most significantly enriched network, as shown, is comprised of 36 genes, of which 20 are coronary heart disease genes.
Intermediate phenotypes such as inflammatory activity or endothelial dysfunction will overlap between several types of CVD, but other constituents may be unique alone or in their specific interplay with neighboring nodes. Previously unknown relations will emerge and provide causal links whose preventive or therapeutic modulation may alter disease occurrence and phenotype. A holistic view as suggested by systems biology, integrating all available knowledge in context with CVD will guide the next steps in cardiovascular genomics and enhance our understanding of disease susceptibility, treatment and monitoring and influence preventive actions.
Prospect of clinical application
Despite the enormous advances in science, genomic information is not widely applied in clinical cardiology. Whereas, for example, in oncology gene expression profiling is actively used in clinical practice to determine cancer entity and guide therapy(90), large-scale transcriptome assessment in CVD has not entered clinical routine owing to limited experience and controversial findings with restricted clinical relevance.
In contrast to the fairly stable primary DNA sequence scaffold, gene-gene, RNA-DNA, protein-DNA interactions and chemical modifications are significant determinants of transcription and gene expression(91), and we are only beginning to understand the complex picture that may shed light on the dark matter of heritability. The wealth of GWAS findings has generated hypotheses in CVD pathophysiology for the clinician. To fully exploit the power of GWAS, functional work-up is needed over the next years to establish causative links. Advances in laboratory medicine with the refinement of genotyping methods through next generation sequencing, the assessment of epigenetics and the transcriptome as well as the identification of novel genomic markers such as miRNAs will rapidly advance genomic cardiovascular medicine and change the definition of complex CVD.
To accelerate clinically relevant discoveries the National Institutes of Health has supported several large database studies involving de-identified electronic health record phenotypes and genomic investigations. Current research efforts are being directed towards issues such as data safety, database architecture, and widely accessible exploration algorithms. Informatics knowledge is essential to compile and manage project-related clinical data for research. The National Institutes of Health supports the i2b2 (Informatics for Integrating Biology and the Bedside) initiative that has been aiming to provide researchers with the informatics tools to accelerate clinical translation of genomic findings. Similarly, the National Institutes of Health sponsors the eMERGE Network to accelerate research integrating DNA biorepositories with electronic medical record (EMR) systems(92).
Pharmacogenomics has advanced the field of drug response assessment, e.g. first experiences with guidance of vitamin K antagonist therapy with the help of CYP2C9 or VKORC1 polymorphisms(93) and cytochrome p-450 polymorphisms in relation to clopidogrel response have entered US Food and Drug Administration recommendations(94). Disease prevention lags behind. Gene chips and modern sequencing allow large-scale interrogation of the genome at the population level and will generate novel hypotheses for disease causation. Furthermore, with the continuing drop in costs of whole genome sequencing the practicing physician may soon be faced with the need to comment on a patient’s over four million sequence variants regarding disease risk before clinical signs occur, a task that no certified genetic counselor could fulfill at present. With the advent of GWAS ethical and practical concerns of reporting genetic research results have become apparent. Initial efforts at defining rules of reporting large-scale association results and to assess the level of evidence have recently been suggested that also apply for next-generation large-scale genomics(95;96). Reports have suggested that consumer side genome-wide genetic profiling in employees of health and technology companies did not change anxiety symptoms, dietary fat intake, or exercise behavior, i.e. lifestyle factors over a six month period(97). However, the risk association of genetic variation and the dissection between objective markers of risk versus risk factors which reside in the causal pathway of disease will need careful assessment before entering clinical decision making(98).
To be of clinical utility the determination of genetic polymorphisms needs to fulfill the basic requirements of a biomarker. As risk predictors they need to account for sufficient absolute or population attributable risk. So far, even the combination of multiple newly identified and replicated loci as shown for blood cholesterol or C-reactive protein concentrations as intermediate phenotypes does not add appreciably optimized risk prediction of CVD(99;100). Concrete diagnostic or interventional steps should result from the determination of genetic variation to serve as pre-clinical markers or indicate pathogenic mechanisms. Whereas modifiability of the DNA code is impractical for genetic variation, epigenetic and transcriptional patterns will be accessible for targeted modifications(43;54;84).
Standardization of measurement methods and determination across populations is desirable and robustly feasible for DNA, however, at present less reliable for gene expression. Full DNA sequencing of the human genome was performed at an error rate less than 1 in 10,000 bases in the Human Genome Projects (http://www.genome.gov/11006943). The biochemical characteristics of miRNA including analyte stability and presence of circulating extracellular molecules render small RNAs interesting targets if relevant disease relations can be established. In addition, gene expression, measured either at the mRNA or protein level, is highly variable over time, as it can show dramatic changes on the scale of hours to minutes. Clinical trials will need to prove utility and provide evidence for cost-benefit assessment before general recommendations regarding the use of genomics information for common diseases can be expected.
To date, unbiased genome-wide approaches have unraveled novel biologic pathways in CVD. Multi-stage, multi-disciplinary (basic scientist, biostatistician, physician) efforts are now necessary to understand the functional annotation of risk alleles and explain residual disease susceptibility (Table 3). With the growing number of databases and the establishment of large-scale consortial cooperation unprecedented opportunities for effective genomic research at the population level have arisen. With accumulating evidence and simplification and standardization of methods, genomic findings will find their translation in clinical practice in the near future.
Table 3.
Resources for the holistic approach of systems biology
| Phenotype |
|
| Data sources |
|
| Bioinformatics and Statistical |
|
| Laboratory resources |
|
Acknowledgements
We hope for the understanding of all authors whose important work in the field could not be cited due to word count limitations. Renate B. Schnabel is supported by Deutsche Forschungsgemeinschaft (German Research Foundation) Emmy Noether Program SCHN 1149/3-1. Emelia J. Benjamin is supported by 1R01HL092577; 1RC1HL101056; 1R01HL102214; 1R01AG028321. Patrick T. Ellinor is supported by 1R01HL092577; 5R21DA027021; 5R01HL104156; 1K24HL105780. This work was partially supported by the Evans Center for Interdisciplinary Biomedical Research ARC on “Atrial Fibrillation at Boston University (http://www.bumc.bu.edu/evanscenteribr/). Andrea Baccarelli is supported by P30ES000002; R21ES019773; R01ES015172.
Glossary
Abbreviations
- CNV
copy number variation
- CVD
cardiovascular disease
- GWAS
genome-wide association study
- miRNA
microRNA
- SNP
single nucleotide polymorphism
Reference List
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banerjee A, Silver LE, Heneghan C, Welch SJ, Mehta Z, Banning AP, Rothwell PM. Relative familial clustering of cerebral versus coronary ischemic events. Circ Cardiovasc Genet. 2011;4:390–396. doi: 10.1161/CIRCGENETICS.110.959114. [DOI] [PubMed] [Google Scholar]
- 3.Marenberg ME, Risch N, Berkman LF, Floderus B, de FU. Genetic susceptibility to death from coronary heart disease in a study of twins. N Engl J Med. 1994;330:1041–1046. doi: 10.1056/NEJM199404143301503. [DOI] [PubMed] [Google Scholar]
- 4.Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 5.Schunkert H, Konig IR, Kathiresan S, Reilly MP, Assimes TL, Holm H, et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. 2011;43:333–338. doi: 10.1038/ng.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer M, Broeckel U, Holmer S, Baessler A, Hengstenberg C, Mayer B, et al. Distinct heritable patterns of angiographic coronary artery disease in families with myocardial infarction. Circulation. 2005;111:855–862. doi: 10.1161/01.CIR.0000155611.41961.BB. [DOI] [PubMed] [Google Scholar]
- 7.Kathiresan S, Melander O, Guiducci C, Surti A, Burtt NP, Rieder MJ, et al. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat Genet. 2008;40:189–197. doi: 10.1038/ng.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McPherson R, Pertsemlidis A, Kavaslar N, Stewart A, Roberts R, Cox DR, et al. A common allele on chromosome 9 associated with coronary heart disease. Science. 2007;316:1488–1491. doi: 10.1126/science.1142447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helgadottir A, Thorleifsson G, Manolescu A, Gretarsdottir S, Blondal T, Jonasdottir A, et al. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science. 2007;316:1491–1493. doi: 10.1126/science.1142842. [DOI] [PubMed] [Google Scholar]
- 10.Gudbjartsson DF, Arnar DO, Helgadottir A, Gretarsdottir S, Holm H, Sigurdsson A, et al. Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature. 2007;448:353–357. doi: 10.1038/nature06007. [DOI] [PubMed] [Google Scholar]
- 11.Hindorff LA, Sethupathy P, Junkins HA, Ramos EM, Mehta JP, Collins FS, Manolio TA. Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc Natl Acad Sci U S A. 2009;106:9362–9367. doi: 10.1073/pnas.0903103106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janssens AC, van Duijn CM. Genome-based prediction of common diseases: advances and prospects. Hum Mol Genet. 2008;17:R166–R173. doi: 10.1093/hmg/ddn250. [DOI] [PubMed] [Google Scholar]
- 13.Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lango AH, Estrada K, Lettre G, Berndt SI, Weedon MN, Rivadeneira F, et al. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature. 2010;467:832–838. doi: 10.1038/nature09410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frazer KA, Murray SS, Schork NJ, Topol EJ. Human genetic variation and its contribution to complex traits. Nat Rev Genet. 2009;10:241–251. doi: 10.1038/nrg2554. [DOI] [PubMed] [Google Scholar]
- 16.Jiang YH, Bressler J, Beaudet AL, et al. Epigenetics and human disease. Annu Rev Genomics Hum Genet. 2004;5:479–510. doi: 10.1146/annurev.genom.5.061903.180014. [DOI] [PubMed] [Google Scholar]
- 17.Musunuru K, Strong A, Frank-Kamenetsky M, Lee NE, Ahfeldt T, Sachs KV, et al. From noncoding variant to phenotype via SORT1 at the 1p13 cholesterol locus. Nature. 2010;466:714–719. doi: 10.1038/nature09266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eid J, Fehr A, Gray J, Luong K, Lyle J, Otto G, et al. Real-time DNA sequencing from single polymerase molecules. Science. 2009;323:133–138. doi: 10.1126/science.1162986. [DOI] [PubMed] [Google Scholar]
- 19.Heintzman ND, Stuart RK, Hon G, Fu Y, Ching CW, Hawkins RD, et al. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet. 2007;39:311–318. doi: 10.1038/ng1966. [DOI] [PubMed] [Google Scholar]
- 20.Daw EW, Chen SN, Czernuszewicz G, Lombardi R, Lu Y, Ma J, et al. Genome-wide mapping of modifier chromosomal loci for human hypertrophic cardiomyopathy. Hum Mol Genet. 2007;16:2463–2471. doi: 10.1093/hmg/ddm202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kidd JM, Cooper GM, Donahue WF, Hayden HS, Sampas N, Graves T, et al. Mapping and sequencing of structural variation from eight human genomes. Nature. 2008;453:56–64. doi: 10.1038/nature06862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kepp K, Org E, Sober S, Kelgo P, Viigimaa M, Veldre G, et al. Hypervariable intronic region in NCX1 is enriched in short insertion-deletion polymorphisms and showed association with cardiovascular traits. BMC Med Genet. 2010;11:15. doi: 10.1186/1471-2350-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poirier O, Georges JL, Ricard S, Arveiler D, Ruidavets JB, Luc G, et al. New polymorphisms of the angiotensin II type 1 receptor gene and their associations with myocardial infarction and blood pressure: the ECTIM study. Etude Cas-Temoin de I'Infarctus du Myocarde. J Hypertens. 1998;16:1443–1447. doi: 10.1097/00004872-199816100-00007. [DOI] [PubMed] [Google Scholar]
- 24.Norton N, Li D, Rieder MJ, Siegfried JD, Rampersaud E, Zuchner S, et al. Genome-wide studies of copy number variation and exome sequencing identify rare variants in BAG3 as a cause of dilated cardiomyopathy. Am J Hum Genet. 2011;88:273–282. doi: 10.1016/j.ajhg.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wineinger NE, Patki A, Meyers KJ, Broeckel U, Gu CC, Rao DC, et al. Genome-wide joint SNP and CNV analysis of aortic root diameter in African Americans: the HyperGEN study. BMC Med Genomics. 2011;4:4. doi: 10.1186/1755-8794-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prakash SK, LeMaire SA, Guo DC, Russell L, Regalado ES, Golabbakhsh H, et al. Rare copy number variants disrupt genes regulating vascular smooth muscle cell adhesion and contractility in sporadic thoracic aortic aneurysms and dissections. Am J Hum Genet. 2010;87:743–756. doi: 10.1016/j.ajhg.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kathiresan S, Voight BF, Purcell S, Musunuru K, Ardissino D, Mannucci PM, et al. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat Genet. 2009;41:334–341. doi: 10.1038/ng.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bock C, Tomazou EM, Brinkman AB, Muller F, Simmer F, Gu H, et al. Quantitative comparison of genome-wide DNA methylation mapping technologies. Nat Biotechnol. 2010;28:1106–1114. doi: 10.1038/nbt.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feinberg AP. Phenotypic plasticity and the epigenetics of human disease. Nature. 2007;447:433–440. doi: 10.1038/nature05919. [DOI] [PubMed] [Google Scholar]
- 30.Kacem S, Feil R. Chromatin mechanisms in genomic imprinting. Mamm Genome. 2009;20:544–556. doi: 10.1007/s00335-009-9223-4. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Valo Z, Bowers CW, Smith DD, Liu Z, Singer-Sam J. Dual DNA methylation patterns in the CNS reveal developmentally poised chromatin and monoallelic expression of critical genes. PLoS ONE. 2010;5:e13843. doi: 10.1371/journal.pone.0013843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Irizarry RA, Ladd-Acosta C, Wen B, Wu Z, Montano C, Onyango P, et al. The human colon cancer methylome shows similar hypo- and hypermethylation at conserved tissue-specific CpG island shores. Nat Genet. 2009;41:178–186. doi: 10.1038/ng.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lister R, Pelizzola M, Dowen RH, Hawkins RD, Hon G, Tonti-Filippini J, et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009;462:315–322. doi: 10.1038/nature08514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris RA, Wang T, Coarfa C, Nagarajan RP, Hong C, Downey SL, et al. Comparison of sequencing-based methods to profile DNA methylation and identification of monoallelic epigenetic modifications. Nat Biotechnol. 2010;28:1097–1105. doi: 10.1038/nbt.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Zhao Q, Rank G, Tan YT, Li H, Moritz RL, Simpson RJ, et al. PRMT5-mediated methylation of histone H4R3 recruits DNMT3A, coupling histone and DNA methylation in gene silencing. Nat Struct Mol Biol. 2009;16:304–311. doi: 10.1038/nsmb.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petronis A. Epigenetics as a unifying principle in the aetiology of complex traits and diseases. Nature. 2010;465:721–727. doi: 10.1038/nature09230. [DOI] [PubMed] [Google Scholar]
- 38.Bollati V, Baccarelli A. Environmental epigenetics. Heredity. 2010;105:105–112. doi: 10.1038/hdy.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Friso S, Choi SW, Girelli D, Mason JB, Dolnikowski GG, Bagley PJ, et al. A common mutation in the 5,10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc Natl Acad Sci U S A. 2002;99:5606–5611. doi: 10.1073/pnas.062066299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brawley L, Itoh S, Torrens C, Barker A, Bertram C, Poston L, Hanson M. Dietary protein restriction in pregnancy induces hypertension and vascular defects in rat male offspring. Pediatr Res. 2003;54:83–90. doi: 10.1203/01.PDR.0000065731.00639.02. [DOI] [PubMed] [Google Scholar]
- 41.Illi B, Scopece A, Nanni S, Farsetti A, Morgante L, Biglioli P, et al. Epigenetic histone modification and cardiovascular lineage programming in mouse embryonic stem cells exposed to laminar shear stress. Circ Res. 2005;96:501–508. doi: 10.1161/01.RES.0000159181.06379.63. [DOI] [PubMed] [Google Scholar]
- 42.Lund G, Andersson L, Lauria M, Lindholm M, Fraga MF, Villar-Garea A, et al. DNA methylation polymorphisms precede any histological sign of atherosclerosis in mice lacking apolipoprotein E. J Biol Chem. 2004;279:29147–29154. doi: 10.1074/jbc.M403618200. [DOI] [PubMed] [Google Scholar]
- 43.Rao X, Zhong J, Zhang S, Zhang Y, Yu Q, Yang P, et al. Loss of Methyl-CpG-Binding Domain Protein 2 Enhances Endothelial Angiogenesis and Protects Mice Against Hind-Limb Ischemic Injury. Circulation. 2011 doi: 10.1161/CIRCULATIONAHA.110.966408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chan Y, Fish JE, D’Abreo C, Lin S, Robb GB, Teichert AM, et al. The cell-specific expression of endothelial nitric-oxide synthase: a role for DNA methylation. J Biol Chem. 2004;279:35087–35100. doi: 10.1074/jbc.M405063200. [DOI] [PubMed] [Google Scholar]
- 45.Johnson AB, Denko N, Barton MC. Hypoxia induces a novel signature of chromatin modifications and global repression of transcription. Mutat Res. 2008;640:174–179. doi: 10.1016/j.mrfmmm.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fish JE, Yan MS, Matouk CC, St BR, Ho JJ, Gavryushova A, et al. Hypoxic repression of endothelial nitric-oxide synthase transcription is coupled with eviction of promoter histones. J Biol Chem. 2010;285:810–826. doi: 10.1074/jbc.M109.067868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zampetaki A, Zeng L, Margariti A, Xiao Q, Li H, Zhang Z, et al. Histone deacetylase 3 is critical in endothelial survival and atherosclerosis development in response to disturbed flow. Circulation. 2010;121:132–142. doi: 10.1161/CIRCULATIONAHA.109.890491. [DOI] [PubMed] [Google Scholar]
- 48.Villeneuve LM, Natarajan R. The role of epigenetics in the pathology of diabetic complications. Am J Physiol Renal Physiol. 2010;299:F14–F25. doi: 10.1152/ajprenal.00200.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuroda A, Rauch TA, Todorov I, Ku HT, Al-Abdullah IH, Kandeel F, et al. Insulin gene expression is regulated by DNA methylation. PLoS ONE. 2009;4:e6953. doi: 10.1371/journal.pone.0006953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kong A, Steinthorsdottir V, Masson G, Thorleifsson G, Sulem P, Besenbacher S, et al. Parental origin of sequence variants associated with complex diseases. Nature. 2009;462:868–874. doi: 10.1038/nature08625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baccarelli A, Rienstra M, Benjamin EJ. Cardiovascular epigenetics: basic concepts and results from animal and human studies. Circ Cardiovasc Genet. 2010;3:567–573. doi: 10.1161/CIRCGENETICS.110.958744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goring HH, Curran JE, Johnson MP, Dyer TD, Charlesworth J, Cole SA, et al. Discovery of expression QTLs using large-scale transcriptional profiling in human lymphocytes. Nat Genet. 2007;39:1208–1216. doi: 10.1038/ng2119. [DOI] [PubMed] [Google Scholar]
- 53.Carthew RW, Sontheimer EJ. Origins and Mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin Z, Murtaza I, Wang K, Jiao J, Gao J, Li PF. miR-23a functions downstream of NFATc3 to regulate cardiac hypertrophy. Proc Natl Acad Sci U S A. 2009;106:12103–12108. doi: 10.1073/pnas.0811371106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Castanotto D, Rossi JJ. The promises and pitfalls of RNA-interference-based therapeutics. Nature. 2009;457:426–433. doi: 10.1038/nature07758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ho-Pun-Cheung A, Bascoul-Mollevi C, Assenat E, Boissiere-Michot F, Bibeau F, Cellier D, et al. Reverse transcription-quantitative polymerase chain reaction: description of a RIN-based algorithm for accurate data normalization. BMC Mol Biol. 2009;10:31. doi: 10.1186/1471-2199-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mattick JS, Taft RJ, Faulkner GJ. A global view of genomic information--moving beyond the gene and the master regulator. Trends Genet. 2010;26:21–28. doi: 10.1016/j.tig.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 58.Richard H, Schulz MH, Sultan M, Nurnberger A, Schrinner S, Balzereit D, et al. Prediction of alternative isoforms from exon expression levels in RNA-Seq experiments. Nucleic Acids Res. 2010;38:e112. doi: 10.1093/nar/gkq041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sultan M, Schulz MH, Richard H, Magen A, Klingenhoff A, Scherf M, et al. A global view of gene activity and alternative splicing by deep sequencing of the human transcriptome. Science. 2008;321:956–960. doi: 10.1126/science.1160342. [DOI] [PubMed] [Google Scholar]
- 60.Seo D, Ginsburg GS, Goldschmidt-Clermont PJ. Gene expression analysis of cardiovascular diseases: novel insights into biology and clinical applications. J Am Coll Cardiol. 2006;48:227–235. doi: 10.1016/j.jacc.2006.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Passerini AG, Polacek DC, Shi C, Francesco NM, Manduchi E, Grant GR, et al. Coexisting proinflammatory and antioxidative endothelial transcription profiles in a disturbed flow region of the adult porcine aorta. Proc Natl Acad Sci U S A. 2004;101:2482–2487. doi: 10.1073/pnas.0305938101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Magid R, Davies PF. Endothelial protein kinase C isoform identity and differential activity of PKCzeta in an athero-susceptible region of porcine aorta. Circ Res. 2005;97:443–449. doi: 10.1161/01.RES.0000179767.37838.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Civelek M, Manduchi E, Riley RJ, Stoeckert CJ, Jr, Davies PF. Coronary artery endothelial transcriptome in vivo: identification of endoplasmic reticulum stress and enhanced reactive oxygen species by gene connectivity network analysis. Circ Cardiovasc Genet. 2011;4:243–252. doi: 10.1161/CIRCGENETICS.110.958926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seo D, Wang T, Dressman H, Herderick EE, Iversen ES, Dong C, et al. Gene expression phenotypes of atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:1922–1927. doi: 10.1161/01.ATV.0000141358.65242.1f. [DOI] [PubMed] [Google Scholar]
- 65.Heidecker B, Kasper EK, Wittstein IS, Champion HC, Breton E, Russell SD, et al. Transcriptomic biomarkers for individual risk assessment in new-onset heart failure. Circulation. 2008;118:238–246. doi: 10.1161/CIRCULATIONAHA.107.756544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma J, Liew CC. Gene profiling identifies secreted protein transcripts from peripheral blood cells in coronary artery disease. J Mol Cell Cardiol. 2003;35:993–998. doi: 10.1016/s0022-2828(03)00179-2. [DOI] [PubMed] [Google Scholar]
- 67.Patino WD, Mian OY, Kang JG, Matoba S, Bartlett LD, Holbrook B, et al. Circulating transcriptome reveals markers of atherosclerosis. Proc Natl Acad Sci U S A. 2005;102:3423–3428. doi: 10.1073/pnas.0408032102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dixon AL, Liang L, Moffatt MF, Chen W, Heath S, Wong KC, et al. A genome-wide association study of global gene expression. Nat Genet. 2007;39:1202–1207. doi: 10.1038/ng2109. [DOI] [PubMed] [Google Scholar]
- 69.Stranger BE, Nica AC, Forrest MS, Dimas A, Bird CP, Beazley C, et al. Population genomics of human gene expression. Nat Genet. 2007;39:1217–1224. doi: 10.1038/ng2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Freedman JE, Loscalzo J. Platelet-monocyte aggregates: bridging thrombosis and inflammation. Circulation. 2002;105:2130–2132. doi: 10.1161/01.cir.0000017140.26466.f5. [DOI] [PubMed] [Google Scholar]
- 71.Zeller T, Wild P, Szymczak S, Rotival M, Schillert A, Castagne R, et al. Genetics and beyond--the transcriptome of human monocytes and disease susceptibility. PLoS ONE. 2010;5:e10693. doi: 10.1371/journal.pone.0010693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wild PS, Zeller T, Schillert A, Szymczak S, Sinning CR, Deiseroth A, et al. A Genome-wide Association Study Identifies LIPA as a Susceptibility Gene for Coronary Artery Disease. Circ Cardiovasc Genet. 2011 doi: 10.1161/CIRCGENETICS.110.958728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rao PK, Toyama Y, Chiang HR, Gupta S, Bauer M, Medvid R, et al. Loss of cardiac microRNA-mediated regulation leads to dilated cardiomyopathy and heart failure. Circ Res. 2009;105:585–594. doi: 10.1161/CIRCRESAHA.109.200451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao Y, Ransom JF, Li A, Vedantham V, von DM, Muth AN, et al. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Cell. 2007;129:303–317. doi: 10.1016/j.cell.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 75.van RE, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, et al. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci U S A. 2006;103:18255–18260. doi: 10.1073/pnas.0608791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fang Y, Shi C, Manduchi E, Civelek M, Davies PF. MicroRNA-10a regulation of proinflammatory phenotype in athero-susceptible endothelium in vivo and in vitro. Proc Natl Acad Sci U S A. 2010;107:13450–13455. doi: 10.1073/pnas.1002120107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Corsten MF, Dennert R, Jochems S, Kuznetsova T, Devaux Y, Hofstra L, et al. Circulating MicroRNA-208b and MicroRNA-499 reflect myocardial damage in cardiovascular disease. Circ Cardiovasc Genet. 2010;3:499–506. doi: 10.1161/CIRCGENETICS.110.957415. [DOI] [PubMed] [Google Scholar]
- 78.Matkovich SJ, Van Booven DJ, Youker KA, Torre-Amione G, Diwan A, Eschenbacher WH, et al. Reciprocal regulation of myocardial microRNAs and messenger RNA in human cardiomyopathy and reversal of the microRNA signature by biomechanical support. Circulation. 2009;119:1263–1271. doi: 10.1161/CIRCULATIONAHA.108.813576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shang LL, Pfahnl AE, Sanyal S, Jiao Z, Allen J, Banach K, et al. Human heart failure is associated with abnormal C-terminal splicing variants in the cardiac sodium channel. Circ Res. 2007;101:1146–1154. doi: 10.1161/CIRCRESAHA.107.152918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fichtlscherer S, De RS, Fox H, Schwietz T, Fischer A, Liebetrau C, et al. Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010;107:677–684. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- 81.Wang GK, Zhu JQ, Zhang JT, Li Q, Li Y, He J, et al. Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur Heart J. 2010;31:659–666. doi: 10.1093/eurheartj/ehq013. [DOI] [PubMed] [Google Scholar]
- 82.Voellenkle C, van RJ, Cappuzzello C, Greco S, Arcelli D, Di VL, et al. MicroRNA signatures in peripheral blood mononuclear cells of chronic heart failure patients. Physiol Genomics. 2010;42:420–426. doi: 10.1152/physiolgenomics.00211.2009. [DOI] [PubMed] [Google Scholar]
- 83.Sethupathy P, Borel C, Gagnebin M, Grant GR, Deutsch S, Elton TS, et al. Human microRNA-155 on chromosome 21 differentially interacts with its polymorphic target in the AGTR1 3’ untranslated region: a mechanism for functional single-nucleotide polymorphisms related to phenotypes. Am J Hum Genet. 2007;81:405–413. doi: 10.1086/519979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 85.Lal A, Pan Y, Navarro F, Dykxhoorn DM, Moreau L, Meire E, et al. miR-24-mediated downregulation of H2AX suppresses DNA repair in terminally differentiated blood cells. Nat Struct Mol Biol. 2009;16:492–498. doi: 10.1038/nsmb.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Folkersen L, Kyriakou T, Goel A, Peden J, Malarstig A, Paulsson-Berne G, et al. Relationship between CAD risk genotype in the chromosome 9p21 locus and gene expression. Identification of eight new ANRIL splice variants. PLoS ONE. 2009;4:e7677. doi: 10.1371/journal.pone.0007677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu Y, Sanoff HK, Cho H, Burd CE, Torrice C, Mohlke KL, et al. INK4/ARF transcript expression is associated with chromosome 9p21 variants linked to atherosclerosis. PLoS ONE. 2009;4:e5027. doi: 10.1371/journal.pone.0005027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Harismendy O, Notani D, Song X, Rahim NG, Tanasa B, Heintzman N, et al. 9p21 DNA variants associated with coronary artery disease impair interferon-gamma signalling response. Nature. 2011;470:264–268. doi: 10.1038/nature09753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Loscalzo J, Kohane I, Barabasi AL. Human disease classification in the postgenomic era: a complex systems approach to human pathobiology. Mol Syst Biol. 2007;3:124. doi: 10.1038/msb4100163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sotiriou C, Pusztai L. Gene-expression signatures in breast cancer. N Engl J Med. 2009;360:790–800. doi: 10.1056/NEJMra0801289. [DOI] [PubMed] [Google Scholar]
- 91.Parker SC, Hansen L, Abaan HO, Tullius TD, Margulies EH. Local DNA topography correlates with functional noncoding regions of the human genome. Science. 2009;324:389–392. doi: 10.1126/science.1169050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McCarty CA, Chisholm RL, Chute CG, Kullo IJ, Jarvik GP, Larson EB, et al. The eMERGE Network: a consortium of biorepositories linked to electronic medical records data for conducting genomic studies. BMC Med Genomics. 2011;4:13. doi: 10.1186/1755-8794-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Anderson JL, Horne BD, Stevens SM, Grove AS, Barton S, Nicholas ZP, et al. Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation. Circulation. 2007;116:2563–2570. doi: 10.1161/CIRCULATIONAHA.107.737312. [DOI] [PubMed] [Google Scholar]
- 94.Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360:354–362. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- 95.Janssens AC, Ioannidis JP, van Duijn CM, Little J, Khoury MJ. Strengthening the reporting of Genetic RIsk Prediction Studies: the GRIPS Statement. PLoS Med. 2011;8 doi: 10.1038/ejhg.2011.25. e1000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fabsitz RR, McGuire A, Sharp RR, Puggal M, Beskow LM, Biesecker LG, et al. Ethical and practical guidelines for reporting genetic research results to study participants: updated guidelines from a National Heart, Lung, and Blood Institute working group. Circ Cardiovasc Genet. 2010;3:574–580. doi: 10.1161/CIRCGENETICS.110.958827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bloss CS, Schork NJ, Topol EJ. Effect of direct-to-consumer genomewide profiling to assess disease risk. N Engl J Med. 2011;364:524–534. doi: 10.1056/NEJMoa1011893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 99.Kathiresan S, Melander O, Anevski D, Guiducci C, Burtt NP, Roos C, et al. Polymorphisms associated with cholesterol and risk of cardiovascular events. N Engl J Med. 2008;358:1240–1249. doi: 10.1056/NEJMoa0706728. [DOI] [PubMed] [Google Scholar]
- 100.Dehghan A, Dupuis J, Barbalic M, Bis JC, Eiriksdottir G, Lu C, et al. Meta-analysis of genome-wide association studies in >80 000 subjects identifies multiple loci for C-reactive protein levels. Circulation. 2011;123:731–738. doi: 10.1161/CIRCULATIONAHA.110.948570. [DOI] [PMC free article] [PubMed] [Google Scholar]