Abstract
The aim of the current study is to examine the protective effect of MGN-3 on overall maintenance of hematopoietic tissue after γ-irradiation. MGN-3 is an arabinoxylan from rice bran that has been shown to be a powerful antioxidant and immune modulator. Swiss albino mice were treated with MGN-3 prior to irradiation and continued to receive MGN-3 for 1 or 4 weeks. Results were compared with mice that received radiation (5 Gy γ rays) only, MGN-3 (40 mg/kg) only and control mice (receiving neither radiation nor MGN-3). At 1 and 4 weeks post-irradiation, different hematological, histopathological and biochemical parameters were examined. Mice exposed to irradiation alone showed significant depression in their complete blood count (CBC) except for neutrophilia. Additionally, histopathological studies showed hypocellularity of their bone marrow, as well as a remarkable decrease in splenic weight/relative size and in number of megakaryocytes. In contrast, pre-treatment with MGN-3 resulted in protection against irradiation-induced damage to the CBC parameters associated with complete bone marrow cellularity, as well as protection of the aforementioned splenic changes. Furthermore, MGN-3 exerted antioxidative activity in whole-body irradiated mice, and provided protection from irradiation-induced loss of body and organ weight. In conclusion, MGN-3 has the potential to protect progenitor cells in the bone marrow, which suggests the possible use of MGN-3/Biobran as an adjuvant treatment to counteract the severe adverse side effects associated with radiation therapy.
Keywords: MGN-3, Biobran, radiation, hematopoeitic cells
INTRODUCTION
Ionizing radiation has a diversity of beneficial uses in medicine including radiotherapy as an important treatment modality for cancer, radiographs for screening, diagnosis and staging of diseases and malignancies. However, effective use of ionizing radiation is compromised by the side effects that result from radiation-induced damage to normal tissue [1]. Vulnerability of exposure to ionizing radiation is of great concern to patients and medical personnel in the occupational setting (radiotherapy technicians, dental assistants, and research personnel). In addition, environmental contamination from accidents like the Ukraine Chernobyl disaster of 26 April 1986, and Fukushima radiation releases in Japan on 11 March 2011, can cause widespread health concerns. Ionizing radiation can cause a series of deleterious side-effects, including oxidative stress [2] and oxidative damage to cellular macromolecules [3–5], which lead to the demise of hematopoietic tissues [6, 7]. The bone marrow and spleen are important in maintaining the peripheral blood cell pool and proper functioning of the immune system. Thus, radiation damage to these vital organs can affect hematopoiesis as well as immune defense, both critical determinants of post-exposure morbidity and mortality [8, 9]. It is unfortunate that the use of most synthetic radioprotective compounds is restricted owing to their toxicity and high cost. These observations instigated a search for alternative agents that are highly effective, less toxic, and not costly.
MGN-3 is an arabinoxylan from rice bran, a polysaccharide containing β-1,4-xylopyronase hemicelluloses, that has been enzymatically treated with extract from shiitake mushrooms [10]. Our work and that of others has shown that MGN-3 is a potent biological response modifier (BRM) known to activate dendritic cells [11, 12], enhance NK cell activity [13, 14], modulate cytokines, and induce apoptosis in tumor tissue [15]. Earlier studies by S. Nakatsugawa (personal communication, 2001) showed that MGN-3 is very effective in enhancing the survival of mice exposed to γ-irradiation in a dose-dependent manner (range, 4.5–8.5 Gy). The deleterious effects of ionizing radiation on living cells are often mediated by increased production of reactive oxygen species (ROS) [3]. Since MGN-3 is an effective antioxidant agent in mice, as manifested by preventing the formation of free radicals, modulating lipid peroxidation, augmenting the antioxidant defense system, and protecting against oxidative stress [16], in this study we examine the protective effect of MGN-3 on the overall maintenance of hematopoietic tissue after γ-irradiation.
MATERIALS AND METHODS
Mice
A total of 57 adult female Swiss albino mice of about 8 weeks of age (average body weight of 24 ± 2 g) were used in this investigation. Mice were housed 5/cage and were allowed free access to standard laboratory cube pellets and water.
MGN-3/Biobran
MGN-3 is modified rice bran extract that is treated enzymatically with an extract from Shiitake mushrooms. It contains polysaccharide β1, 4-xylopyronase hemicellulose. The main chemical structure of MGN-3 is arabinoxylan, with a xylose in its main chain and an arabinose polymer in its side chain [10]. MGN-3 was freshly prepared by solution in 0.9% saline, and 40 mg/kg body weight/day was given to mice intraperitoneally (i.p.) every other day via a single 0.1 ml shot [15]. Treatment commenced on Day 0 and continued throughout the experimental period. MGN-3 was kindly provided by Daiwa Pharmaceuticals Co Ltd., Tokyo Japan.
Irradiation
Whole body-irradiation was performed on mice at the NCRRT, Cairo, Egypt, using the Gamma cell-40 (Caesium-137 source). Animals were placed in well-ventilated containers and irradiated at an acute single dose level of 5 Gy, delivered at a dose rate of 0.45 Gy/min.
Experimental design
The 57 mice were divided into four groups (G1–G4). Group G1 served as the untreated vehicle saline control group (receiving neither irradiation nor MGN-3). Group G2 received only MGN-3 every other day. Group G3 received only whole body γ-irradiation at 2 weeks after the beginning of the experiment. Group G4 was pretreated with MGN-3 for 2 weeks then exposed to irradiation and continued to receive MGN-3 every other day. Animals from all groups were euthanized at 1 and 4 weeks after radiation. Parameters under investigation include fluctuations in body and organ weights, complete blood count (CBC), histopathology of multiple organs and evaluation of oxidative stress biomarkers.
Body weight changes
The initial body weight of the animals in the four different groups was determined and then measured at the intervals of 1 and 4 weeks post-irradiation. The significance of the differences in body weight (BW) due to the different treatments—MGN-3, irradiation, MGN + irradiation—was compared to untreated mice.
Organ weight changes
At 1 and 4 weeks post-exposure to irradiation, a range of organs from G1–G4 mice were examined. These included: liver, heart, lungs, spleen, kidney, testes and brain.
Hematology studies
At 1 and 4 weeks post-exposure to radiation, blood samples from G1–G4 were drawn by heart puncture using heparinized plastic syringes. Blood was quickly transferred into anticoagulation test tubes for CBC analysis including hemoglobin (Hb), hematocrit (Hct), total red blood cell count (RBCs), total platelet count (PLT) and total white blood cell (WBCs) with differential counts.
Histopathology
A range of organs, such as bone marrow and spleen, of each mouse were examined for histopathological changes at 1 and 4 weeks post-exposure to γ-irradiation. Organs were fixed in 10% formalin solution, sectioned and submitted into cassettes, fixed overnight, sectioned into 4 µm-thick sections, and stained with hematoxylin and eosin (H&E). The numbers of megakaryocytes in the spleens were determined under light microscopy.
Biochemical analysis
The ability of MGN-3 to exert antioxidative activity in whole-body irradiated mice was investigated. Spleens from mice under different treatment conditions were dissected at 1 and 4 weeks post-exposure to radiation and analyzed for different parameters of oxidative stress. Spleens were homogenized in ice-cold phosphate buffer (0.1 mol/l, pH 7.4) using a Potter-Elvehjem homogenizer to give a 10% homogenate. The lipid peroxidation (LPx) product, malondialdehyde (MDA), was measured using a thiobarbituric acid test [17]. The MDA content of the spleen tissue was determined colorimetrically from the absorbance at 535 nm. The level of peroxidation products was expressed as the amount of MDA in tissue (µmol/g). The GSH content was determined as previously described [18]; the method is based on determination of a yellow hue that develops when 5,5 dithiobis-(2-nitrobenzoic acid) (DTNB) is added to sulfhydryl compounds. The developed color was measured spectrophotometrically at 412 nm. Results were expressed as mg/g of tissue.
Statistical analysis
Data were analyzed using one-way analysis of variance (ANOVA) using the SPSS 16 program, followed by Newman-Keuls post hoc test for multiple comparisons. The data were expressed as the mean ± standard error of the mean (SEM). Differences were considered significant at the P < 0.05 level.
RESULTS
Mice under different treatment conditions were examined for the following parameters: body and organ weights, CBC parameters, histopathology of multiple organs, and oxidative stress.
Body weight change
The data in Table 1 show body weight changes in mice at 1 and 4 weeks post-irradiation. The groups of mice without irradiation (control and MGN-3-only) showed comparable weight gain from Day 0 to the end of the experiment. Irradiated mice showed early weight loss at 1 week post-irradiation (–20% of control) (P < 0.01), which was maintained at 4 weeks post-irradiation (P < 0.05). However, treatment with MGN-3 prevented the early weight loss in irradiated mice, and maintained normal body weight throughout the 4 weeks.
Table 1.
Body weight change/gm in mice at 1 and 4 weeks post-irradiation
| Groups |
||||
|---|---|---|---|---|
| Control | MGN-3 | Irradiation | MGN-3 + irradiation | |
| Number of mice/group | 13 | 14 | 15 | 15 |
| Initial body weight (no treatment) | 21.01 ± 0.48 | 21.68 ± 0.56 (3.16%) |
22.37 ± 0.53 (6.44%) |
22.01 ± 0.32 (4.74%) |
| 2weeks post-MGN-3 treatment | 24.40 ± 0.66 | 25.42 ± 0.47 (4.20%) |
24.15 ± 0.44 (–1.02%) |
25.12 ± 0.61 (2.96%) |
| 1 week post- irradiation | 27.02 ± 0.82 | 28.75 ± 0.57*,†† (6.39%) |
21.61 ± 0.49** (–20.03%) |
26.64 ± 0.40#,†† (–1.41%) |
| Number of mice/group | 6 | 6 | 6 | 7 |
| 4 weeks post-irradiation | 29.30 ± 0.79 | 31.45 ± 0.75* (7.32%) |
27.02 ± 0.65*,## (–7.79%) |
29.14 ± 0.63†,# (–0.54%) |
| Number of mice/group | 7 | 8 | 9 | 8 |
*Significantly different from control group at 0.05 level. **Significantly different from control group at 0.01 level. #Significantly different from MGN-3 group at 0.05 level. ##Significantly different from MGN-3 group at 0.01 level. †Significantly different from irradiation group at 0.05 level. ††Significantly different from irradiation group at 0.01 level (% change of control group). Data represent changes in body weight ± SE.
Organ weight change
The organ weight change in mice was examined. Table 2 demonstrates a differential response among organs toward exposure to irradiation. Irradiation at 1 week caused significant weight loss of liver, kidney and testes, while other organs such as heart, lungs and brain showed no significant change. In contrast, treatment with MGN-3 provided protection against organ weight loss in the irradiated mice 1 week post-irradiation. The weight of all organs at 4 weeks showed no change in mice under the different treatment conditions.
Table 2.
Organ weight change/gm in mice at 1 and 4 weeks post-irradiation
| Groups | ||||||||
|---|---|---|---|---|---|---|---|---|
| Control | MGN-3 | Irradiation | MGN-3+ Irradiation | |||||
| Group | 1 week | 4 weeks | 1 week | 4 weeks | 1 week | 4 weeks | 1 week | 4 weeks |
| Number of mice | 6 | 7 | 6 | 8 | 6 | 9 | 7 | 8 |
| Liver | 1.09 ± 0.05 | 0.94 ± 0.07 | 1.10 ± 0.05†† (1.2%) | 0.92 ± 0.04 (−1.35%) | 0.81 ± 0.024** (−25.51%) | 0.87 ± 0.04 (−6.59%) | 0.99 ± 0.061† (−8.58%) | 0.88 ± 0.04 (−5.77%) |
| Heart | 0.11 ± 0.01 | 0.10 ± 0.005 | 0.12 ± 0.003 (3.24%) | 0.11 ± 0.006 (15.61%) | 0.10 ± 0.07 (−14.26%) | 0.11 ± 0.009 (13.61%) | 0.12 ± 0.01 (8.91%) | 0.10 ± 0.007 (−2.96%) |
| Lungs | 0.175 ± 0.004 | 0.174 ± 0.009 | 0.186 ± 0.003 (6.4%) | 0.176 ± 0.012 (1.13%) | 0.152 ± 0.007 (−12.99%) | 0.177 ± 0.009 (1.43%) | 0.171 ± 0.03 (−1.84%) | 0.179 ± 0.006 (2.85%) |
| Kidney | 0.161 ± 0.007 | 0.122 ± 0.007 | 0.168 ± 0.01†† (3.93%) | 0.136 ± 0.008 (11.42%) | 0.124 ± 0.006** (−23.19%) | 0.130 ± 0.006 (6.49%) | 0.169 ± 0.009†† (5.04%) | 0.123 ± 0.005 (0.48%) |
| Testes | 0.132 ± 0.005 | 0.158 ± 0.007 | 0.136 ± 0.01† (2.91%) | 0.162 ± 0.01 (2.39%) | 0.105 ± 0.007* (−20.25%) | 0.155 ± 0.014 (−1.67%) | 0.124 ± 0.008 (−5.61%) | 0.159 ± 0.011 (0.72%) |
| Brain | 0.348 ± 0.018 | 0.396 ± 0.009 | 0.355 ± 0.017 (1.87%) | 0.411 ± 0.007 (3.55%) | 0.337 ± 0.02 (−3.11%) | 0.380 ± 0.019 (−4.20%) | 0.357 ± 0.016 (2.71%) | 0.413 ± 0.002 (4.09%) |
*Significantly different from the corresponding control group at 0.05 level.
**Significantly different from the corresponding control at 0.01 level.
†Significantly different from the corresponding irradiation group at 0.05 level.
††Significantly different from the corresponding irradiation group at 0.01 level (% change of control group).
Hematological studies
Several hematological parameters were examined to demonstrate the protective effect of MGN-3 after 1 and 4 weeks post-exposure to γ-irradiation. These included RBC indices, total WBCs with differential counts, and PLT counts.
RBC indices
We examined the effect of irradiation and MGN-3 treatment on the RBC indices, including the RBC count, Hb and Hct. Fig. 1 summarizes the changes of the RBC indices at 1 and 4 weeks post-irradiation. Irradiation caused anemia (lower RBC count, Hb and Hct) in mice at 1 and 4 weeks. However, MGN-3 treatment prior to irradiation prevented the decrease of these parameters at 1 week and maintained them at 4 weeks. Mice treated with MGN-3 alone showed RBC indices within the values of control untreated mice.
Fig. 1.
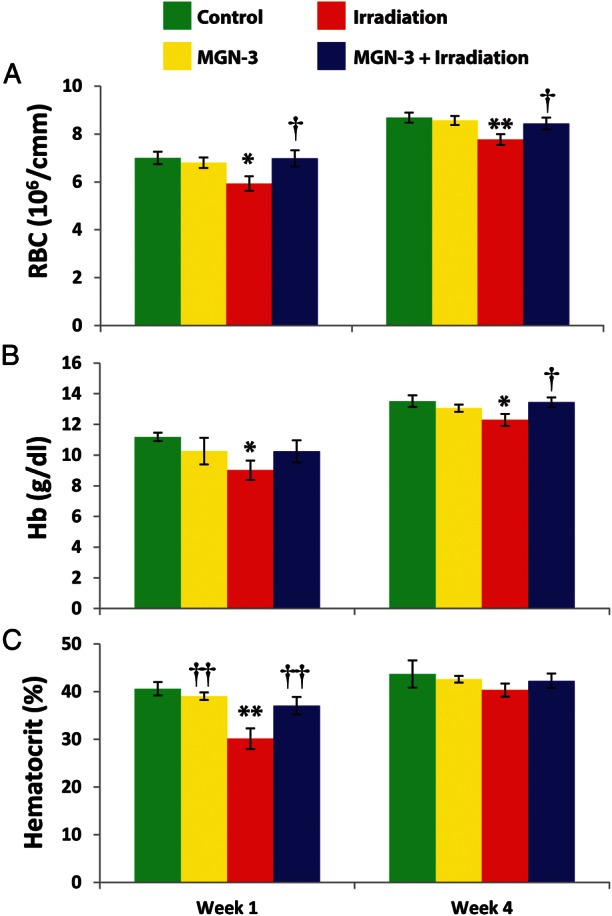
RBC series in mice under different treatments at 1 and 4 weeks post-irradiation. A) RBC count, B) hemoglobin content (Hb), and C) hematocrit (Hct) readings at 1 and 4 weeks after initial treatment. Number of mice per group is 6–9. One asterisk indicates significant difference from control group at the 0.05 level. Two asterisks indicate significant difference from the control group at the 0.01 level. One dagger indicates significant difference from the irradiation group at the 0.05 level. Two daggers indicate significant difference from the irradiation group at the 0.01 level (% difference from the control group).
WBC counts and differential
Data shown in Fig. 2 are summaries of changes in the WBC counts and the percentages of lymphocytes, monocytes, and neutrophils in mice under different treatment conditions. The data show that irradiation caused leukopenia in mice at 1 week (P < 0.01). However, MGN-3 treatment prevented leukopenia of irradiated mice (P < 0.05). These values were maintained but not significant at 4 weeks.
Fig. 2.
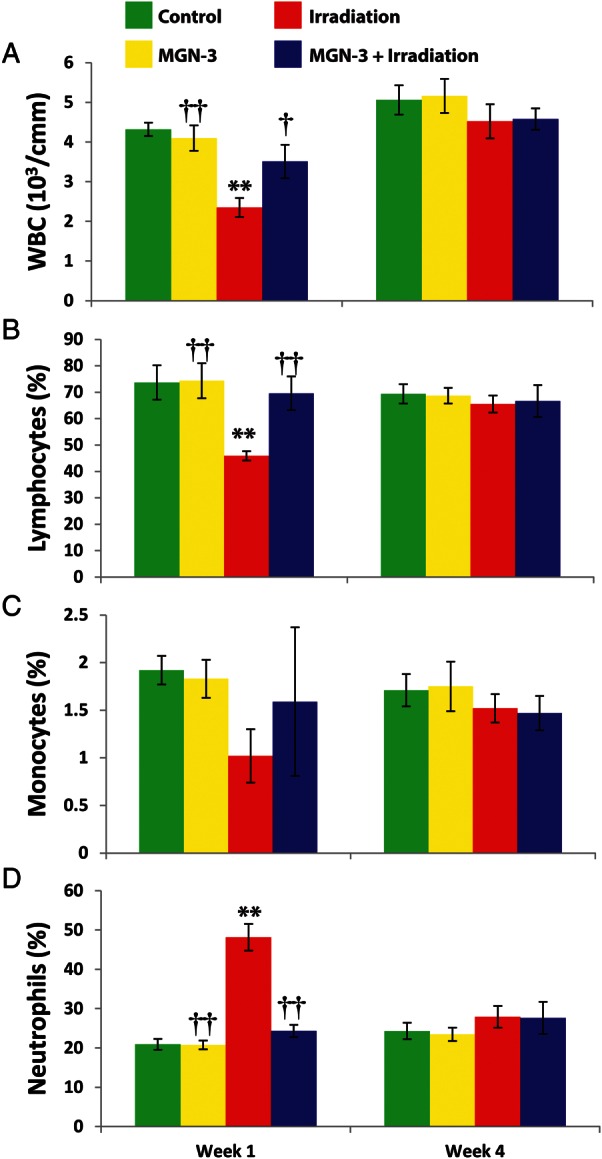
WBC series in mice under different treatments at 1 and 4 weeks post-irradiation. A) Total WBC count. The percent B) lymphocytes, C) monocytes, and D) neutrophils were determined at 1 and 4 weeks after initial treatment. Number of mice per group is 6–9. Two asterisks indicate significant difference from the corresponding control group at the 0.01 level. One dagger indicates significant difference from the irradiation group at the 0.05 level. Two daggers indicate significant difference from the irradiation group at the 0.01 level (% difference from the control group).
Lymphopenia is also noted in the irradiated mice at 1 week post-irradiation, and again treatment with MGN-3 protected its level (P < 0.01). These values were also noted at 4 weeks but were not significant. Irradiation caused an early neutrophilia at 1 week (P < 0.01). However, MGN-3 limited the marked neutrophilia caused by irradiation to half its value. At 4 weeks, these values were not significant. In addition, irradiation caused a decreased monocyte count at 1 week that was prevented by treatment with MGN-3.
PLT count
The data shown in Fig. 3 indicate the presence of thrombocytopenia in mice post-exposure to irradiation at 1 and 4 weeks. In contrast, treatment of mice with MGN-3 prior to irradiation provided protection against thrombocytopenia at 1 and 4 weeks post-irradiation. We also observed thrombocytosis in non-irradiated mice treated with MGN-3 alone at 1 week (2.44%), which was maximized at 4 weeks (8.73%) compared with control mice.
Fig. 3.
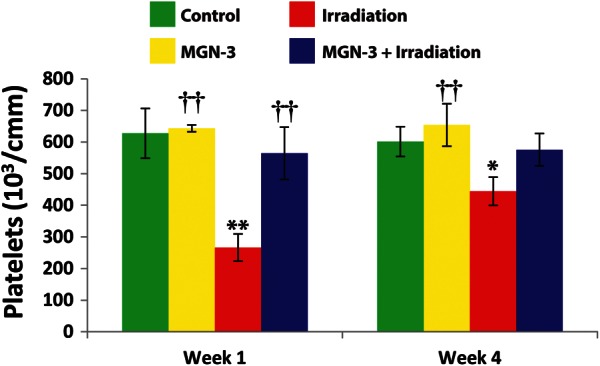
Platelet content in mice under different treatments at 1 and 4 weeks post-irradiation. Number of mice per group is 6–9. One asterisk indicates significant difference from the corresponding control group at the 0.05 level. Two asterisks indicate significant difference from the corresponding control group at the 0.01 level. Two daggers indicate significant difference from the irradiation group at the 0.01 level (% difference from the control group).
Histopathological studies
Mice were examined histopathologically for possible changes in their bone marrow and spleen due to exposure to irradiation and treatment with MGN-3.
Bone marrow
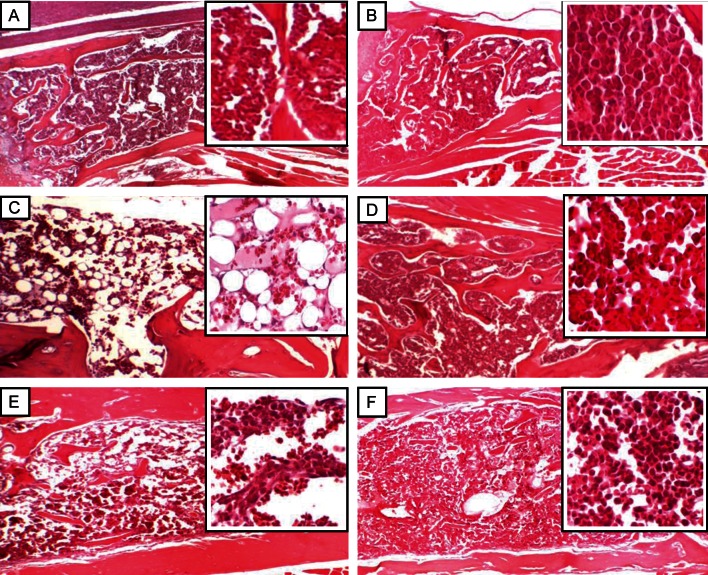
The data illustrated in Fig. 4 indicate significant hypocellularity in the bone marrow of the irradiated mice at 1 week, as indicated by the mostly absent bone marrow cellularity. In contrast, treatment with MGN-3 prior to irradiation provided full protection of the bone marrow cellularity. At 4 weeks post-irradiation, partial recovery of bone marrow cellularity occurred in the mice exposed to irradiation.
Fig. 4.
Histological sections of bone marrow from mice exposed to γ-irradiation, with or without treatment of MGN-3, at 1 and 4 weeks post-irradiation. Each cross-section contains an enlarged image. A) Control group, no treatment with irradiation or MGN-3 (100% marrow cellularity). B) Mice treated with MGN-3 alone (100% marrow cellularity). C) Mice exposed to irradiation alone, 1 week post-irradiation (marked decreased to absent marrow cellularity). D) Mice treated with MGN-3 and exposed to irradiation, 1 week post-irradiation (100% marrow cellularity). E) Mice exposed to irradiation alone, 4 weeks post-irradiation (partial recovery of bone marrow cellularity). F) Mice treated with MGN-3 and exposed to irradiation, 4 weeks post-irradiation (80% marrow cellularity).
Spleen
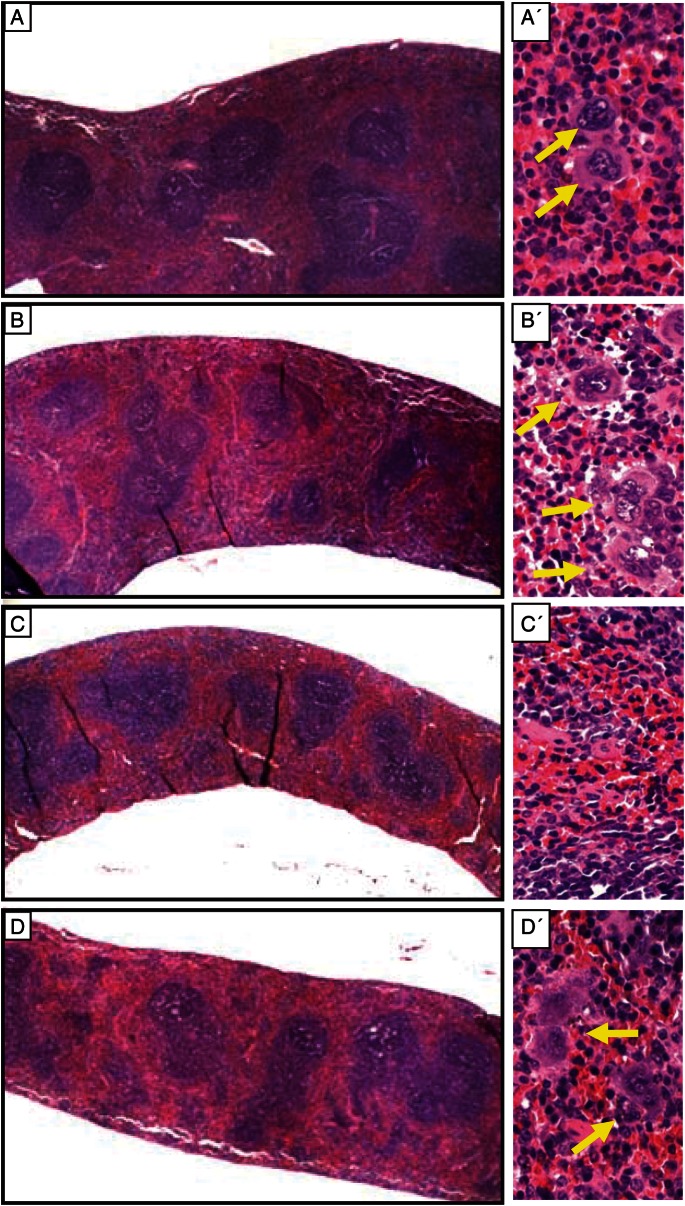
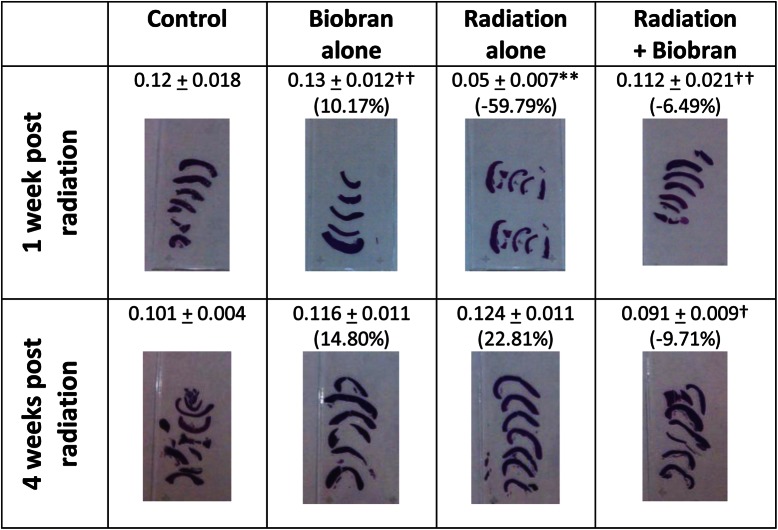
Changes of spleen weight/relative size in mice exposed to γ-irradiation in the presence or absence of treatment with MGN-3 are shown in Figs 5 and 6. Exposure to irradiation resulted in remarkable changes in the spleens of mice at 1 week post-irradiation, as manifested by a 60% decrease in spleen weight/relative size as compared with control mice (P < 0.01). These results are also associated with a 75% decrease in the number of megakaryocytes, as compared with control mice. On the other hand, MGN-3 treatment prior to irradiation provided full protection of the weight/relative size, as well as the number of megakaryocytes in the spleen. At 4 weeks post-irradiation, these changes became less significant.
Fig. 5.
Histological sections of spleens from mice exposed to irradiation, with or without treatment with MGN-3, at 1 week post-irradiation. Cross-sections (A–D left panel) of the spleens and higher magnifications of each (A′–D′ right panel). A) Control group, no exposure to irradiation or treatment with MGN-3. B) Mice treated with MGN-3 alone. C) Mice exposed to irradiation alone. D) Mice treated with MGN-3 and exposed to irradiation. Please notice the yellow arrows pointing to the megakaryocytes.
Fig. 6.
Changes of spleen (weight/relative size) from mice exposed to γ-irradiation, with or without treatment with MGN-3, at 1 and 4 weeks post-irradiation. Two asterisks indicate significant difference from the corresponding control at the 0.01 level. One dagger indicates significant difference from the corresponding irradiation group at the 0.05 level. Two daggers indicate significant difference from the corresponding irradiation group at the 0.01 level (% difference from the control group). The data are the mean ± SEM from 6–9 mice. Each of the photos is representative of the mice spleens in each group (comprised of 6–9 mice).
Determination of oxidative stress biomarkers
LPx level
The effect of MGN-3 treatment and/or irradiation on the level of lipid peroxidation, measured in terms of MDA in spleen tissues, is shown in Fig. 7A. There was no significant difference between the untreated control group and the group treated with MGN-3 alone. Mice exposed to irradiation showed a marked elevation in MDA content. These values were 106.34% (P < 0.01) at 1 week, and 43.44% at 4 weeks, as compared to the normal control group. Animals pretreated with MGN-3 prior to irradiation exhibited protection of MDA content at 1 week. In addition, MGN-3 markedly ameliorated the increase in MDA content at 4 weeks.
Fig. 7.
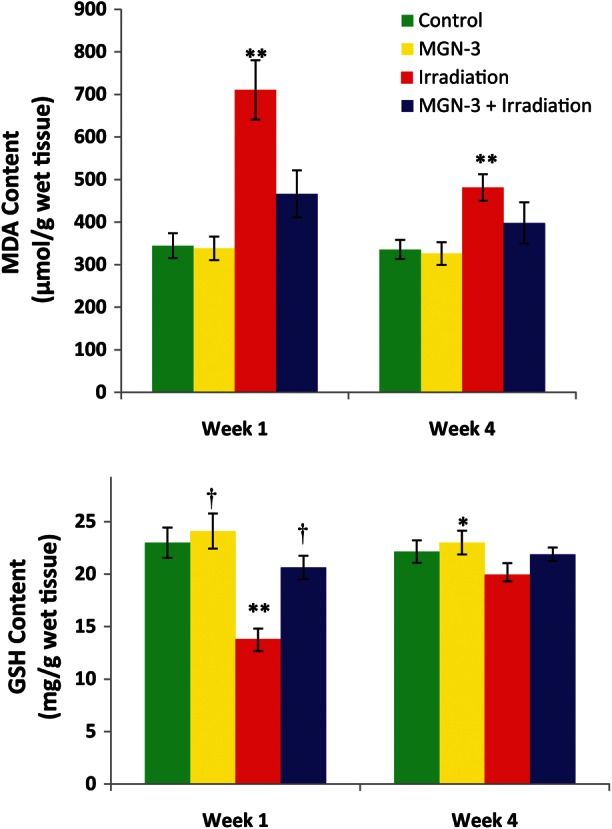
Effects of MGN-3 treatment on A) MDA content (µmol/g wet tissue), and B) GSH content (mg/g wet tissue) in spleens of mice at 1 and 4 weeks post-irradiation. Each value represents the mean ± SEM of six mice/group. One asterisk indicates significant difference from the corresponding irradiation group at the 0.05 level. Two asterisks indicate significant difference from the corresponding control group at the 0.01 level. One dagger indicates significant difference from the corresponding irradiated group at the 0.01 level.
Glutathione content
The effect of MGN-3 treatment and/or irradiation on the level of glutathione (GSH) in spleen tissues, as a function of time, is shown in Fig. 7B. There was no significant difference between the non-treated control group and the group treated with MGN-3 alone. Irradiated mice showed a significant decline (–40%, P < 0.01) in the GSH level in their spleens at 1 week post-irradiation, as compared to the non-treated control group. In contrast, MGN-3 treatment prevented irradiation-induced GSH depletion in the spleens of mice at 1 week. The trends in GSH levels continued for each group, but the differences were less significant at 4 weeks.
DISCUSSION
Results of the current study indicate that MGN-3 has the ability to enhance the blood cellular radioresistance; this property coupled with the ability of MGN-3 to restore the survival rate of γ-irradiated mice (personal communication from S. Nakatsugawa, 2001) suggests that this agent could be used as a potential radioprotector. Earlier studies showed that hematopoietic tissues are the most radiosensitive tissues to ionizing radiation through induction of oxidative damage to cellular macromolecules [3–5], leading to demise of the hematopoietic system [3, 5, 6, 7]. In the current study, we demonstrate that exposure to a sub-lethal dose of ionizing radiation causes significant decrease in the CBC, which correlates well with the expected loss/reduction of hematopoietic cells in the bone marrow. On the other hand, treatment with MGN-3 results in full protection against irradiation-induced damage to all parameters of the CBC, and complete bone marrow cellularity and splenic weight/relative size. Thus MGN-3 protects the progenitor cells, enabling proliferation to occur.
The exact mechanism(s) underlying the effect of MGN-3 is/are not fully understood, but it/they could be associated with both antioxidant properties and immunomodulatory effects of MGN-3. Regarding the antioxidant activity, data of the current study indicate that MGN-3 exerts antioxidative activity in whole-body irradiated mice. Lipid peroxidation analysis (in terms of MDA levels) in spleen tissues shows that treatment with MGN-3 exhibits significant protection against the radiation-induced elevation of MDA content. In addition, MGN-3 treatment prevents irradiation-induced GSH depletion in the spleens of mice. The antioxidant activity of MGN-3 has also been reported in other models. For example, mice with antioxidant disturbances due to tumor growth, exposed to MGN-3 have shown augmented GSH contents, enhanced activity of antioxidant scavenging enzymes, and reduction in lipid peroxidation and free radical levels [16]. Furthermore, MGN-3 has been shown to demonstrate antioxidative activity in hypoxanthine-xanthine oxidase, ferrous sulfate-hydrogen peroxide, and UV light reaction systems [19]. The mechanism of MGN-3 as an antioxidant is not fully understood, but it could be associated with the presence of β-glucans [20]. Several studies have shown that β-glucan possesses antioxidant activity [21–23]. MGN-3 is a polysaccharide that contains β-1,3-glucans which are constituents of fungi, algae, and higher plants [24], and are known to demonstrate free radical scavenging activity [25]. β-glucan also shows protection against burn-induced oxidative damage [26].
The immune suppressive effect of irradiation is well documented. Our work and that of others has shown that exposure to irradiation causes a decrease in WBC counts, and dysfunction of different populations of immune cells [27–31]. The current results show that treatment with MGN-3 can protect the WBC count and overall maintenance of hematopoietic tissues against γ-irradiation. Most notably, the bone marrow histopathology is well protected. Additionally, the changes in the weight/relative size of, and the number of megakaryocytes in, the spleen were maintained. MGN-3 has been shown to be a potent BRM, which causes activation of dendritic cells [11, 12] and NK cells [13, 32], an increase in the proliferation of T and B cells [10], enhancement of phagocytic activity of macrophages [33], modulation of cytokines [15, 32], and induction of apoptosis in tumor tissues [15]. This characteristic suggests that the use of MGN-3 may prevent immune dysfunction associated with irradiation, i.e. MGN-3 acts as both a radioprotector and an immune modulator. MGN-3 would therefore provide additional advantages over other synthetic compounds.
The data of the current study indicate the presence of thrombocytopenia in mice post-exposure to irradiation for 1 and 4 weeks. This data is attributed to a significant decrease in the number of megakaryocytes. The megakaryocytes are bone marrow cells responsible for the production of blood platelets, which are necessary for normal blood clotting. However, treatment with MGN-3 protected blood platelet levels at 1 and 4 weeks post-irradiation, which was associated with the normalization of the number of megakaryocytes.
In the present study, decreases in RBCs and Hct were observed in animals after 1 week of ionizing radiation exposure. Hct is the percentage of red blood cells in whole blood and its decrease below normal levels indicates anemia. Another measure of anemia is a decrease in the Hb content [34]. In the current study, it was observed that Hb levels declined significantly following irradiation exposure. These observations are in accordance with the findings of others [35]. The decrease in Hb content is attributed to the decline in the number of RBCs. MGN-3 treatment offered protection against the γ-irradiation-induced decrease in hemoglobin values.
The safety of many radioprotectors is still a major concern. While some natural products have been safely used to protect against gamma-irradiation [36, 37], several synthetic compounds also have a radioprotective effect but are known to be toxic. These include aminothiol, zinc aspartate, MPG (2-mercaptopropionylglycine), cysteamine and its derivative WR-2721 (S-2-(3-aminopropylamino) ethyl phosphorothioic acid) [38–40]. Contrary to synthetic compounds, MGN-3 is a naturally-occurring arabinoxylan extracted from rice bran [10]. MGN-3 has been shown to be a safe, nontoxic agent as manifested by the following: the LD50 (lethal dose, 50%) of MGN-3 is greater than 36 g/kg; the Ames test for mutagenicity was negative; and the subchronic toxicity study in rats, antigenicity study, and genotoxic testing all demonstrate that MGN-3 is nontoxic [41, 42]. Furthermore, MGN-3 was shown to be safe when investigated in humans using blood chemistry analysis including liver enzymes (SGOT and SGPT) [13], and in clinical trials on cancer patients. A recent 3-year randomized clinical trial of the anticancer activity of MGN-3 against hepatocellular carcinoma (HCC) was carried out [43]. Patients that were treated with conventional therapy (CT) plus MGN-3, as compared with CT alone, showed: lower alpha fetoprotein (AFP) level, lower alanine transaminase (ALT) level, reduced tumor size, less recurrence of cancer, and higher survival rate. In another clinical study, involving patients with different types of malignancies, treatment with chemotherapy plus MGN-3 demonstrated a prolonged life expectancy, and an improved quality of life (QOL) as characterized by a decrease in pain, nausea, and malaise and an increase in appetite [44]. MGN-3 is currently distributed in 49 countries and is sold as a pharmacologically active natural product.
We conclude that MGN-3 is an effective protector against γ-irradiation-induced hematopoietic damage in mice. It is a safe agent that possesses radical scavenging and immune modulatory properties. This suggests that MGN-3 might be a useful adjuvant for preventing the severe adverse side effects that are associated with radiation therapy.
FUNDING
This work was supported by Diawa Pharmaceutical Co. Ltd, Tokyo, Japan; Grant #T0099108.
ACKNOWLEDGEMENTS
MGN-3 was provided by Daiwa Pharmaceuticals Co. Ltd, Tokyo, Japan.
REFERENCES
- 1.Petersen C, Würschmidt F. Late toxicity of radiotherapy: a problem or a challenge for the radiation oncologist? Breast Care (Basel) 2011;6:369–74. doi: 10.1159/000334220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badr El-Din NK. Protective role of sanumgerman against γ-irradiation-induced oxidative stress in Ehrlich carcinoma-bearing mice. Nutr Res. 2004;24:271–91. [Google Scholar]
- 3.Karbownik M, Reiter RJ. Antioxidative effects of melatonin in protection against cellular damage caused by ionizing radiation. Proc Soc Exp Biol Med. 2000;225:9–22. doi: 10.1177/153537020022500102. [DOI] [PubMed] [Google Scholar]
- 4.Karran P. DNA double strand break repair in mammalian cells. Curr Opin Genet Dev. 2000;10:144–50. doi: 10.1016/s0959-437x(00)00069-1. [DOI] [PubMed] [Google Scholar]
- 5.Kalpana KB, Devipriya N, Srinivasan M, et al. Evaluating the radioprotective effect of hesperidin in the liver of Swiss albino mice. Eur J Pharmacol. 2011;658:6–12. doi: 10.1016/j.ejphar.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 6.Uma Dev P. Radiosensitivity of the developing haemopoietic system in mammals and its adult consequences: animal studies. British J Radiol. 2003;76:366–72. doi: 10.1259/bjr/42623440. [DOI] [PubMed] [Google Scholar]
- 7.Fliedner TM, Graessle DH. Hematopoietic cell renewal systems: mechanisms of coping and failing after chronic exposure to ionizing radiation. Radiat Environ Biophys. 2008;47:63–9. doi: 10.1007/s00411-007-0148-6. [DOI] [PubMed] [Google Scholar]
- 8.Gridley DS, Pecaut MJ, Miller GM, et al. Dose and dose rate effects of whole-body gamma-irradiation: II. Hematological variables and cytokines. In Vivo. 2001;15:209–16. [PubMed] [Google Scholar]
- 9.Gridley DS, Pecaut MJ. Whole-body irradiation and long-term modification of bone marrow-derived cell populations by low- and high LET radiation. In Vivo. 2006;20:781–9. [PubMed] [Google Scholar]
- 10.Ghoneum M. Anti-HIV activity in vitro of MGN-3, an activated arabinoxylane from rice bran. Biochem Biophys Res Commun. 1998;243:25–91. doi: 10.1006/bbrc.1997.8047. [DOI] [PubMed] [Google Scholar]
- 11.Cholujova D, Jakubikova J, Sedlak J. BioBran-augmented maturation of human monocyte-derived dendritic cells. Neoplasma. 2009;56:89–95. doi: 10.4149/neo_2009_02_89. [DOI] [PubMed] [Google Scholar]
- 12.Ghoneum M, Agrawal S. Activation of human monocyte-derived dendritic cells in vitro by biological response modifier arabinoxylan rice bran (MGN-3/Biobran) Int J Immunopathol Pharmcol. 2011;24:941–8. doi: 10.1177/039463201102400412. [DOI] [PubMed] [Google Scholar]
- 13.Ghoneum M. Enhancement of human natural killer cell activity by modified arabinoxylan from rice bran (MGN-3) Int J Immunother. 1998;14:89–99. [Google Scholar]
- 14.Ghoneum M, Abedi S. Enhancement of natural killer cell activity of aged mice by modified arabinoxylan rice bran (MGN-3/Biobran) J Pharm Pharmacol. 2004;56:1581–8. doi: 10.1211/0022357044922. [DOI] [PubMed] [Google Scholar]
- 15.Badr El-Din NK, Noaman E, Ghoneum M. In vivo tumor inhibitory effects of nutritional rice bran supplement MGN-3/biobran on Ehrlich carcinoma–bearing mice. Nutr Cancer. 2008;60:235–44. doi: 10.1080/01635580701627285. [DOI] [PubMed] [Google Scholar]
- 16.Noaman E, Badr El-Din NK, Bibars MA, et al. Antioxidant potential by arabinoxylan rice bran, MGN-3/biobran, represents a mechanism for its oncostatic effect against murine solid Ehrlich carcinoma. Cancer Lett. 2008;268:348–59. doi: 10.1016/j.canlet.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Yoshioka T, Kawada K, Shimada T, et al. Lipid peroxidation in maternal and cord blood and protective mechanism against activated oxygen toxicity in the blood. Am J Obstet Gynecol. 1979;135:372–6. doi: 10.1016/0002-9378(79)90708-7. [DOI] [PubMed] [Google Scholar]
- 18.Beutler E, Duron O, Kelly BM. Improved method of the determination of blood glutathione. J Lab Clin Med. 1963;61:882–8. [PubMed] [Google Scholar]
- 19.Tazawa K, Namikawa H, Oida N, et al. Scavenging activity of modified arabinoxylan from rice bran (BioBran/MGN-3) with natural killer cell activity on free radicals. Biotherapy. 2000;14:493–5. [Google Scholar]
- 20.Mura T, Chiba M, Miyazaki Y. In: Chemical Structure of the Component Involved in Immunoregulation. BioBran/MGN-3; Basic and Clinical Application to Integrative Medicine. Tazawa K, editor. Tokyo: Iyakushuppan Co; 2006. [Google Scholar]
- 21.Deng C, Hu Z, Fu H, et al. Chemical analysis and antioxidant activity in vitro of a β-d-glucan isolated from Dictyophora indusiata. Int J Biol Macromol. 2012;51:70–5. doi: 10.1016/j.ijbiomac.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Kofuji K, Aoki A, Tsubaki K, et al. Antioxidant activity of β-glucan. ISRN Pharm. 2012;2012:125864. doi: 10.5402/2012/125864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lattimer JM, Haub MD. Effects of dietary fiber and its components on metabolic health. Nutrients. 2010;2:1266–89. doi: 10.3390/nu2121266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laroche C, Michaud P. New developments and prospective applications for beta (1,3) glucans. Recent Pat Biotechnol. 2007;1:59–73. doi: 10.2174/187220807779813938. [DOI] [PubMed] [Google Scholar]
- 25.Pourahmad J, Shaki F, Tanbakosazan F, et al. Protective effects of fungal β-(1→3)-D-glucan against oxidative stress cytotoxicity induced by depleted uranium in isolated rat hepatocytes. Hum Exp Toxicol. 2011;30:173–81. doi: 10.1177/0960327110372643. [DOI] [PubMed] [Google Scholar]
- 26.Toklu HZ, Sener G, Jahovic N, et al. β-glucan protects against burn-induced oxidative organ damage in rats. Int Immunopharmacol. 2006;6:156–69. doi: 10.1016/j.intimp.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 27.Ghoneum M, Egami N. Effect of X-irradiation on adult and embryo of Oryzias Latipes on thymus size. In: Egami N, editor. Radiation Effects of Aquatic Organisms. Baltimore MD, USA: University Park Press; 1980. pp. 135–7. [Google Scholar]
- 28.Ghoneum M, Egami N, Ijiri K. Effects of acute gamma-irradiation on the development of the thymus in embryos and fry of Oryzias latipes. Int J Radiat Biol Relat Stud Phys Chem Med. 1981;39:339–44. doi: 10.1080/09553008114550431. [DOI] [PubMed] [Google Scholar]
- 29.Ghoneum M, Ijiri K, Egami N. Effects of gamma-rays on morphology of the thymus of the adult fish of Oryzias latipes. J Radiat Res. 1982;23:253–9. doi: 10.1269/jrr.23.253. [DOI] [PubMed] [Google Scholar]
- 30.Yang R, Pei X, Wang J, et al. Protective effect of a marine oligopeptide preparation from chum salmon (Oncorhynchus keta) on radiation-induced immune suppression in mice. J Sci Food Agric. 2010;90:2241–8. doi: 10.1002/jsfa.4077. [DOI] [PubMed] [Google Scholar]
- 31.Assayed ME. Radioprotective effects of black seed (Nigella sativa) oil against hemopoietic damage and immunosuppression in gamma-irradiated rats. Immunopharmacol Immunotoxicol. 2010;32:284–96. doi: 10.3109/08923970903307552. [DOI] [PubMed] [Google Scholar]
- 32.Ghoneum M, Jewett A. Production of tumor necrosis factor-alpha and interferon-gamma from human peripheral blood lymphocytes by MGN-3, a modified arabinoxylan from rice bran, and its synergy with interleukin-2 in vitro. Cancer Detect Prev. 2000;24:314–24. [PubMed] [Google Scholar]
- 33.Ghoneum M, Matsuura M. Augmentation of macrophage phagocytosis by modified arabinoxylan rice bran (MGN-3/biobran) Int J Immunopathol Pharmacol. 2004;17:283–92. doi: 10.1177/039463200401700308. [DOI] [PubMed] [Google Scholar]
- 34.Patil S, Kansase A, Kulkarni PH. Antianaemic properties of ayurvedic drugs, raktavardhak, punarnavasav and navayas Louh in albino rats during phenylhydrazine induced hemolytic anaemia. Ind J Exp Bio. 2000;38:253–7. [PubMed] [Google Scholar]
- 35.Xu W, Shen X, Yang F, et al. Protective effect of polysaccharides isolated from Tremella fuciformis against radiation-induced damage in mice. J Radiat Res. 2012;53:353–60. doi: 10.1269/jrr.11073. [DOI] [PubMed] [Google Scholar]
- 36.Devi PU, Ganasoundari A. Radioprotective effect of leaf extract of Indian medicinal plant Ocimum sanctum. Indian J Exp Biol. 1995;33:205–8. [PubMed] [Google Scholar]
- 37.Ganasoundari A, Devi PU, Rao MN. Protection against radiation-induced chromosome damage in mouse bone marrow by Ocimum sanctum. Mutat Res. 1997;373:271–6. doi: 10.1016/s0027-5107(96)00208-4. [DOI] [PubMed] [Google Scholar]
- 38.Yuhas JM, Spellman JM, Culo F. The role of WR-2721 in radiotherapy and/or chemotherapy. Cancer Clin Trials. 1980;3:211–6. [PubMed] [Google Scholar]
- 39.Cairnie AB. Adverse effects of the radioprotector WR2721. Radiat Res. 1983;94:221–6. [PubMed] [Google Scholar]
- 40.Floersheim GL, Bieri A. Further studies on selective radioprotection by organic zinc salts and synergism of zinc aspartate with WR 2721. Br J Radiol. 1990;63:468–75. doi: 10.1259/0007-1285-63-750-468. [DOI] [PubMed] [Google Scholar]
- 41.Daiwa Pharmaceutical Co., Ltd. BioBran rice bran arabinoxylan compound. http://www.daiwa-pharm.com/eng/bio-1.html. (3 December 2012, date last accessed) [Google Scholar]
- 42.Jacoby HI, Wnorowski G, Sakata K, et al. The effect of MGN-3 on cisplatin and adriamycin induced toxicity in the rat. J Nutraceut Funct Med Foods. 2001;3:3–11. [Google Scholar]
- 43.Bang MH, Van Riep T, Thinh NT, et al. Arabinoxylan rice bran (MGN-3/Biobran) enhances the effects of interventional therapies for the treatment of hepatocellular carcinoma: a three-year randomized clinical trial. Anticancer Res. 2010;30:5145–51. [PubMed] [Google Scholar]
- 44.Takahara K, Sano K. The life prolongation and QOL improvement effect of rice bran arabinoxylan derivative (MGN-3/Bio-Bran) for progressive cancer. Clin Pharmacol Ther. 2004;14:267–71. [Google Scholar]