Abstract
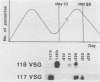
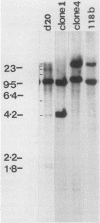
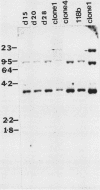
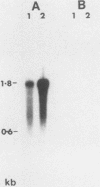
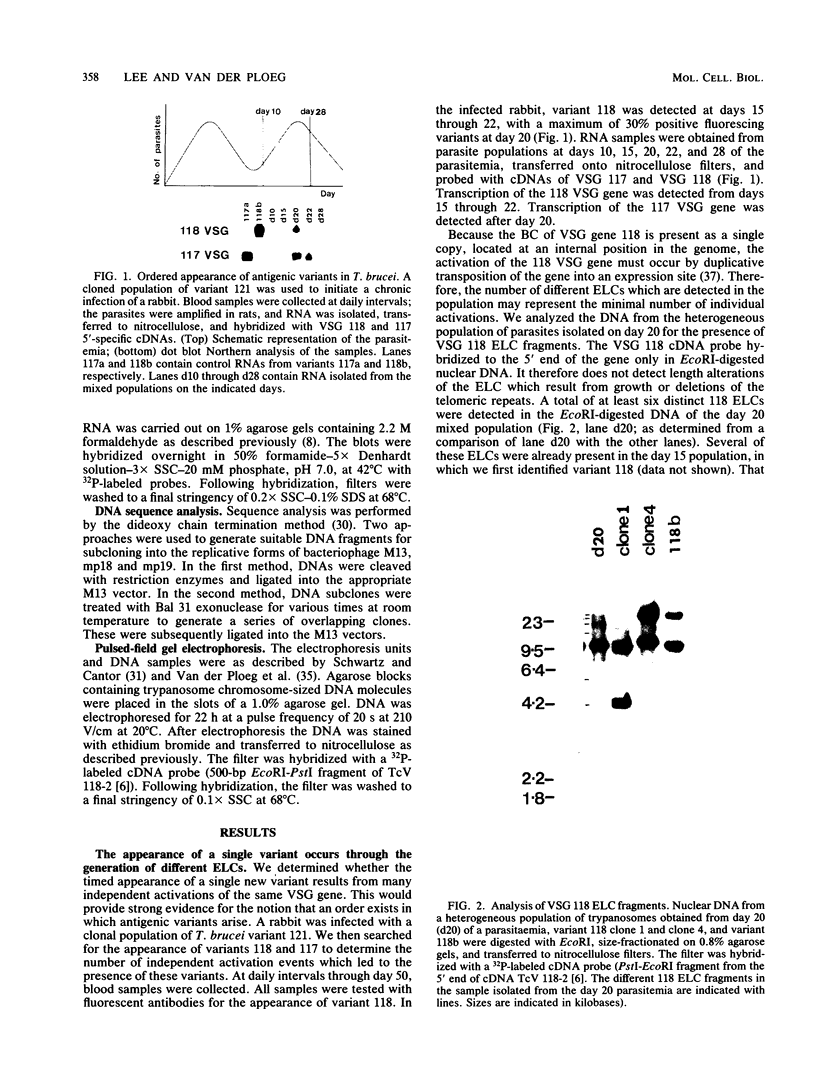
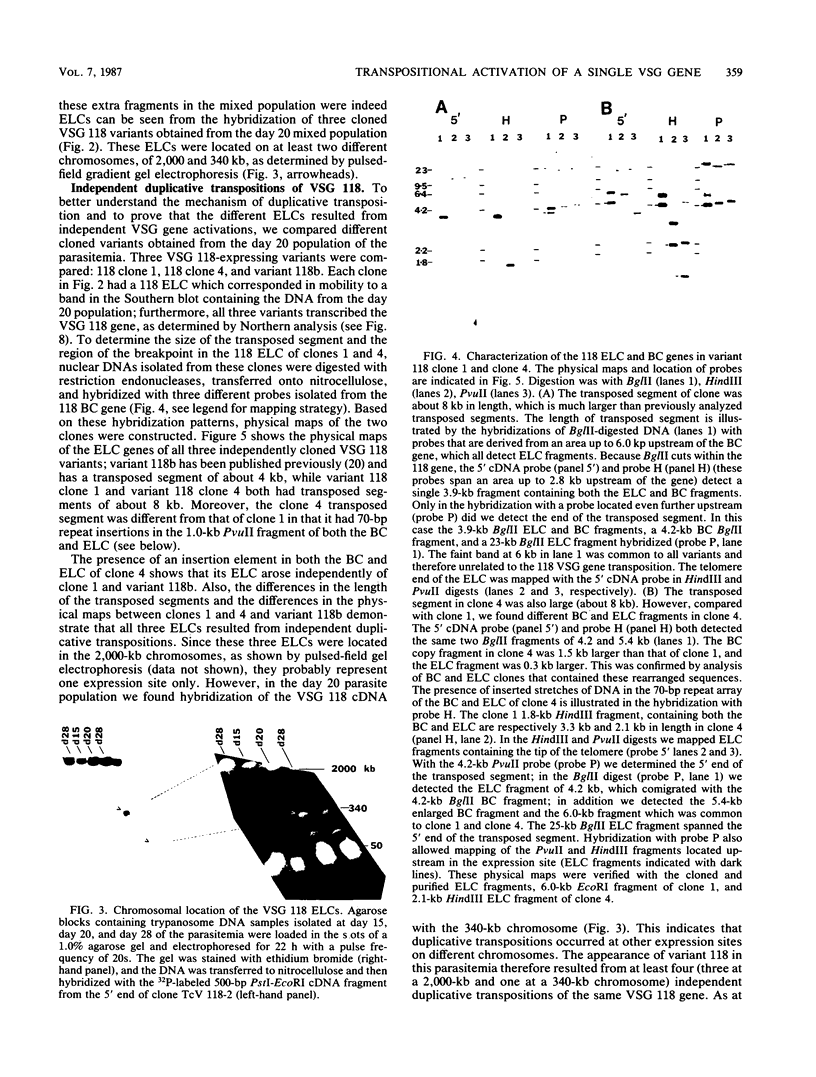
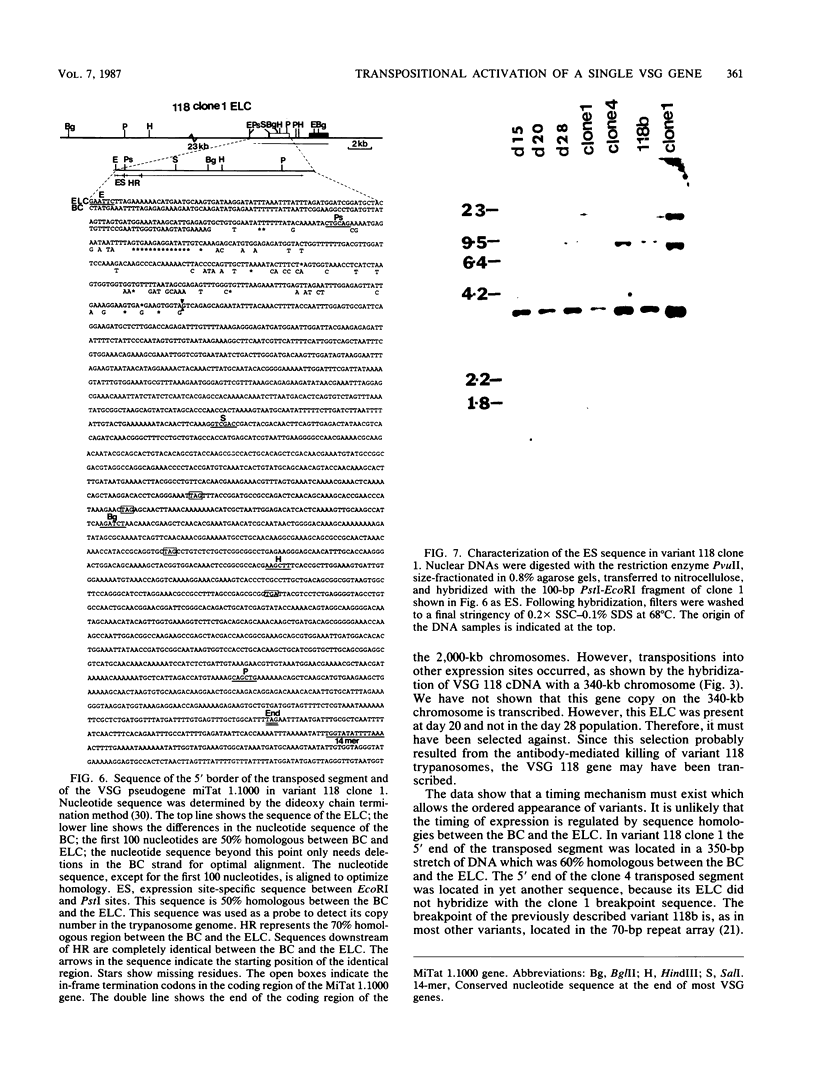
The expression of several surface antigen genes in Trypanosoma brucei is mediated by the duplicative transposition of a basic-copy variant surface glycoprotein (VSG) gene into an expression site. We determined that the appearance of variant 118, in a parasitemia, resulted from at least four independent duplicative transpositions of the same VSG 118 gene. Variants 117 and 118 both appeared at specific periods but resulted from multiple independent activations. Antigenic variants thus occur in an ordered manner. We show that in the duplicative transpositions of VSG genes, the ends of the transposed segments were homologous between the basic copy and the expression site. Sequences other than the previously reported 70-base-pair (bp) repeats could be involved. In one variant, 118 clone 1, the homology was between a sequence previously transposed into the expression site and a sequence located 6 kilobases upstream of the VSG 118 gene. In variant 118b the homology was presumably in 70-bp repeat arrays, while in a third 118 variant yet another sequence was involved. The possibility that the 70-bp repeats are important in the initial steps of the recombinational events was illustrated by a rearrangement involving a 70-bp repeat array. The data provide strong evidence for the notion that gene conversion mediates the duplicative transposition of VSG genes. We discuss a model that explains how the process of duplicative transposition can occur at random and still produce an ordered appearance of variants.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aline R., Jr, MacDonald G., Brown E., Allison J., Myler P., Rothwell V., Stuart K. (TAA)n within sequences flanking several intrachromosomal variant surface glycoprotein genes in Trypanosoma brucei. Nucleic Acids Res. 1985 May 10;13(9):3161–3177. doi: 10.1093/nar/13.9.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Baltz T., Giroud C., Baltz D., Roth C., Raibaud A., Eisen H. Stable expression of two variable surface glycoproteins by cloned Trypanosoma equiperdum. Nature. 1986 Feb 13;319(6054):602–604. doi: 10.1038/319602a0. [DOI] [PubMed] [Google Scholar]
- Bernards A., De Lange T., Michels P. A., Liu A. Y., Huisman M. J., Borst P. Two modes of activation of a single surface antigen gene of Trypanosoma brucei. Cell. 1984 Jan;36(1):163–170. doi: 10.1016/0092-8674(84)90085-0. [DOI] [PubMed] [Google Scholar]
- Bernards A., Kooter J. M., Borst P. Structure and transcription of a telomeric surface antigen gene of Trypanosoma brucei. Mol Cell Biol. 1985 Mar;5(3):545–553. doi: 10.1128/mcb.5.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernards A., Van der Ploeg L. H., Frasch A. C., Borst P., Boothroyd J. C., Coleman S., Cross G. A. Activation of trypanosome surface glycoprotein genes involves a duplication-transposition leading to an altered 3' end. Cell. 1981 Dec;27(3 Pt 2):497–505. doi: 10.1016/0092-8674(81)90391-3. [DOI] [PubMed] [Google Scholar]
- Bernards A., Van der Ploeg L. H., Gibson W. C., Leegwater P., Eijgenraam F., De Lange T., Weijers P., Calafat J., Borst P. Rapid change of the repertoire of variant surface glycoprotein genes in trypanosomes by gene duplication and deletion. J Mol Biol. 1986 Jul 5;190(1):1–10. doi: 10.1016/0022-2836(86)90070-7. [DOI] [PubMed] [Google Scholar]
- Borst P., Bernards A., van der Ploeg L. H., Michels P. A., Liu A. Y., de Lange T., Kooter J. M. The control of variant surface antigen synthesis in trypanosomes. Eur J Biochem. 1983 Dec 15;137(3):383–389. doi: 10.1111/j.1432-1033.1983.tb07840.x. [DOI] [PubMed] [Google Scholar]
- Campbell D. A., van Bree M. P., Boothroyd J. C. The 5'-limit of transposition and upstream barren region of a trypanosome VSG gene: tandem 76 base-pair repeats flanking (TAA)90. Nucleic Acids Res. 1984 Mar 26;12(6):2759–2774. doi: 10.1093/nar/12.6.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capbern A., Giroud C., Baltz T., Mattern P. Trypanosoma equiperdum: etude des variations antigéniques au cours de la trypanosomose experimentale du lapin. Exp Parasitol. 1977 Jun;42(1):6–13. doi: 10.1016/0014-4894(77)90055-8. [DOI] [PubMed] [Google Scholar]
- Cross G. A. Antigenic variation in trypanosomes. Proc R Soc Lond B Biol Sci. 1978 Jun 5;202(1146):55–72. doi: 10.1098/rspb.1978.0057. [DOI] [PubMed] [Google Scholar]
- Cross G. A. Identification, purification and properties of clone-specific glycoprotein antigens constituting the surface coat of Trypanosoma brucei. Parasitology. 1975 Dec;71(3):393–417. doi: 10.1017/s003118200004717x. [DOI] [PubMed] [Google Scholar]
- De Lange T., Borst P. Genomic environment of the expression-linked extra copies of genes for surface antigens of Trypanosoma brucei resembles the end of a chromosome. Nature. 1982 Sep 30;299(5882):451–453. doi: 10.1038/299451a0. [DOI] [PubMed] [Google Scholar]
- Esser K. M., Schoenbechler M. J. Expression of two variant surface glycoproteins on individual African trypanosomes during antigen switching. Science. 1985 Jul 12;229(4709):190–193. doi: 10.1126/science.3892689. [DOI] [PubMed] [Google Scholar]
- Fairlamb A. H., Weislogel P. O., Hoeijmakers J. H., Borst P. Isolation and characterization of kinetoplast DNA from bloodstream form of Trypanosoma brucei. J Cell Biol. 1978 Feb;76(2):293–309. doi: 10.1083/jcb.76.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. A physical map of the DNA regions flanking the rabbit beta-globin gene. Cell. 1977 Oct;12(2):429–439. doi: 10.1016/0092-8674(77)90119-2. [DOI] [PubMed] [Google Scholar]
- Liu A. Y., Van der Ploeg L. H., Rijsewijk F. A., Borst P. The transposition unit of variant surface glycoprotein gene 118 of Trypanosoma brucei. Presence of repeated elements at its border and absence of promoter-associated sequences. J Mol Biol. 1983 Jun 15;167(1):57–75. doi: 10.1016/s0022-2836(83)80034-5. [DOI] [PubMed] [Google Scholar]
- Longacre S., Hibner U., Raibaud A., Eisen H., Baltz T., Giroud C., Baltz D. DNA rearrangements and antigenic variation in Trypanosoma equiperdum: multiple expression-linked sites in independent isolates of trypanosomes expressing the same antigen. Mol Cell Biol. 1983 Mar;3(3):399–409. doi: 10.1128/mcb.3.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels P. A., Bernards A., Van der Ploeg L. H., Borst P. Characterization of the expression-linked gene copies of variant surface glycoprotein 118 in two independently isolated clones of Trypanosoma brucei. Nucleic Acids Res. 1982 Apr 10;10(7):2353–2366. doi: 10.1093/nar/10.7.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels P. A., Liu A. Y., Bernards A., Sloof P., Van der Bijl M. M., Schinkel A. H., Menke H. H., Borst P., Veeneman G. H., Tromp M. C. Activation of the genes for variant surface glycoproteins 117 and 118 in Trypanosoma brucei. J Mol Biol. 1983 Jun 5;166(4):537–556. doi: 10.1016/s0022-2836(83)80283-6. [DOI] [PubMed] [Google Scholar]
- Michels P. A., Van der Ploeg L. H., Liu A. Y., Borst P. The inactivation and reactivation of an expression-linked gene copy for a variant surface glycoprotein in Trypanosoma brucei. EMBO J. 1984 Jun;3(6):1345–1351. doi: 10.1002/j.1460-2075.1984.tb01975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. N., Turner M. J. Analysis of antigenic types appearing in first relapse populations of clones of Trypanosoma brucei. Parasitology. 1981 Feb;82(1):63–80. doi: 10.1017/s0031182000041871. [DOI] [PubMed] [Google Scholar]
- Myler P. J., Allison J., Agabian N., Stuart K. Antigenic variation in African trypanosomes by gene replacement or activation of alternate telomeres. Cell. 1984 Nov;39(1):203–211. doi: 10.1016/0092-8674(84)90206-x. [DOI] [PubMed] [Google Scholar]
- Nasmyth K. A. Molecular genetics of yeast mating type. Annu Rev Genet. 1982;16:439–500. doi: 10.1146/annurev.ge.16.120182.002255. [DOI] [PubMed] [Google Scholar]
- Pays E., Van Assel S., Laurent M., Darville M., Vervoort T., Van Meirvenne N., Steinert M. Gene conversion as a mechanism for antigenic variation in trypanosomes. Cell. 1983 Sep;34(2):371–381. doi: 10.1016/0092-8674(83)90371-9. [DOI] [PubMed] [Google Scholar]
- Pays E., Van Assel S., Laurent M., Dero B., Michiels F., Kronenberger P., Matthyssens G., Van Meirvenne N., Le Ray D., Steinert M. At least two transposed sequences are associated in the expression site of a surface antigen gene in different trypanosome clones. Cell. 1983 Sep;34(2):359–369. doi: 10.1016/0092-8674(83)90370-7. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Schwartz D. C., Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984 May;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Bernards A., Rijsewijk F. A., Borst P. Characterization of the DNA duplication-transposition that controls the expression of two genes for variant surface glycoproteins in Trypanosoma brucei. Nucleic Acids Res. 1982 Jan 22;10(2):593–609. doi: 10.1093/nar/10.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Bernards A., Rijsewijk F. A., Borst P. Characterization of the DNA duplication-transposition that controls the expression of two genes for variant surface glycoproteins in Trypanosoma brucei. Nucleic Acids Res. 1982 Jan 22;10(2):593–609. doi: 10.1093/nar/10.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Cornelissen A. W., Michels P. A., Borst P. Chromosome rearrangements in Trypanosoma brucei. Cell. 1984 Nov;39(1):213–221. doi: 10.1016/0092-8674(84)90207-1. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Schwartz D. C., Cantor C. R., Borst P. Antigenic variation in Trypanosoma brucei analyzed by electrophoretic separation of chromosome-sized DNA molecules. Cell. 1984 May;37(1):77–84. doi: 10.1016/0092-8674(84)90302-7. [DOI] [PubMed] [Google Scholar]
- Vickerman K. Antigenic variation in trypanosomes. Nature. 1978 Jun 22;273(5664):613–617. doi: 10.1038/273613a0. [DOI] [PubMed] [Google Scholar]
- Vickerman K. On the surface coat and flagellar adhesion in trypanosomes. J Cell Sci. 1969 Jul;5(1):163–193. doi: 10.1242/jcs.5.1.163. [DOI] [PubMed] [Google Scholar]
- Williams R. O., Young J. R., Majiwa P. A. Genomic rearrangements correlated with antigenic variation in Trypanosoma brucei. Nature. 1979 Dec 20;282(5741):847–849. doi: 10.1038/282847a0. [DOI] [PubMed] [Google Scholar]
- Young J. R., Donelson J. E., Majiwa P. A., Shapiro S. Z., Williams R. O. analysis of genomic rearrangements associated with two variable antigen genes of Trypanosoma brucei. Nucleic Acids Res. 1982 Feb 11;10(3):803–819. doi: 10.1093/nar/10.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]