Abstract
The purpose of this review of clinical guidelines and best practices literature is to suggest prevention options and a treatment approach for intermittent catheter users that will minimize urinary tract infections (UTI). Recommendations are based both on evidence in the literature and an understanding of what is currently attainable within the Alberta context. This is done through collaboration between both major tertiary care centres (Edmonton and Calgary) and between various professionals who regularly encounter these patients, including nurses, physiatrists and urologists.
Bladder management in the context of a spinal cord injury
A pervasive issue in most neurologic diseases (Parkinson’s disease, multiple sclerosis, diabetes, stroke and spinal cord injury [SCI]) is bladder dysfunction (neurogenic bladder) or neurogenic lower urinary tract dysfunction (NLUTD). These dysfunctions result in symptoms of urgency, increased daytime and nighttime frequency, urinary retention, incontinence and urinary tract infection (UTI). Treatment involves non-invasive continence management through toileting, fluid management, containment products, medications and intermittent catheterization. UTIs have substantial negative physical and psychological effects and are a major burden on the health care system. Frequent UTI in the SCI population is defined clinically as 3 or more infections per year where both symptoms of infection and a positive urine culture are present. Despite the variety of treatment approaches to emptying the neurogenic bladder, UTI remains a complex and challenging clinical problem.
Intermittent catheterization (IC) is recognized as the gold standard for treating voiding disorders associated with the neurogenic bladder.1,2 Self IC is the preferred method of bladder emptying because it minimizes the risk of infection and decreases the risk of calcium phosphate and struvite stone formation.2 Intermittent catheterization involves the use of a short (15 to 40 cm) flexible catheter which is inserted up the urethra into the bladder to drain urine. IC has contributed to increased life expectancy of people with SCI. This was realized with the introduction of clean intermittent catheterization (CIC), which was simpler, faster and less costly than the original sterile method; people with SCI can benefit from CIC.1
Clarity is often lacking in the literature regarding which type of IC technique is being used by study participants. In this article, CIC refers to a method of catheterization where the hands and urethral area are cleaned with soap and the catheter is either washed or newly removed from sterile packaging. In sterile IC the urethral area is cleaned with an antiseptic. Sterile gloves are worn and a sterile field is maintained. A sterile single-use all-in-one collection device may or may not be used as part of this technique after cleaning the urethral area.
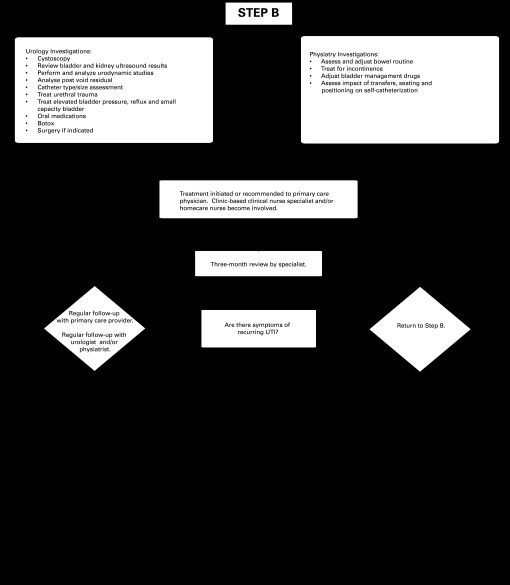
In Alberta, an individual’s choice of sterile or clean method depends on a number of factors, one of which is the availability of funding to purchase the products. See Fig. 1a, Fig. 1b for an assessment and treatment approach to urinary tract infection that is supported by current evidence and clinical best practice in Alberta. Alberta Aids to Daily Living (AADL) is a provincially funded program that provides financial assistance to Albertans with a long-term disability, chronic illness or terminal illness to buy medical equipment and supplies. AADL covers the cost of one sterile polyvinyl chloride (PVC) type catheter per day. Users are directed to wash the catheter and store it for up to 4 subsequent catheterizations. Individuals with SCI and some healthcare professionals have raised questions about whether the risk of UTI is increased when catheters are reused.
Fig. 1a.
A flow diagram of evidence-based assessment and treatment in the Alberta context. UTI: urinary tract infection.
Fig. 1b.
Current treatments for recurring bladder infections: Information for individuals with neurogenic bladder due to spinal cord injury or other causes. UTI: urinary tract infection.
The purpose of this review of clinical guidelines and best practices literature is to suggest prevention options and a treatment approach for intermittent catheter users that will minimize UTI. Recommendations are based both on evidence in the literature and an understanding of what is currently attainable within the Alberta context, through collaboration between both major tertiary care centres (Edmonton and Calgary) and between various professionals who regularly encounter these patients, including nurses, physiatrists and urologists. Key references used to prepare this document included Canadian sources, such as SCIRE (Spinal Cord Injury Rehabilitation Evidence), along with the American Urological Association (AUA) and European Urological Association (EUA) documents on the topic and resources from Paralyzed Veterans of America. Currently, there are no documents on neurogenic bladder management with the Canadian Urological Association (see Table 1 for more resources). Finally, the impetus for preparing these protocols arose due to concerns identified by a survey of individuals with SCI regarding perceived gaps in knowledge and practice among caregivers and physicians about SCI and UTI prevention and management.
Table 1.
Additional resources
| American Urological Association (AUA) http://www.auanet.org/content/homepage/homepage.cfm |
| European Urological Association (EUA) http://www.uroweb.org/ |
| Paralyzed Veterans of America http://www.pva.org/site/c.ajIRK9NJLcJ2E/b.6305401/k.BCBB/Home.htm |
| SCIRE Rehabilitation Evidence http://www.scireproject.com/rehabilitation-evidence/bladder-management |
| Geneva Foundation for Medical Education and Research http://www.gfmer.ch/Guidelines/Urinary_tract_infections_urinalysis/Catheter-associated-urinary-tract-infection.htm |
| Spinal Cord Connections and SCI University http://www.spinalcordconnections.ca/default.aspx |
| The Canadian Paraplegic Association (Alberta) www.cpa-ab.org
|
What constitutes UTI in the spinal cord injured population?
In the young able-bodied adult with a UTI, protocols for treatment and follow-up are well-documented. However, for those with a neurogenic bladder, diagnosis and follow-up procedures are complicated by the presence of comorbid conditions, decreased pain sensation or other potential sources of infection. A key message is that asymptomatic bacteriuria is not a disease and that the presence of bacteria in the urine is not unusual in the intermittent catheter user. Bacteriuria may be a sign of poor hydration or infrequent voiding (catheterizing) and can often be addressed by changing hydration and voiding/bladder management routines.2,3
UTI is defined by the presence of physical symptoms and high amounts of bacteria in the urine. Neither urine odour nor the presence of pyuria (i.e., pus, white blood cells or leukocytes) indicates a sufficient volume of harmful bacteria to constitute an infection. Individuals can be free of symptoms despite high levels of bacteria in the urine.
A treatable level of infection requires one or more of the following physical symptoms: new onset or worsening of fever, rigors; altered mental status; malaise or lethargy with no other identified cause; acute hematuria; pelvic discomfort; discomfort or pain over the kidney or bladder or during urination; development of, or increase of urinary incontinence; increased frequency of catheterization/voiding; increased spasticity; autonomic dysreflexia; and a sense of unease. It has been shown that 60% of individuals using IC to empty their bladders are chronically colonized (that is, they have a significant quantity of bacteria in their urine), however, without the physical symptoms to accompany positive urinalysis, they are considered “asymptomatic” and are not candidates for antibiotics.4
Where one or more of the above-mentioned symptoms exist, the urine should be tested by microscope (microscopy), urine dip (macroscopy) and by a culture and sensitivity (C&S) test. Numbers of bacteria are reported in colony forming units per litre (cfu/L). Urine cultures show the type and number of bacteria. Sensitivity indicates to which antibiotic the bacteria are “sensitive.”
Tests for bacteria or pyuria do not establish a diagnosis of UTI, but are important aspects of the diagnosis when symptoms are present.3 Both the SCIRE5 and the Scottish Intercollegiate Guidelines Network (SIGN)3 indicate that ≥108 (cfu/L) represents significant bacteriuria and that antibiotics should be considered when symptoms are present. However, both groups note that false positive tests can occur depending on the method of urine sample collection. The only sample that should be considered for urinalysis is one that is collected with a new, sterile catheter, drained into a sterile container and taken to the laboratory immediately. When these conditions are met, SCIRE5 suggests that ≥105 cfu/L in an intermittent catheter specimen represents sufficient bacteriuria to consider antibiotic treatment (with symptoms present). In clean-voided specimens from catheter-free men using condom catheters, ≥107 (cfu/L) is a sufficient level.
How should UTI be treated in the SCI population?
Treatment with antibiotics is initiated on the basis of a positive urine C&S and the presence of clinical symptoms as described above.6 It is generally understood that treating asymptomatic colonization of the urine with antibiotics does not benefit the patient, except in patients with urea-splitting bacteria (e.g., proteus), which can help to prevent bladder calculi (bladder stones).
Which antibiotics should be considered?
The oral drugs often recommended, depending on bacterial sensitivity, are ciprofloxacin or ofloxacin administered over either a 3- or 7-day treatment regimen. In clinical practice, a 7-day treatment course is often used due to the complex and recurrent nature of the UTI in those with NLUTD. (Recurrent UTI is defined clinically as 3 or more UTIs per year and should be treated as a complicated infection. In this case, antibiotic treatment for a course less than 7 days is not recommended.) Ciprofloxacin has also been studied in this population with a 14-day administration period with some indications of a reduced re-infection rate.5 Ofloxacin has higher bladder bacterial biofilm eradication rates than trimethoprim-sulfamethoxazole.
In Alberta, resistant patterns for all of these antibiotics are becoming increasingly common.5 Norfloxacin places an individual at risk for subsequent bacterial resistance and individuals need to be monitored.5 High rates of bacterial resistance in Alberta to both amoxicillin and trimethoprim-sulfamethoxazole make them poor first-line choices. Amoxicillin in combination with clavulanic acid, however, is a reasonable choice, as the potassium clavulanate imparts increased efficacy and lower resistance rates.
In certain cases where quinolone drugs cannot be used and/or resistant organisms are present, cephalosporins, such as cephalexin (first generation) and cefuroxime (second generation) may be used. Nitrofurantoin is acceptable for simple bladder infections, however it is not recommended for more serious deep tissue infections, like prostatitis or pyelonephritis, because it is exclusively excreted in the urine with no tissue penetration. For complex UTI, aminoglycoside antibiotics may be administered intravenously. Occasionally, intermittent bladder irrigation with neomycin/polymyxin has been used, but the evidence to support this practice is weak.5
What is the relationship between type and method of catheterization and UTI?
Three types of intermittent catheter products are available for CIC: standard PVC catheters (pre-lubricated or not); single-use hydrophilic-coated catheters; and all-in-one urine collection systems (which may be pre-lubricated or hydrophilic-coated). Of these three types, only the standard PVC catheters can be reused after being washed thoroughly with soap and water. Hydrophilic-coated and catheters with an attached urine collection system may only be used once and then discarded.
There is some recent evidence involving hospitalized individuals. In these patients, it was found that UTIs are lower and antibiotic treatment is reduced when single-use pre-lubricated or hydrophilic catheters are used as compared to single-use sterile non-hydrophilic catheters.1 Pre-lubricated catheters may have the added benefit of reducing the urethral microtrauma compared to PVC catheters; however, the clinical relevance of this finding is unclear, particularly for community dwelling (i.e., not hospitalized) individuals who independently perform self-catheterization.5
Social and environmental factors play a role in UTI, as does choice of catheter. For example, the method and type of catheterization must be matched to patients’ ability to maintain cleanliness and to their access to facilities that enable them to catheterize frequently and in a sanitary manner. Financial resources also play a role. For individuals with limited access to wheelchair accessible bathroom facilities and running water throughout the day, single-use catheters may be more appropriate.
The current literature and the most recent Cochrane systematic review7 indicate that there is inadequate evidence to state with certainty that sterile single-use IC or sterile hydrophilic-coated catheters are better at reducing UTI than multi-use PVC catheters. According to the Cochrane Review,7 there are no definitive studies showing the incidence of UTI is improved with any catheter technique, type or strategy.8 However, the EAU Guidelines9 state that sterile IC significantly reduces the risk of UTI and/or bacteriuria compared with clean IC.10–12 Although Wyndaele is quoted as stating, “Based on the current data, it is not possible to state that one catheter method is better than another and further research on the topic is strongly recommended.”10 Guttmann is clearly in favour of sterile IC based on in-patient research with the endpoint of urine sterility on discharge from hospital.12
It must be noted, however, that until recently, studies on NLUTD were limited by sample size, sample heterogeneity and imprecise outcome measures, particularly with respect to defining UTI.5 SCIRE also indicates that both sterile (single-use) and clean (multi-use) approaches to IC have similar rates of UTI, but the studies upon which this conclusion is based were conducted primarily with an in-patient rehabilitation population.5
At present, there is no gold standard for cleaning reusable PVC catheters for IC, but the practice typically recommended by clinicians in Alberta is to clean them thoroughly with liquid dish soap (i.e., Sunlight, The Sun Products Canada Corporation, Etobicoke, ON), air dry and store them in a clean plastic bag or container. Further randomized controlled trials are desperately needed to provide answers to this important clinical question.13
Why should we review product, technique and hygiene with the patient?
A variety of bladder management techniques can reduce UTI risk in community-dwelling persons with SCI, although limited evidence exists as to which approach is the most effective (Table 2).5 Poor technique includes inadequately cleansing catheters, inadequate perineal hygiene or hand washing, excessively long or too short intervals between catheterization, or the inability to insert the catheter without contaminating it in the process.
Table 2.
Current treatments for recurring bladder infections: Information for individuals with neurogenic bladder due to spinal cord injury or other causes
| Treatment | Description |
|---|---|
| Cranberry, d-mannose, vitamin C | Many people use these supplements to prevent and treat UTI. Taken orally, it is believed that they reduce bacterial growth by acidifying the urine and preventing bacteria from adhering to the bladder wall. |
| Oral antibiotics and other medications | Antibiotics are commonly used to treat UTI. In some cases, they may be used over the long-term, although the effectiveness of this approach is not well supported by research. |
| Anti cholinergic medications (ditropan, detrol, vesicare) | Medications used to improve bladder function and muscle tone, and to treat problems with urinary frequency, urgency, and incontinence. |
| Onabotulinum toxin | Onabotulinum toxin is a long-acting nerve signal disruptor that reduces muscle tension and spasms in the bladder wall and bladder sphincter muscle. Onabotulinum toxin injections can reduce spasticity, incontinence, reflux, urgency and frequency of urination. |
| Bacterial interference | Bacterial interference involves putting a benign (harmless) strain of E-coli bacteria into the bladder to compete with and reduce the growth of harmful bacteria. |
| Sterile intermittent catheterization | Sterile intermittent catheterization is a method of emptying the bladder using a single-use sterile catheter rather than cleaning and reusing it. |
| Sterile pre-lubricated hydrophilic catheter | Using this kind of catheter has been shown to cause less damage to the urethra than standard vinyl catheters, however there is no clear evidence that reducing damage to the urethra also reduces the chance of getting a UTI. |
| Sterile closed system catheter | A sterile closed system catheter is a catheter with a bag attached. Some also include an introducer tip (a small tube that is inserted into the urethra and through which the catheter slides). There is some evidence that an introducer tip can reduce the movement of bacteria from the urethra into the bladder. It is unclear, however, if the bacteria found in the urethra cause UTI. |
| Foley catheter | A Foley catheter consists of a catheter and a small “balloon” that are inserted through the urethra to the bladder. The balloon is filled with water which then holds the catheter in place. Foley catheters are sometimes called “indwelling” catheters because they can stay in place for several days or weeks without needing to be changed. |
| Suprapubic catheter | Suprapubic catheters are inserted surgically through the abdomen and into the bladder. They drain urine through a small hole in the abdomen. |
| Mitrofanoff procedure | This is a surgical procedure in which the appendix is used to create a channel between the bladder and a small opening in the abdomen (called a stoma). Urine is drained through the opening using intermittent catheterization. |
| Bladder augmentation | Bladder augmentation involves surgically enlarging the bladder with tissue grafts (called anastomosis) from the small intestine (ileum). |
| Ileal conduit urinary diversion | This is a surgical procedure that involves detaching the ureters from the bladder and connecting them to a section of the intestine (called ureteroenteric anastomosis). The urine then flows into the small intestine (ileum). The end of the ileum is brought out through a small opening (stoma) in the abdominal wall. Urine drains out into a collection bag. |
| Cystectomy | Cystectomy is a surgical procedure in which the bladder is removed. Urine is diverted through a small opening (stoma) in the abdomen. The urine travels to the hole through a channel called an ileal conduit (see “Ileal conduit urinary diversion” above). |
UTI: urinary tract infection.
If UTIs persist and there is an indication of consistently high bladder volumes (generally in excess of 500 mL), adjust fluid intake and/or increase the frequency of IC.14 Generally in the NLUTD population, catheterization with a 12 to 14 French catheter is needed 4 to 6 times per day. Less frequent catheterization results in higher bladder-storage volumes and an increased risk of UTI.3 More frequent catheterizations increase the risk of cross-infection.3 If the UTIs persist and a consistent catheterization routine (with respect to frequency and technique) and adequate hydration are not possible, a less demanding bladder management technique should be considered, such as using a condom catheter with external collection device (ECD) or an indwelling urethral Foley or suprapubic catheter.14
The role of indwelling catheterization
For those with unacceptable infections with CIC or for those who do not have the functional ability to self-catheterize (and do not have access to a caregiver), Foley catheters are first considered as they are easy to insert and care for, and provide exceptional convenience for most individuals. For some, however, urethral erosion or patient preference results in the choice of a suprapubic tube, which is more invasive to insert initially. Also, if a suprapubic tube accidentally falls out without being replaced expediently, the tract through the abdominal wall actually closes, necessitating another invasive procedure. In terms of infection risk, the EUA also cautions that indwelling transurethral catheterisation and suprapubic cystostomy are to be avoided because they are risk factors for UTI and have significant long-term complications.3 On choice of indwelling catheter material, the EUA states that silicone catheters have advantages over latex catheters.3 Medications to reduce bladder overactivity may also address frequent UTI, with both CIC and indwelling catheterization.
Why are annual evaluations suggested?
The Consortium of Spinal Cord Medicine (CSCM) and the AUA update on urological care in the out-patient NLUTD indicates that best practice is to evaluate the kidneys (upper urinary tract) and bladder (lower urinary tract) in individuals who perform intermittent catheterization.2,15 In general, the recommendation is for urodynamic studies (UDS) to be conducted to assess bladder capacity and pressure, bladder and sphincter functioning, and the presence of reflux. Upper urinary tract evaluations include tests that evaluate function (such as nuclear medicine renal scans) and tests that evaluate anatomy (such as ultrasound and computed tomography [CT] scans). Ultrasound scans are frequently used to screen the upper tract because they are not user-dependent, do not have a risk of allergic reactions, do not require bowel prep, and cause much less radiation exposure than a CT scan.
Unfortunately, history, level of injury and signs and symptoms alone are not enough to determine if a person is experiencing high bladder (intravesical) pressures or reflux, which may cause frequent UTI as well as renal complications over time.15 To evaluate bladder pressures and voiding dynamics, UDS are indicated. UDS may be done with or without video fluoroscopy (video UDS or VUDS). The advantage of video is the ability to simultaneously study function and anatomy, allowing for improved diagnosis of findings, such as vesicoureteral reflux from high pressures, external sphincter dyssynergia and bladder diverticula. UDS should be done during the first year after injury, as well as after any change in bladder management or symptoms.
The lower urinary tract is also assessed by ultrasound to measure residual volumes after voiding or self-catheterization and to highlight any abnormalities of the urinary tract structure, such as thickening of the bladder wall or the presence of diverticula or bladder calculi.15 Cystoscopy is indicated when a UTI recurs, bladder function has changed, cancer concern arises (e.g., significant haematuria) or if anatomic abnormalities are suggested by imaging. Regular cystocopy and bladder biopsy are also recommended to screen for malignancy in long-term indwelling catheter users.16
If a recurrent UTI develops, the patient needs renal and bladder ultrasound tests and blood work (or have results reviewed from previous tests) to identify significant changes in the structure and function of the urinary tract. Hydration, catheterization technique and hygiene should have already been discussed with the patient; if this is not the case, it is a priority to refer the patient to a home care or a continence clinic to receive this information.14 If not already done, the patient should also be referred to a urologist or rehabilitation physician.
Are other preventive or adjunctive therapies effective?
Cranberry juice and tablets
It is uncertain if cranberry is effective in preventing UTIs in people with SCI.5 It is believed that drinking cranberry juice reduces the proliferation of bacteria by acidifying the urine;17 however there is no agreement on the amount of cranberry juice or tablets required to achieve this effect, or if a single dose or multiple doses are necessary. Routine use of cranberry juice in the concentration required to achieve a clinical effect may be contraindicated in patients prone to obesity or oxalate or uric acid calculi (bladder stones). Cranberry juice is also not recommended in patients on anticoagulation therapy. Although cranberry juice is not for everyone, it is a relatively safe and natural remedy, which might provide symptomatic and therapeutic relief for patients with UTIs or excessive mucus formation and high glomerular filtration rate.17,18
D-mannose
D-mannose is thought to be effective in dislodging E. coli bacteria from the bladder wall and may improve many UTIs caused by this bacterium, including those in individuals with SCI. D-mannose is a naturally occurring sugar that is similar in structure to glucose (a component of table sugar). Because the body metabolizes only small amounts of d-mannose and excretes the rest in the urine, it does not interfere with blood-sugar regulation, even in diabetics. D-mannose does not kill any bacteria, but simply helps to displace them. Unfortunately, evidence for the use of d-mannose to treat bacteria in the urine is weak and based on studies in rats which were performed in the 1980s.19
Vitamin C
Dietary supplementation with vitamin C is frequently recommended as a way to reduce UTIs by increasing urine acidity; however, there are no clinical studies which demonstrate effectiveness of vitamin C in improving symptoms or UTI incidence.
Portable bladder scanners
Portable bladder scanners are a recent development and have been studied as a method of self-monitoring in an attempt to determine self-catheterization frequency.6 Although this approach has promise, it is expensive and has not been demonstrated to reduce the rate of UTIs.6
Bacterial interference
With the bacterial interference approach to combating UTI, innocuous bacteria are allowed to colonize the bladder, which in turn, inhibits colonization of the bacteria that cause the symptomatic infection.5 Any antibiotic treatment during colonization would kill off the protective bacteria.
Conclusion
Urine colonization (asymptomatic bacteriuria) is common in individuals with SCI. In the absence of symptoms, treatment should not be initiated. If symptomatic UTIs persist after treatment, individuals require full assessment by a urologist to rule out bladder pathology that may be contributing to problems. Annual monitoring of the structure and function of the urinary tract is recommended for people with neurogenic bladder with support from specialist nurses and physicians as problems arise.
IC is the preferred method of emptying the neurogenic bladder for those with the capability to do so and has the support of over 50 years of research and clinical practice. Unfortunately there is limited research evidence to support selecting one type of catheter over another7 or recommending single-use catheterization over multi-use catheters. Although catheters are reused often in Alberta, the research is inconclusive whether clean versus sterile catheter use is best practice. Definitive evidence on the ideal method of cleaning and storage is unavailable.7
According to the Cochrane Review,7 available data on IC do not provide convincing evidence that any specific technique (sterile or clean), catheter type (coated or uncoated), method (single-use or multiple-use), person (self or other), or strategy is better than any other for all clinical settings.8
Evidence for many of the adjunctive therapies used in UTI prevention is weak. We need more studies to answer these and other questions.
Acknowledgments
This report is a project of the Alberta Spinal Cord Injury Initiative, a collaborative effort by Albertans with SCI, service providers, researchers and decision-makers committed to improving the lives of people affected by SCI and similar physical disabilities. We gratefully acknowledge the Government of Alberta who recognized the value of the vision for the Alberta SCI community.
Special thanks: Special thanks go to the working group members who spearheaded the development of this paper. They are: Lauran Chittim (Alberta Aids to Daily Living), Teren Clarke (Canadian Paraplegic Association (Alberta)), Guy Coulombe (Canadian Paraplegic Association (Alberta)), Tim Hill (Canadian Paraplegic Association (Alberta)) (project coordinator), Bev Matthiessen (Alberta Committee of Citizens with Disabilities), Katherine Moore (University of Alberta); Raj Parmar (Foothills Medical Centre).
Footnotes
Competing interests: None declared.
This paper has been peer-reviewed.
References
- 1.De Ridder DJMK, Everaert K, Garcia Fernandez L, et al. Intermittent catheterisation with hydrophilic-coated catheters (SpeediCath) reduces the risk of clinical urinary tract infection in spinal cord injured patients: A prospective randomised parallel comparative trial. Eur Urol. 2005;48:991–5. doi: 10.1016/j.eururo.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 2.DeFade B, Kennelly M, Deem S. Urological Care of the Neurologically Impaired Patient in the Outpatient Setting. AUA Update Series. 2011;30 Lesson 4. [Google Scholar]
- 3.Scottish Intercollegiate Guidelines Network (SIGN) Edinburgh: SIGN; 2012. Management of suspected bacterial urinary tract infection in adults. (SIGN publication no. 88). [July 2012]. http://www.sign.ac.uk. Accessed February 25, 2013. [Google Scholar]
- 4.Cain M, King S, Rink R. Managing recalcitrant urinary tract infections in patients with bladder augmentation. Eur Urol Rev. 2008;3:127–8. [Google Scholar]
- 5.Wolfe DL, Ethans K, Hill D, et al. Bladder Health and Function Following Spinal Cord Injury. In: Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, Connolly SJ, Mehta S, Sakakibara BM, editors. Spinal Cord Injury Rehabilitation Evidence. Vancouver, BC: 2010. 2010. pp. 1–19. Version 3.0. [Google Scholar]
- 6.Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 international clinical practice guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:625–63. doi: 10.1086/650482. [DOI] [PubMed] [Google Scholar]
- 7.Moore K, Fader M, Getliffe K. Long-term bladder management by intermittent catheterisation in adults and children. Cochrane Database Syst Rev. 2007;17:CD006008. doi: 10.1002/14651858.CD006008.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Newman DK, Willson MM. Review of intermittent catheterization and current best practices. Urol Nurs. 2011;31:12–28. [PubMed] [Google Scholar]
- 9.Stohrer M, Blok B, Castro-Diaz D, et al. EAU Guidelines on Neurogenic Lower Urinary Tract Dysfunction. Eur Urol. 2009;56:81–8. doi: 10.1016/j.eururo.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 10.Wyndaele JJ, Castro D, Madersbacher H, et al. Neurologic urinary and faecal incontinence. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence. Vol. 2. Plymouth, UK: Health Publications; 2005. pp. 1059–162. [Google Scholar]
- 11.Wyndaele JJ. Complications of intermittent catheterization: their prevention and treatment. Spinal Cord. 2002;40:536–41. doi: 10.1038/sj.sc.3101348. [DOI] [PubMed] [Google Scholar]
- 12.Guttmann L, Frankel H. The value of intermittent catheterisation in the early management of traumatic paraplegia and tetraplegia. Paraplegia. 1966;4:63–84. doi: 10.1038/sc.1966.7. [DOI] [PubMed] [Google Scholar]
- 13.Cardenas DD, Moore KN, Dannels-McClure A, et al. Intermittent catheterization with a hydrophilic-coated catheter delays urinary tract infections in acute spinal cord injury: A prospective, randomized, multicenter trial. PM R. 2011;3:408–17. doi: 10.1016/j.pmrj.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Consortium for Spinal Cord Medicine . Bladder management following spinal cord injury: What you should know. Washington DC: Paralyzed Veterans of America; 2010. www.pva.org. Accessed February 25, 2013. [Google Scholar]
- 15.Consortium of Spinal Cord Medicine (2006) Bladder management for adults with spinal cord injury: A clinical practice guideline for health-care providers. J Spinal Cord Med. 2006;29:527–73. [PMC free article] [PubMed] [Google Scholar]
- 16.Casey RG, Cullen IM, Crotty T, et al. Intermittent self-catheterization and the risk of squamous cell cancer of the bladder: An emerging clinical entity? Can Urol Assoc J. 2009;3:E51–4. doi: 10.5489/cuaj.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geng V, Emblem E, Gratzl S, et al. Urethral catheterization, section 2, male, female and paediatric intermittant catheterization. Arnhem: European Association of Urology Nurses; 2006. http://www.uroweb.org/fileadmin/user_upload/EAUN/EAUN1.pdf. Accessed February 25, 2013. [Google Scholar]
- 18.Hess MJ, Hess PE, Sullivan MR, et al. Evaluation of cranberry tablets for the prevention of urinary tract infections in spinal cord injured patients with neurogenic bladder. Spinal Cord. 2008;46:622–6. doi: 10.1038/sc.2008.25. Epub 2008 Apr 8. [DOI] [PubMed] [Google Scholar]
- 19.Michaels E, Chmiel J, Plotkin B, et al. Effect of d-mannose and d-glucose on escherichia coli bacteriuria in rats. Urol Res. 1983;11:97–102. doi: 10.1007/BF00256954. [DOI] [PubMed] [Google Scholar]