Abstract
Despite more than a decade of research on the endocrine system, there have been no published studies about the effects of concurrent exposure of radiofrequency electromagnetic fields (RF-EMF) on this system. The present study investigated the several parameters of the endocrine system including melatonin, thyroid stimulating hormone, stress hormone and sex hormone after code division multiple access (CDMA, 849 MHz) and wideband code division multiple access (WCDMA, 1.95 GHz) signals for simultaneous exposure in rats. Sprague-Dawley rats were exposed to RF-EMF signals for 45 min/day, 5 days/week for up to 8 weeks. The whole-body average specific absorption rate (SAR) of CDMA or WCDMA was 2.0 W/kg (total 4.0 W/kg). At 4 and 8 weeks after the experiment began, each experimental group's 40 rats (male 20, female 20) were autopsied. Exposure for 8 weeks to simultaneous CDMA and WCDMA RF did not affect serum levels in rats of melatonin, thyroid stimulating hormone (TSH), triiodothyronine (T3) and thyroxin (T4), adrenocorticotropic hormone (ACTH) and sex hormones (testosterone and estrogen) as assessed by the ELISA method.
Keywords: CDMA, WCDMA, combined exposure, melatonin, hormone
INTRODUCTION
The steadily increasing use of radio frequency (RF) communication systems has raised public concerns about the safety of electromagnetic fields (EMF). Daily, over three billion people in more than 200 countries are exposed to EMF [1]. Most studies to date do not indicate a health risk of RF exposure. However, controversy has been stimulated by some epidemiologic studies reporting an association between magnetic field exposure and human diseases [2]. Moreover, a recent International Agency for Research on Cancer (IARC) report suggested that RF-EMF is regarded as a category 2B carcinogen [3]. Animal studies have shown that exposure to RF-EMF may alter the endocrine or nervous systems [4–6]. Melatonin is a hormone, secreted by the pineal gland, which plays an important role in central nervous system. Melatonin levels vary in a circadian rhythm in both rats and humans, sunsetted to a 12 h light–dark (LD) cycle. The LD cycle causes blood and pineal body melatonin levels to increase during the dark period and decrease during the light period. Thyroid activity is regulated by the thyroid stimulating hormone (TSH) secreted by the pituitary gland. Elevated TSH levels induce the thyroid to elaborate triiodothyronine (T3) and thyroxin (T4), a hormone that functions in at least 20 enzyme systems, influencing the acceleration of protein synthesis. It has been suggested that exposure to RF-EMF may alter thyrotropin secretion [7].
Experimental data have shown that RF-EMF can act on the emotional state of people and on the anxiety-related behavior of animals [8–10]. Both epidemiological and experimental studies indicate that ELF-EMF could increase anxiety in women [10] and enhance anxiety-like behavior in rats [8, 9].
It is well known that activation of the central corticotrophin-releasing factor system and secretion of glucocorticoids can evoke negative emotional states and can potentiate fear- and anxiety-related behaviors [11]. Therefore, it is reasonable to attribute elevation in anxiety level to the stimulating effect of RF-EMF on the hypothalamic–pituitary–adrenal system function. Adrenocorticotropic hormone (ACTH) triggers the secretion of glucocorticoids from the adrenal cortex, and overall functioning is controlled by several negative feedback loops [12]. ACTH, stress- induced hormone, is regarded as a major hallmark of the stress-activated hypothalamic–pituitary–adrenal axis. Despite these concerns, there have been few in vivo experiments on the effects of RF-EMF from personal telecommunication devices on the endocrine system. Moreover, the literature is contradictory and leaves open the question of whether RF-EMF may affect the endocrine system [7, 13]. Human subjects use various types of telecommunication systems, raising concerns regarding the harmful effects of multi-signal RF-EMF exposure on human health. Despite more than a decade of research in this field, to the best of our knowledge there have been no published studies about the effects of concurrent exposure of RF-EMF on the endocrine system, and no such studies have been identified in the endocrine experiment database. The present study investigated several parameters of the endocrine system such as melatonin, thyroid-related hormone, stress hormone and sex hormones. code division multiple access (CDMA, 849 MHz) and wideband code division multiple access (WCDMA) signals were selected for simultaneous exposure, because CDMA is used in classical cellular telephones in Korea and WCDMA is utilized in new types of mobile telecommunication systems.
MATERIALS AND METHODS
Animals and animal husbandry
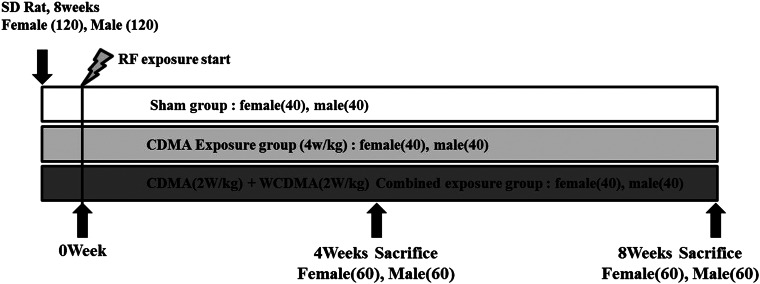
A total of 240 specific pathogen-free male (120) and female (120) Sprague-Dawley (SD) rats were obtained from the Japan SLC, Inc (Hamamatsu, Shizuoka, Japan) at an age of 8 weeks. The temperature and relative humidity in the exposure facility were maintained at 22 ± 2°C and 50 ± 10%, respectively, and were continuously monitored. Fluorescent lighting was provided for 12 h daily. The studies were performed according to the guidelines for use and care of laboratory animals and were approved by the Institutional Animal Care and Use Committee of the Korea Institute Radiological and Medical Sciences (KIRAMS).
RF multi-exposure system
A reverberation chamber was designed as a whole-body exposure system for in vivo experiments to allow simultaneous exposure to multiple mobile phone frequencies. The multiple-frequency whole-body exposure system generates CDMA signals and WCDMA signals simultaneously. To generate the signals, a microprocessor unit (MPU) chip with prewritten CDMA and WCDMA format codes was used. The MPU, in turn, controlled a central processing unit (CPU) to generate real CDMA and WCDMA test signals. For the cellular system, a real CDMA signal, at 848.5 MHz, was generated and subsequently amplified using a high power amplifier module (DCS60WHPA_CW; Kortcom, Anyang-si, Korea) after passing through a digital attenuator. For the WCDMA system, a real WCDMA signal at 1950 MHz was generated and subsequently amplified using another high power amplifier module (PCS60WHPA_CW; Kortcom) after passing through a separate digital attenuator. We used an 11-bit digital PIN diode attenuator (Model 349; General Microwave, Farmingdale, NY, USA) and the attenuator controlled the output power level. The maximum available input power was 60 W. The transmitting antennae used for the CDMA and the WCDMA frequencies are commercial products (patch type; KCAN0800PA for CDMA, KCAN1900PA for WCDMA, Korea telecommunication component, Kyunggi-do, South Korea). A computer controlled the exposure level and the exposure time schedule. The external dimensions of the reverberation chamber were 2295 mm × 2293 mm × 1470 mm, and the thickness of the walls was 2.3 mm. These dimensions satisfy the requirements for the minimum number of modes required at the lowest useable frequency (LUF) of interest. Additionally, the stainless wall of the chamber functioned as an electromagnetic shield. Eight cages were placed on the table (test area), which was located inside the exposed chamber and the field uniformity in the test area was confirmed by measuring the field strength for 1 min at 24 points. The field uniformity in the cage was also confirmed by measuring the field strength for 1 min at 27 points on the surface of the cage at a typical exposure location. The electric field distribution was measured inside the chamber using a three-axis isotropic probe (HI-6005; ETS-Lindgren, Cedar Park, TX, USA). The field distribution of both 848.5 and 1950 MHz was well within 3 dB in the region of interest. Assuming the field vectors were uniform in all directions, the SAR distribution for a caged rat was calculated using a finite difference time domain (FDTD) tool (XFDTD version 6.5; Remcom, State College, PA, USA) in six orthogonal directions. Two independent polarizations were used for each direction. The rat model (Chungnam National University, Daejon, Korea) used for simulation had 40 tissues and a 1-mm voxel size. The calculated SAR values were then averaged and multiplied by the measured root mean square electric field to estimate the real SAR values for a given input power. For 2.0 W/kg of whole-body averaged SAR, the power output was controlled at 30 W for CDMA and at 18 W for WCDMA [14, 15].
Experimental design
The experimental design is shown in Fig. 1. RF exposure was performed by whole-body exposure in the exposure chamber for 45 min/day, 5 days per week for a total of 8 weeks from 9 a.m. to 2 p.m. CDMA exposed rats were exposed to CDMA (4.0 W/kg) signals. Combined RF exposed rats were simultaneously exposed to CDMA (2.0 W/kg) and WCDMA (2.0 W/kg) signals, 4 W/kg in total. All rats (five rats per cage) were housed in autoclaved polycarbonate cages (420 × 260 × 180 mm) on certified hardwood bedding. The position of the cages and turn of exposure were changed every day. Sham-exposed rats were placed in the exposure chamber without exposure to magnetic field signals. Experimental animals were euthanized with CO2 gas. The data analysis was blinded to prevent treatment bias. Experimental animals were sacrificed in the morning, because of serum melatonin level.
Fig. 1.
Schematic of the experimental procedure described in Materials and Methods.
Enzyme linked immunosorbent assay (ELISA) assay
Melatonin (Uscn Life Science Inc., Wuhan, P.R. China), TSH (Cusabio Biotech Co., Wuhan, P.R. China), T3, T4 (Calbiotech Inc., CA, USA), ACTH (Cusabio Biotech Co.), testosterone (Enzo Life Science, NY, USA) and estrogen (Cusabio Biotech Co.) in the serum were measured using an ELISA Complete Kit according to the manufacturer's instruction sheet. Rat sera were collected from the rat abdominal aorta blood.
Briefly, standard solution or rat sera at various dilutions (testosterone; 1:20, estrogen; 1:100, T3, T4, TSH; 1:2, ACTH; 1:200, melatonin; 1:10) were added to each well and incubated at 37°C for 30–60 min. After washing three times with PBST (0.05%, Tween 20), Horseradish peroxidase (HRP)-labeled anti-rat testosterone, estrogen, T3, T4, TSH, ACTH and melatonin secondary antibody was added and incubated at 37°C for 1 h. After removing liquid from all wells, the wells were washed three times and the plates developed with 3,3′, 5,5′ tetra-methylbenzidine (TMB) color development solution. Sulfuric acid (2 mmol/10 ml) was added after the plates were allowed to react for 15 min at room temperature in the dark. Plates were read at 450 nm by ELISA reader (Multiskan MS photometer type 352, Labsystems, Helsinki, Finland). The final hormone concentration was calculated based on a standard curve constructed for each assay using recombinant hormone standards.
Statistical analysis
Data are presented as mean ± SEM. The significance of the differences between group means of hormone levels was determined by one-way analysis of variance (ANOVA) followed by post hoc testing using the least significant difference (LSD) method, performed with the SPSS 12.0 for Windows statistical package (SPSS, Inc., Chicago, IL, USA). The significance of the differences between the means of each age (4 weeks and 8 weeks) in the same gender was determined by Student's t-test. Differences were considered statistically significant at P < 0.05.
RESULTS
Body weights and histopathological analysis
No deaths occurred during the 8-week experimental period. When compared to gender-matched controls after 4 and 8 weeks of exposure, there was no difference in body weight among the sham, CDMA alone and combined RF-exposed groups (Table 1). Histopathological analysis of all the organs including brain was performed and no significant alteration was found (data not shown).
Table 1.
The body weights in RF-exposed rats
| Body weight (g) | Sham | CDMA | P-valuea | CDMA + WCDMA | P-valuea | F-valueb |
|---|---|---|---|---|---|---|
| Male (weeksc) | ||||||
| 4 | 414.73 ± 32.12 | 415.90 ± 27.59 | 0.902 | 425.25 ± 27.94 | 0.276 | 0.465 |
| 8 | 454.73 ± 70.11 | 465.55 ± 60.80 | 0.605 | 468.80 ± 52.29 | 0.476 | 0.751 |
| Female (weeks3) | ||||||
| 4 | 267.40 ± 20.95 | 263.33 ± 16.81 | 0.502 | 266.60 ± 18.11 | 0.898 | 0.767 |
| 8 | 288.10 ± 26.13 | 279.95 ± 26.64 | 0.335 | 286.03 ± 35.55 | 0.835 | 0.669 |
Values represent mean ± SD, aP-value: Student's t-test, bF-value: one-way ANOVA test, cWeeks after exposure.
Serum melatonin levels
The effect of combined exposure on serum melatonin levels of both male and female SD rats was studied. Rats were exposed to RF-EMF during day time and autopsied at day time. The serum melatonin concentration was slightly lower at 8 weeks after exposure than 4 weeks in male rats, however, there were no significant differences. No alteration by exposure of CDMA alone or simultaneous exposure of CDMA and WCDMA was shown in serum melatonin levels (Table 2).
Table 2.
The levels of melatonin in RF-exposed rat serum
| Melatonin (pg/ml) | Sham | CDMA | P-valuea | CDMA + WCDMA | P-valuea | F-valueb |
|---|---|---|---|---|---|---|
| Male (weeksc) | ||||||
| 4 | 525.31 ± 41.03 | 523.30 ± 38.50 | 0.704 | 521.11 ± 51.50 | 0.671 | 0.982 |
| 8 | 498.49 ± 27.84 | 501.20 ± 22.36 | 0.898 | 499.95 ± 39.46 | 0.929 | 0.986 |
| Female (weeksc) | ||||||
| 4 | 508.71 ± 41.69 | 503.11 ± 20.39 | 0.839 | 506.76 ± 39.07 | 0.729 | 0.962 |
| 8 | 505.95 ± 32.96 | 507.98 ± 45.13 | 0.682 | 506.76 ± 36.24 | 0.940 | 0.994 |
Values represent mean ± SD, aP-value: Student's t-test, bF-value: one-way ANOVA test, cWeeks after exposure.
Serum TSH, T3 and T4 levels
The effects of CDMA alone or combined exposure of CDMA and WCDMA on serum TSH and T3, T4 hormones levels of adult male and female SD rats were studied. Table 3 showed TSH concentrations of sham-exposed, CDMA alone-exposed and combined RF exposed groups, after autopsy at 4 and 8 weeks post-exposure. Serum TSH concentration increased with age in both male and female rats, but only female TSH level in CDMA alone-exposed rats showed statistical significance. Moreover, there were no significant differences between sham and exposed groups (Table 3). Similarly, serum T3 and T4 hormone levels of CDMA alone or combined RF-exposed rats did not show any significant differences compared with sham exposed rats (Table 3).
Table 3.
The levels of thyroid-stimulating hormone (TSH), thyroxine 3 (T3) and 4 (T4) in -F exposed rat serum
| TSH (μU/ml) | Sham | CDMA | P-valuea | CDMA + WCDMA | P-valuea | F-valueb |
|---|---|---|---|---|---|---|
| Male (weeksc) | ||||||
| 4 | 3.68 ± 0.99 | 3.43 ± 2.62 | 0.572 | 3.67 ± 1.47 | 0.810 | 0.956 |
| 8 | 4.91 ± 2.31 | 4.61 ± 2.31 | 0.374 | 4.99 ± 2.26 | 0.694 | 0.923 |
| Female (weeks3) | ||||||
| 4 | 2.85 ± 1.13 | 2.59 ± 1.01 | 0.716 | 2.76 ± 0.50 | 0.697 | 0.883 |
| 8 | 4.06 ± 1.93 | 4.28 ± 1.48* | 0.616 | 4.11 ± 1.90 | 0.830 | 0.964 |
| T3 (ng/ml) | ||||||
| Male (weeksc) | ||||||
| 4 | 19.33 ± 1.96 | 20.05 ± 0.49 | 0.472 | 19.42 ± 0.84 | 0.929 | 0.699 |
| 8 | 19.38 ± 1.41 | 19.92 ± 0.81 | 0.352 | 19.14 ± 1.17 | 0.712 | 0.350 |
| Female (weeksc) | ||||||
| 4 | 18.57 ± 1.93 | 18.86 ± 1.05 | 0.816 | 18.85 ± 0.39 | 0.897 | 0.907 |
| 8 | 16.68 ± 3.12 | 16.81 ± 2.91 | 0.716 | 16.20 ± 1.69 | 0.849 | 0.898 |
| T4 (ng/dl) | ||||||
| Male (weeksc | ||||||
| 4 | 31.81 ± 1.69 | 31.35 ± 1.36 | 0.709 | 30.16 ± 0.51 | 0.768 | 0.731 |
| 8 | 31.55 ± 1.42 | 31.81 ± 3.53 | 0.618 | 31.47 ± 0.34 | 0.814 | 0.967 |
| Female (weeksc) | ||||||
| 4 | 33.46 ± 4.31 | 33.66 ± 1.69 | 0.897 | 34.09 ± 2.09 | 0.694 | 0.895 |
| 8 | 33.26 ± 4.04 | 33.87 ± 3.24 | 0.899 | 33.47 ± 2.24 | 0.765 | 0.923 |
Values represent mean ± SD, aP-value: Student's t-test, bF-value: one-way ANOVA test, cWeeks after exposure.
*Significant difference from the corresponding values of 4-week-old rats according to Student's t-test.
Serum ACTH level
Serum ACTH levels were assayed using the ELISA method. Serum ACTH levels significantly decreased with age in both male and female rats. However, CDMA alone or simultaneous combined exposure did not affect serum ACTH levels when compared with those of sham exposed rats (Table 4).
Table 4.
The levels of adrenocorticotropic hormone (ACTH) in RF-exposed rat serum.
| ACTH (ng/ml) | Sham | CDMA | P-valuea | CDMA + WCDMA | P-valuea | F-valueb |
|---|---|---|---|---|---|---|
| Male (weeksc) | ||||||
| 4 | 7.18 ± 0.26 | 7.21 ± 0.17 | 0.728 | 7.14 ± 0.38 | 0.802 | 0.853 |
| 8 | 6.28 ± 0.28* | 6.42 ± 0.11* | 0.884 | 6.39 ± 0.38* | 0.423 | 0.643 |
| Female (weeksc) | ||||||
| 4 | 6.32 ± 0.23 | 6.40 ± 0.24 | 0.545 | 6.44 ± 0.20 | 0.883 | 0.614 |
| 8 | 5.07 ± 0.47* | 5.10 ± 0.50* | 0.713 | 5.04 ± 0.49* | 0.880 | 0.957 |
Values represent mean ± SD, aP-value: Student's t-test, bF-value: one-way ANOVA test, cWeeks after exposure.
*Significant differentcefrom the corresponding values of 4-week-old rats according to Student's t-test.
Serum sex hormone levels
Sex hormones (testosterone in male rats and estrogen in female rats) were assayed. These sex hormone levels increased with age however, only estrogen level in the group of combined exposed rats showed statistical significance. CDMA alone or combined exposure of CDMA and WCDMA, total SAR 4 W/kg, did not alter these serum hormone levels (Table 5).
Table 5.
The levels of sexual hormones in RF-exposed rat serum
| Testosterone (ng/ml) (weeksc) | Sham | CDMA | P-valuea | CDMA + WCDMA | P-valuea | F-valueb |
|---|---|---|---|---|---|---|
| 4 | 12.39 ± 2.6 | 12.86 ± 2.4 | 0.558 | 12.30 ± 3.2 | 0.861 | 0.787 |
| 8 | 13.45 ± 2.6 | 13.84 ± 2.8 | 0.971 | 13.28 ± 1.5 | 0.332 | 0.751 |
| Estrogen (ng/ml) (weeksc) | ||||||
| 4 | 69.66 ± 4.06 | 68.51 ± 4.34 | 0.701 | 68.26 ± 3.71 | 0.543 | 0.576 |
| 8 | 71.40 ± 5.75 | 71.32 ± 8.73 | 0.474 | 72.99 ± 3.58* | 0.522 | 0.805 |
Values represent mean ± SD, aP-value: Student's t-test, bF-value: one-way ANOVA test, cWeeks after exposure.
*Significant difference from the corresponding values of 4-week-old rats according to Student's t-test.
DISCUSSION
In the present study, we investigated the effects of simultaneous combined exposure of CDMA and WCDMA RF-EMF, especially focusing on serum hormone levels. We exposed rats to CDMA alone or to a combination of CDMA and WCDMA with total 4 W/kg for 8 weeks, a relatively long exposure in animal endocrine system experiments [7, 16, 17].
There is some research indicating that low frequency EMF decreases serum and pineal melatonin levels [18, 19]. However, RF-EMF has not been often reported to affect melatonin level [20–22]. One explanation could be the relatively low SAR level and short-term exposure duration. However, our long-term exposure (8 weeks) and higher SAR level (4 W/kg) did not alter serum melatonin level either. One limitation of our study is the timing of exposure and the timing of sacrifice. We exposed RF-EMF to rats at day time and sacrificed at day time. Therefore, if we were to change the timing of exposure or sacrifice, the results may be different. While prior research shows that dark phase exposure does not affect serum and pineal melatonin level [16], exposure was limited to only 6 h of exposure with a SAR value of 2.0 W/kg, so more detailed experiments for combined exposure with longer exposure and higher SAR values were needed.
There is little prior work describing the relationship between RF-EMF and pituitary hormones such as TSH, T3 and T4 hormones in animals. GSM-EMF significantly lowered serum TSH and T3, T4 values [7] and increased thyroxine and triiodothyronine secretion when the thyroids of dogs were exposed to varying levels of 2.45 GHz RF-EMF at estimated very high SARs of 58–190 W/kg. Whole-body exposure to RF-EMF at 4 W/kg in rats caused rectal temperature rise to 40°C resulting in decreased circulating thyroxine and TSH levels [23]. However, 1.29 GHz RF-EMF at 3–4 W/kg did not change serum thyroxin [24]. Our data indicated that combined exposure to CDMA and WCDMA at 4 W/kg SAR for 8 weeks did not alter TSH, T3 and T3 hormone levels, and did not increase rectal temperature [25].
ACTH is a hormone induced by stress in the hypothalamic–pituitary–adrenal axis (HPA). In a previous study 120 min of exposure of universal mobile telecommunications system (UMTS) to full brain-exposed rats with 10 W/kg SAR increased the plasma corticosterone level and ACTH level [26]. However, our 4 W/kg SAR with whole-body exposure for 8 weeks did not affect serum ACTH level, even with combination exposure of CDMA and WCDMA, suggesting higher SAR levels with focused exposure to the brain may be necessary to alter ACTH levels.
Serum testosterone levels were not changed by RF-EMF according to several papers [14, 27]. However, there are suggestions that damage to the testis may occur, resulting in reduced fertility potential of men [28–30] and experimental data have suggested that RF-EMF caused a significant increase in sperm cell death and abnormal clumping of sperm cells [31]. Others found no such adverse effects of RF-EMF on testicular function including testosterone level in animal experiments [25, 32–34]. Consistently with these latter findings, our data show no alteration of serum testosterone level from combined exposure of CDMA and WCDMA with 4 W/kg SAR and 8 weeks of exposure, a higher SAR level and longer exposure time. The literature on estrogen effects is sparse. Short-term exposure (4 h) of time division multiple access (TDMA) with higher SAR levels of 6.1 W/kg did not change estrogenic levels in rats [35]. Similarly, our combined exposure to CDMA and WCDMA with total 4 W/kg SAR for 8 weeks did not alter serum estrogen level.
In this study, we examined the various endocrine hormone levels in serum after 4 or 8 weeks' exposure to RF-EMF. Even though age-related changes between 4- and 8-week-old rats were found in all the hormone levels we examined in this study, using the combination of CDMA and WCDMA as well as CDMA alone, a 4 W/kg SAR value did not affect hormone secretion in hormones such as melatonin, thyroid-related hormone, ACTH and sex hormone when they were detected in the serum of rats. However, our negative results are limited by the experimental sensitivity for the detection of hormone levels and the absence of a positive control; therefore, more detailed experiments are needed in the future. In summary, our findings indicate that simultaneous combined exposure of CDMA and WCDMA with a total SAR dose of 4.0 W/kg for 45 min/day for 8 weeks, which is a relatively high SAR level and longer duration of exposure than the basic restrictions recommended by ICNIRP for humans (frequency range of 100 kHz–10 GHz, 0.4 W/kg is the occupational exposure limit of whole-body average SAR and 0.08 W/kg is the general public exposure limit) has no significant effects on rat serum hormone levels in our limited experimental systems.
We also compared the effects between CDMA alone and a combination of CDMA and WCDMA with the same total SAR value (4 W/kg). Our results suggested that neither 4 W/kg of CDMA alone nor a combination of CDMA and WCDMA affected rat serum hormone levels.
ACKNOWLEDGEMENTS
A Grant from the Korea Communications Commission (2011) supported this work. Further support was provided by the Ewha Global Top 5 Grant 2011 of Ewha Womans University.
REFERENCES
- 1.Fragopoulou A, Grigoriev Y, Johansson O, et al. Scientific panel on electromagnetic field health risks: consensus points, recommendations, and rationales. Rev Environ Health. 2010;25:307–17. [PubMed] [Google Scholar]
- 2.Schüz J, Lagorio S, Bersani F. Electromagnetic fields and epidemiology: an overview inspired by the fourth course at the International School of Bioelectromagnetics. Bioelectromagnetics. 2009;30:511–24. doi: 10.1002/bem.20510. [DOI] [PubMed] [Google Scholar]
- 3.IARC. International Agency for Research on Cancer; 2011. IARC classifies radiofrequency electromagnetic fields as possibly carcinogenic to humans. [Google Scholar]
- 4.Lu ST, Lebda N, Pettit S, et al. Microwave-induced temperature, corticosterone, and thyrotropin inter relationships. J Appl Physiol. 1981;50:399–405. doi: 10.1152/jappl.1981.50.2.399. [DOI] [PubMed] [Google Scholar]
- 5.Lu ST, Lebda N, Michaelson SM, et al. Serum-thyroxine levels in microwave-exposed rats. Radiat Res. 1985;101:413–23. [PubMed] [Google Scholar]
- 6.Lu ST, Lebda NA, Lu SJ, et al. Effects of microwaves on three different strains of rats. Radiat Res. 1987;110:173–91. [PubMed] [Google Scholar]
- 7.Koyu A, Cesur G, Ozguner F, et al. Effects of 900 MHz electromagnetic field on TSH and thyroid hormones in rats. Toxicol Lett. 2005;157:257–62. doi: 10.1016/j.toxlet.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Tamasidze AG. Influence of the chronic exposure to network frequency electromagnetic field on rats under interrupted and continuous action of EMF. [Article in Russian] Georgian Med News. 2006;140:91–3. [PubMed] [Google Scholar]
- 9.Semenova TP, Medvinskaia NI, Bliskovka GI, et al. Influence of electromagnetic fields on the emotional behaviour of rats. [Article in Russian] Radiats Biol Radioecol. 2000;40:693–5. [PubMed] [Google Scholar]
- 10.Boscolo P, Di Giampaolo L, Di Donato A, et al. The immune response of women with prolonged exposure to electromagnetic fields produced by radiotelevision broadcasting stations. Int J Immunopathol Pharmacol. 2006;19:43–8. [PubMed] [Google Scholar]
- 11.Szemerszky R, Zelena D, Barna I, et al. Stress-related endocrinological and psychopathological effects of short- and long-term 50Hz electromagnetic field exposure in rats. Brain Res Bull. 2010;81:92–9. doi: 10.1016/j.brainresbull.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Nussey S, Whitehead S. Endocrinology: An Integrated Approach. Oxford: BIOS Scientific Publishers; 2001. Chapter 4. [PubMed] [Google Scholar]
- 13.Black DR, Heynick LN. Radiofrequency (RF) effects on blood, cells, cardiac, endocrine and immunological functions. Bioelectromagnetics. 2003;6:187–95. doi: 10.1002/bem.10166. [DOI] [PubMed] [Google Scholar]
- 14.Lee HJ, Lee JS, Pack JK, et al. Lack of teratogenicity after combined exposure of pregnant mice to CDMA and WCDMA radiofrequency electromagnetic fields. Radiat Res. 2009;172:648–52. doi: 10.1667/RR1771.1. [DOI] [PubMed] [Google Scholar]
- 15.Jin YB, Lee HJ, Seon Lee J, et al. One-year, simultaneous combined exposure of CDMA and WCDMA radiofrequency electromagnetic fields to rats. Int J Radiat Biol. 2011;87:416–23. doi: 10.3109/09553002.2010.537428. [DOI] [PubMed] [Google Scholar]
- 16.Hata K, Yamaguchi H, Tsurita G, et al. Short term exposure to 1439 MHz pulsed TDMA field does not alter melatonin synthesis in rats. Bioelectromagnetics. 2005;26:49–53. doi: 10.1002/bem.20080. [DOI] [PubMed] [Google Scholar]
- 17.Bakos J, Kubinyi G, Sinay H, et al. GSM modulated radiofrequency radiation does not affect 6-sulfatoxymelatonin excretion of rats. Bioelectromagnetics. 2003;24:531–4. doi: 10.1002/bem.10172. [DOI] [PubMed] [Google Scholar]
- 18.Grota LJ, Reiter RJ, Keng P, et al. Electric field exposure alters serum melatonin but not pineal melatoninsynthesis in male rats. Bioelectromagnetics. 1994;15:427–37. doi: 10.1002/bem.2250150506. [DOI] [PubMed] [Google Scholar]
- 19.Kato M, Honma K, Shigemitsu T, et al. Circularly polarized 50-Hz magnetic field exposure reduces pineal gland and blood melatonin concentrations of Long-Evans rats. Neurosci Lett. 1994;17:59–62. doi: 10.1016/0304-3940(94)90840-0. [DOI] [PubMed] [Google Scholar]
- 20.Vollrath L, Spessert R, Kratzsch T, et al. No short-term effects of high-frequency electromagnetic fields on the mammalian pineal gland. Bioelectromagnetics. 1997;18:376–87. [PubMed] [Google Scholar]
- 21.Radon K, Parera D, Rose DM, et al. No effects of pulsed radio frequency electromagnetic fields on melatonin, cortisol, and selected markers of the immune system in man. Bioelectromagnetics. 2001;22:280–7. doi: 10.1002/bem.51. [DOI] [PubMed] [Google Scholar]
- 22.Bortkiewicz A, Pilacik B, Gadzicka E, et al. The excretion of 6-Hydroxyl-melatonin sulfate in healthy young men exposed to electromagnetic fields emitted by cellular phone—an experimental study. Neuro Endocrinol Lett. 2002;23(Suppl 1):88–91. [PubMed] [Google Scholar]
- 23.Lu ST, Lebda N, Pettit S, et al. Delineating acute neuroendocrine responses in microwave-exposed rats. J Appl Physiol. 1980;48:927–32. doi: 10.1152/jappl.1980.48.6.927. [DOI] [PubMed] [Google Scholar]
- 24.Lotz WG, Podgorski RP. Temperature and adrenocortical responses in rhesus monkeys exposed to microwaves. J Appl Physiol. 1982;53:1565–71. doi: 10.1152/jappl.1982.53.6.1565. [DOI] [PubMed] [Google Scholar]
- 25.Lee HJ, Jin YB, Kim TH, et al. The effects of simultaneous combined exposure to CDMA and WCDMA electromagnetic fields on rat testicular function. Bioelectromagnetics. 2012;33:356–64. doi: 10.1002/bem.20715. [DOI] [PubMed] [Google Scholar]
- 26.Prochnow N, Gebing T, Ladage K, et al. Electromagnetic field effect or simply stress? Effects of UMTS exposure on hippocampal longterm plasticity in the context of procedure related hormone release. PLoS One. 2011;5(6(5)):e19437. doi: 10.1371/journal.pone.0019437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Djeridane Y, Touitou Y, de Seze R. Influence of electromagnetic fields emitted by GSM-900 cellular telephones on the circadian patterns of gonadal, adrenal and pituitary hormones in men. Radiat Res. 2008;169:337–43. doi: 10.1667/RR0922.1. [DOI] [PubMed] [Google Scholar]
- 28.Fejes I, Zavaczki Z, Szollosi J, et al. Is there a relationship between cell phone use and semen quality? Arch Androl. 2005;51:385–93. doi: 10.1080/014850190924520. [DOI] [PubMed] [Google Scholar]
- 29.Agarwal A, Deepinder F, Sharma RK, et al. Effect of cell phone usage on semen analysis in men attending infertility clinic: an observational study. Fertil Steril. 2008;89:124–8. doi: 10.1016/j.fertnstert.2007.01.166. [DOI] [PubMed] [Google Scholar]
- 30.Agarwal A, Desai NR, Makker K, et al. Effects of radiofrequency electromagnetic waves (RF-EMW) from cellular phones on human ejaculated semen: an in vitro pilot study. Fertil Steril. 2009;92:1318–25. doi: 10.1016/j.fertnstert.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 31.Yan JG, Agresti M, Bruce T, et al. Effects of cellular phone emissions on sperm motility in rats. Fertil Steril. 2007;88:957–64. doi: 10.1016/j.fertnstert.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 32.Dasdag S, Zulkuf Akdag M, Aksen F, et al. Whole body exposure of rats to microwaves emitted from a cell phone does not affect the testes. Bioelectromagnetics. 2003;24:182–8. doi: 10.1002/bem.10083. [DOI] [PubMed] [Google Scholar]
- 33.Dasdag S, Akdag MZ, Ulukaya E, et al. Mobile phone exposure does not induce apoptosis on spermatogenesis in rats. Arch Med Res. 2008;39:40–4. doi: 10.1016/j.arcmed.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 34.Wang XW, Ding GR, Shi CH, et al. Effect of electromagnetic pulse exposure on permeability of blood–testicle barrier in mice. Biomed Environ Sci. 2008;21:218–21. doi: 10.1016/S0895-3988(08)60032-X. [DOI] [PubMed] [Google Scholar]
- 35.Yamashita H, Hata K, Yamaguchi H, et al. Short-term exposure to a 1439-MHz TDMA signal exerts no estrogenic effect in rats. Bioelectromagnetics. 2010;31:573–5. doi: 10.1002/bem.20593. [DOI] [PubMed] [Google Scholar]