Abstract
To compare the incidence and degree of hematological toxicity between innovator and generic cisplatin formulations, decreases in white blood cell (WBC) count (leukopenia) and platelet counts (thrombocytopenia) were retrospectively examined, using the Common Toxicity Criteria for Adverse Events ver. 4.0, in patients with uterine cervical cancer treated with concurrent chemoradiotherapy using innovator (innovator group, n = 22) or generic (generic group, n = 22) cisplatin formulations. There were no significant differences in patient characteristics except in the technique of external irradiation; larger numbers of patients in the innovator and generic groups were irradiated using the parallel-opposed two-field technique and the four-field box technique, respectively (P = 0.00012), which is in line with the historical progress of external beam radiation therapy. The numbers of patients showing Grade 1, 2, 3 and 4 leukopenia were 1 (4.5%), 14 (64%), 7 (32%) and 0 (0.0%) in the innovator group, and 1 (4.5%), 6 (27%), 13 (59%) and 2 (9.0%) in the generic group, respectively. The number of patients showing Grade 3–4 leukopenia was significantly greater in the generic group than in the innovator group (P = 0.034). There was no significant relationship between the incidence of Grade 3–4 leukopenia and the technique of external irradiation. There were no significant differences in the incidence and degree of thrombocytopenia between the two groups. These results indicate the possibility that the generic cisplatin formulation may have a different toxicity profile compared to the innovator formulation in terms of the incidence of leukopenia.
Keywords: generic drug, cisplatin, leukopenia, uterine cervical cancer, chemoradiotherapy
INTRODUCTION
Cisplatin has been used as a key chemotherapeutic agent for many types of malignant tumors for more than 30 years because of its significant therapeutic efficacy [1]. In locally advanced cervical cancer, the combination use of cisplatin-based chemotherapy and radiation therapy is considered as standard therapy [2]. A recent meta-analysis demonstrated that concurrent chemoradiotherapy (CCRT) using cisplatin significantly reduced the incidence of pelvic recurrence and distant metastasis, with a 12% benefit in overall survival and a 16% benefit in progression-free survival [3]. On the other hand, in patients treated with CCRT using cisplatin, the incidence of severe leukopenia has been reported to be significantly higher than that observed in the control group [4]. Leukopenia is one of the major dose-limiting factors in cisplatin administration [4–5], and is an issue of major importance in the clinical field.
Generic substitutes serve as lower-cost alternatives to the more costly brand-name drugs in clinical medicine today [6]. If it can be shown that a generic formulation is essentially identical in qualitative and quantitative composition to an innovator preparation, the formulation can be marketed as generic without the need for expensive regulatory clinical trials. However, whether generic cisplatin formulations are truly therapeutically identical to, and interchangeable with, the innovator formulation of the drug has not been fully elucidated. In this study, we compared the incidence and degree of acute hematological toxicity of CCRT for uterine cervical cancer patients between an innovator cisplatin formulation and a generic substitute.
MATERIALS AND METHODS
Patient selection
Patients were retrospectively selected according to the following criteria: (i) a histological diagnosis of uterine cervical cancer; (ii) neither paraaortic lymph node metastasis nor distant metastasis; (iii) neither prior chemotherapy nor radiation therapy; (iv) receiving treatment at the Gunma University Hospital between October 2006 and June 2012; and (v) receiving chemoradiotherapy with a regimen containing 40 mg/m2 of weekly cisplatin. During this period, the innovator cisplatin formulation, RANDA INJ., was administered between October 2006 and October 2009, and a generic cisplatin formulation, CISPLATIN for i.v. infusion (MARUKO), was administered thereafter.
Cisplatin administration
Cisplatin was administered at a fixed dose of 40 mg/m2 in every cycle of chemotherapy for all patients. Cisplatin was administered by i.v. infusion over 60 min on Day 1 in combination with 2200 ml of hydration. On Days 2 and 3, 1100 ml and 600 ml of i.v. infusion fluids were administered, respectively. Antiemetics, such as 5-hydroxytryptamine3 (5-HT3) receptor antagonist, metoclopramide, and dexamethasone were administered on Days 1–3. These treatments were repeated weekly for up to 5 courses, concurrently with external beam irradiation. The toxicities were classified, by the Common Toxicity Criteria for Adverse Events ver. 4.0 (CTCAE), in each patient every week until the end of the treatment. Chemotherapy was interrupted when patients developed a white blood cell (WBC) count <3000/mm3, a platelet count <75 000 /mm3, fever >38.0°C, or Grade 3–4 non-hematological toxicities. Dose reduction of cisplatin in any cycle of chemotherapy was not performed. Administration of cisplatin was resumed when hematological and non-hematological toxicities recovered to Grade 1.
Radiation therapy
Patients were treated with a combination of external beam radiation therapy and high-dose rate intracavitary brachytherapy (ICBT). External whole pelvic irradiation (WP) was performed using parallel-opposed two-field (2 ports) or box four-field (4 ports) technique with a dose of 2.0 Gy per fraction, five times per week, up to 30 or 40 Gy. Thereafter, pelvic irradiation with central shielding (CS) using a midline block was performed with doses of 2.0 Gy per fraction to a total dose of 50 Gy. Along with CS, ICBT was started with a remote after-loading system using high-dose rate 192Ir sources. Four or five fractions of ICBT were carried out once a week with a fraction dose of 4.5–8.0 Gy (median, 6.0 Gy) at point A, with the total dose ranging from 20–36 Gy (median, 24 Gy). If the patient had pelvic lymph node metastasis, an additional irradiation boost of 6.0–10 Gy per 3–5 fractions was given.
Data collection and statistical analyses
Patient characteristics, including age, performance status (PS), body height and weight, International Federation of Gynecology and Obstetrics (FIGO) stage, number of cisplatin cycles, total dose delivered by WP, technique of external irradiation (2 ports or 4 ports), and addition of irradiation boost to pelvic lymph nodes, were retrospectively obtained from medical charts. Decreases in WBC counts (leukopenia) and platelet (Plt) counts (thrombocytopenia) were chosen as the endpoint of hematological toxicity. For these endpoints, pretreatment CTCAE grades, and the highest grades during the 3 months of the treatment, defined as acute toxicity, were recorded.
Anonymity of the patients was preserved. Informed consent for the treatment and research was appropriately obtained for all patients. The current study was approved by the Gunma University Hospital, Gunma, Japan. The institutional ethics committee exempted the current study from the usual review process because of its retrospective nature.
Continuous variables and categorical variables were analyzed by a Mann-Whitney test and a Fisher's exact test, respectively, using StatMateIII ver. 3.17 software (ATMS, Tokyo, Japan). A P value <0.05 was considered significant.
RESULTS
A total of 44 patients met the inclusion criteria. Of these, 22 were treated with innovator (innovator group) and 22 were treated with generic (generic group) cisplatin formulations. There were no significant differences in patient characteristics including age, PS, height, weight, FIGO stage, number of cisplatin cycles, total dose delivered by WP, addition of irradiation boost to pelvic lymph nodes, pretreatment WBC count and pretreatment Plt count between the two groups (Table 1). Regarding the external irradiation technique, larger numbers of patients in the innovator and generic groups were irradiated using the 2-port and 4-port techniques, respectively (P = 0.000 12), which was related to the historical transition of the irradiation technique from 2 ports to 4 ports, the latter being superior in small bowel sparing [7], along with the increasing usage of computed tomography-based treatment planning over time.
Table 1.
Patient characteristics
| Characteristics | Innovator (n = 22) | Generic (n = 22) | P-value |
|---|---|---|---|
| Age (years) | |||
| Median (range) | 53 (35–74) | 51 (32–74) | 0.41 |
| PS | |||
| 0 | 8 | 8 | 1.00 |
| 1 | 13 | 13 | |
| 2 | 1 | 1 | |
| Height (cm) | |||
| Median (range) | 155 (146–168) | 154 (146–163) | 0.56 |
| Weight (kg) | |||
| Median (range) | 56 (45–73) | 52 (42–73) | 0.26 |
| FIGO stage | |||
| I–II | 12 | 13 | 1.00 |
| III–IV | 10 | 9 | |
| Cisplatin cycles | |||
| Median (range) | 4 (3–5) | 4 (3–5) | 0.15 |
| WP dose | |||
| Median (range) | 30 (30–40) | 30 (30–40) | 0.59 |
| Technique of external irradiation | |||
| Parallel-opposed two field | 20 | 7 | 0.000 12 |
| Box four-field | 2 | 15 | |
| Irradiation boost to LN metastasis | |||
| (+) | 11 | 9 | 0.76 |
| (–) | 11 | 13 | |
| Pretreatment WBC count (103/μl) | |||
| Median (range) | 7.0 (4.0–13.5) | 7.5 (3.0–11.4) | 0.99 |
| Pretreatment leukopenia level | |||
| Grade 0 | 22 | 21 | 1.00 |
| Grade 1 | 0 | 1 | |
| Pretreatment platelet count (103/μl) | |||
| Median (range) | 288 (153–532) | 292 (220–558) | 0.75 |
| Pretreatment thrombocytopenia level | |||
| Grade 0 | 22 | 22 | 1.00 |
| Grade 1 | 0 | 0 |
PS = performance status, FIGO = International Federation of Gynecology and Obstetrics, WP = external whole pelvis irradiation, LN = lymph nodes, WBC = white blood cell. Leukopenia (decrease in WBC count) and thrombocytopenia (decrease in platelet count) were evaluated by Common Toxicity Criteria for Adverse Events ver. 4.0.
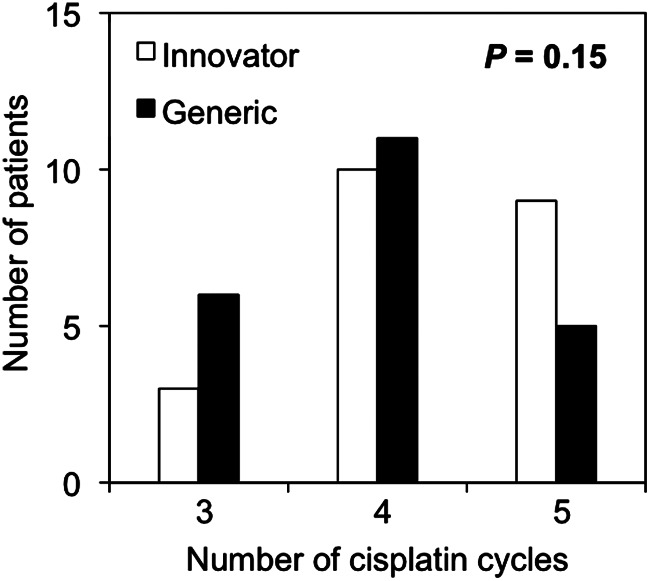
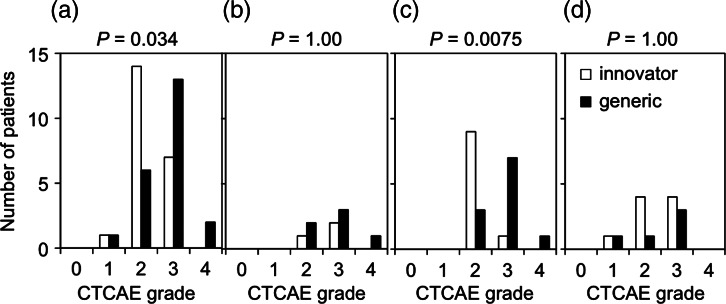
The median number of cisplatin cycles administered was 4 in both groups, although patients in the generic group tended to be administered less cycles than those in the innovator group (P = 0.15) (Fig. 1). All interruptions of cisplatin administration were due to leukopenia. The numbers of patients with Grade 1, 2, 3 and 4 leukopenia were 1 (4.5%), 14 (64%), 7 (32%) and 0 (0.0%) in the innovator group, and 1 (4.5%), 6 (27%), 13 (59%) and 2 (9.0%) in the generic group, respectively (Fig. 2a). A significantly larger number of patients in the generic group showed Grade 3–4 leukopenia (P = 0.034). In order to consider the impact of the dose of cisplatin administered on the severity of leukopenia, we divided the patients into groups by the number of chemotherapy cycles administered (3, 4 and 5 cycles) and compared the leukopenia grade between patients treated with the innovator and generic cisplatin formulations in each group (Fig. 2b–d). Analysis of leukopenia observed in 21 patients treated with 4 cycles of chemotherapy showed that the number of patients who developed Grade 3–4 leukopenia was significantly greater in the generic group than in the innovator group (P = 0.0075) (Fig. 2c). There was no significant relationship between the incidence of Grade 3–4 leukopenia and the external irradiation technique.
Fig. 1.
Number of cisplatin cycles administered in patients treated with innovator and generic cisplatin formulations.
Fig. 2.
Leukopenia observed in patients treated with innovator and generic cisplatin formulations. (a) All patients. (b–d) Patients received 3 (b), 4 (c) and 5 (d) cycles of chemotherapy. Toxicity was evaluated by the Common Terminology Criteria for Adverse Events ver. 4.0 (CTCAE) grades. Incidences of Grade 3–4 and Grade 0–2 leukopenia were compared.
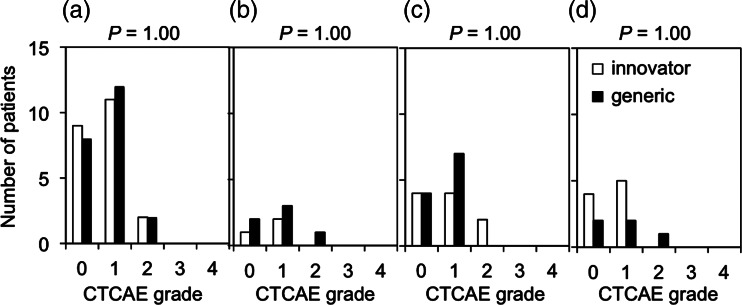
The numbers of patients showing Grade 1, 2, 3 and 4 thrombocytopenia were 11 (50%), 2 (9.1%), 0 (0.0%) and 0 (0.0%) in the innovator group, and 12 (55%), 2 (9.1%), 0 (0.0%) and 0 (0.0%) in the generic group, respectively (Fig. 3a). The incidence of Grade 1–2 thrombocytopenia was not significantly different between the two groups (P = 1.00). Analysis of thrombocytopenia in patients receiving the same number of chemotherapy cycles also showed that the incidence of Grade 1–2 thrombocytopenia was not significantly different between the two groups (P = 1.00, 1.00, 1.00, in patients receiving 3, 4 and 5 chemotherapy cycles, respectively) (Fig. 3b–d).
Fig. 3.
Thrombocytopenia observed in patients treated with innovator and generic cisplatin formulations. (a) All patients. (b–d) Patients received 3 (b), 4 (c) and 5 (d) cycles of chemotherapy. Toxicity was evaluated by the Common Terminology Criteria for Adverse Events ver. 4.0 (CTCAE) grades. Incidences of Grade 1–2 and Grade 0 thrombocytopenia were compared.
DISCUSSION
The current study demonstrated a significantly higher incidence of Grade 3–4 leukopenia with the use of the generic cisplatin formulation than with that of the innovator formulation in combination with radiation therapy for cervical cancer.
Generic drugs are approved without testing with clinical trials because of their identicalness in basic composition and property with innovator formulations [8]. However, Sekine et al. showed that renal toxicity of generic cisplatin was significantly higher than that of the innovator formulation (20.9% vs 9.4%, P < 0.001) in male patients with thoracic malignancies [8], indicating that innovator and generic drugs are not therapeutically identical. In line with their report, our data suggest that generic cisplatin formulations may be more toxic, at least in respect to leukopenia. Furthermore, in the present study, the number of completed cisplatin cycles tended to be less in the generic group, indicating that the more severe leukopenia induced by the generic formulation might influence compliance with the weekly cisplatin regimen. From another point of view, there is a possibility that the anti-tumor effect of the generic cisplatin formulation is also higher than that of the innovator formulation. To further investigate these issues, the long-term clinical outcome of CCRT for cervical cancer using both the generic and innovator cisplatin formulations should be examined in future studies.
In the current study, there was a bias in the technique of external irradiation (Table 1), which was attributable to the retrospective nature of the study. However, a previous study showing that the 4-port technique is better in pelvic bone sparing than the 2-port technique [9] would indicate that the greater number of patients irradiated using the 4-port technique in the generic group than in the innovator group would likely not be related to the higher incidence of Grade 3–4 leukopenia observed in the generic group.
A major limitation of the present study is that the generic and innovator cisplatin formulations were not allocated to the study population in a randomized fashion, but rather depended on the historical period of the treatment. Another weakness of our study is the small number of patients (n = 44). In conclusion, these results point to the possibility that generic cisplatin formulations may have a different toxicity profile compared to innovator formulations in terms of the incidence of leukopenia. Further randomized studies employing larger study populations are needed to validate our results.
FUNDING
This work was supported by Grants-in-Aid from the Japan Society for the Promotion of Science for Scientific Research (C) KAKENHI [23591833].
ACKNOWLEDGEMENTS
We thank Kohta Torikai, Sohei Ohshima and Daisuke Ozaki of Gunma University Hospital for their technical assistance.
REFERENCES
- 1.Reed E. Cisplatin and its analogues. In: DeVita V, Lawrence T, Roesnberg S, editors. Cancer: Principles and Practice of Oncology. 8th edn. Philadelphia: Wolters Kluwer Lippincott Williams & Wilkins; 2008. pp. 419–26. [Google Scholar]
- 2.Nakano T, Ohno T, Ishikawa H, et al. Current advancement in radiation therapy for uterine cervical cancer. J Radiat Res. 2010;51:1–8. doi: 10.1269/jrr.09132. [DOI] [PubMed] [Google Scholar]
- 3.Green JA, Kirwan JM, Tierny JF, et al. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: a systematic review and meta-analysis. Lancet. 2001;358:781–6. doi: 10.1016/S0140-6736(01)05965-7. [DOI] [PubMed] [Google Scholar]
- 4.Kirwan JM, Symonds P, Green JA, et al. A systematic review of acute and late toxicity of concomitant chemoradiation for cervical cancer. Radiother Oncol. 2003;68:217–26. doi: 10.1016/s0167-8140(03)00197-x. [DOI] [PubMed] [Google Scholar]
- 5.Ohno T, Kato S, Wakatsuki M, et al. Incidence and temporal pattern of anorexia, diarrhea, weight loss, and leucopenia in patients with cervical cancer treated with concurrent radiation therapy and weekly cisplatin: Comparison with radiation therapy alone. Gynecol Oncol. 2006;103:94–9. doi: 10.1016/j.ygyno.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 6.Haas JS, Phillips KA, Gestenberger EP, et al. Potential savings from substituting generic drugs for brand-name drugs: medical expenditure panel survey, 1997–2000. Ann Intern Med. 2005;142:891–7. doi: 10.7326/0003-4819-142-11-200506070-00006. [DOI] [PubMed] [Google Scholar]
- 7.Ahmad A, D'Souza W, Salehpour M, et al. Intensity-modulated radiation therapy after hysterectomy: comparison with conventional treatment and sensitivity of the normal-tissue-sparing effect to margin size. Int J Radiat Oncol Biol Phys. 2005;62:1117–24. doi: 10.1016/j.ijrobp.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 8.Sekine I, Kubota K, Tamura Y, et al. Innovator and generic cisplatin formulations: comparison of renal toxicity. Cancer Sci. 2011;102:162–5. doi: 10.1111/j.1349-7006.2010.01764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oike T, Ohno T, Wakatsuki M, et al. The benefit of small bowel and pelvic bone sparing in excluding common iliac lymph node region from conventional radiation fields in patients with uterine cervical cancer: a dosimetric study. J Radiat Res. 2010;51:715–21. doi: 10.1269/jrr.10046. [DOI] [PubMed] [Google Scholar]