Abstract
The retrieval of a memory places it into a plastic state, as a result of which the memory can be disrupted or even enhanced by experimental treatment. This phenomenon has been conceptualised within a framework of memories being reactivated and then reconsolidated in repeated rounds of cellular processing. The reconsolidation phase has been seized upon as critical for the understanding of memory stability, and more recently as a potential therapeutic target in the treatment of disorders such as post-traumatic stress and drug addiction. However, little is known about the reactivation process, nor what might be the adaptive function of retrieval-induced plasticity. Reconsolidation has long been proposed to mediate memory updating, but only recently has this hypothesis been supported experimentally. Here, the adaptive function of memory reconsolidation is explored in more detail, with a strong emphasis on its role in updating memories to maintain their relevance.
Introduction
An introspective analysis of memory clearly suggests that human memories are neither constant in content, nor of fixed strength. Memory retrieval is often triggered by a similar experience that subsequently intermeshes with and modifies future recollections. The reconstructive nature of memory retrieval and memory malleability in general is recognised within the domains of cognitive psychology and neuroscience [1, 2]. However, this cognitive level of analysis is not immediately reconciled with the molecular neurobiological understanding of learning and memory. The interest generated by the study of synaptic plasticity in both hippocampal slices and simpler invertebrate models has led to a surge in knowledge at the cellular level. While mammalian behavioural studies are equally plentiful, the neurobiological analysis has largely been conducted within the theoretical framework of memory consolidation, the time-dependent stabilisation of synaptic plasticity [3], an implicit assumption of which is that memories are subsequently stable and fixed.
The phenomenon of “memory reconsolidation” [4] has the potential to bridge the divide between cellular and cognitive accounts of long-term memory. I will not provide a comprehensive review of the extensive literature on the cellular and behavioural mechanisms of memory reconsolidation (reviews of this nature are relatively plentiful, e.g. [5-10]). Instead, I will focus on a particular viewpoint, namely that reconsolidation serves to enable the updating of memories, ultimately to maintain their predictive and adaptive relevance. Therefore, it is important to note that reconsolidation does not simply represent an automatic restabilisation of a retrieved memory, but is a special process that presents an opportunity for adaptive modification. Moreover, the numerous negative findings in relation to reconsolidation can be argued to result from the use of experimental conditions that fail to engage a memory updating process (thereby explaining the existence of boundary conditions on memory reconsolidation). Given the emphasis on normal learning and memory processes, it is also possible to integrate ideas on reconsolidation and memory updating with traditional learning theories, and I will focus on the potential role of surprise and prediction error. Finally, I will conclude with the broad implications of a memory updating hypothesis of reconsolidation, both in terms of how reconsolidation itself might be viewed as an epiphenomenom, and the conclusion that experimental amnesia must reflect storage impairments.
Reconsolidation and memory updating
The reconsolidation phenomenon is an increasingly studied area in learning and memory, and describes a fundamental finding that the retrieval (or “reactivation”) of a previously stable memory renders that memory vulnerable to the disruptive effects of amnestic agents. Thus retrieval returns memories to a plastic state. While much has been learned regarding the mechanisms of reconsolidation, the search for an endogenous function of the process remains a fundamental issue. As noted by Dudai [11], reconsolidation might not serve any function, especially given the remote chance of encountering in real life the kinds of agents used experimentally to induce amnesia. Nevertheless, interference is a potent cause of amnesia in reconsolidation studies [12-14], and stress can also be detrimental to reactivated memories [15, 16], suggesting that retrieval-induced plasticity does place a memory genuinely at risk of disruption.
It has often been suggested that reconsolidation may enable memories to be modified or updated [5, 8, 9, 13, 17, 18]. Memories are retrieved often in situations presenting additional complementary information. Thus the capacity for plastic changes in memory strength or content following memory retrieval seems potentially adaptive in terms of maintaining a memory’s relevance in guiding future behaviour. Three studies are of direct relevance to the hypothesis that reconsolidation mediates memory updating (see Box 1 for brief experimental details of the following tasks). Firstly, in human episodic memories, interference congruent with retrieval of a prior memory results in an incorrectly updated memory for a list of items [13]. This finding is consistent with, though not directly demonstrative of, a role of reconsolidation in updating memories. Moreover, a prior study of inhibitory avoidance learning in rats did not provide evidence that reconsolidation is functionally involved in linking new information to a reactivated memory [19]. Using the doubly dissociable mechanisms of inhibitory avoidance memory consolidation and reconsolidation, Tronel et al. showed that second-order conditioning recruited consolidation processes selectively [19]. However, linking new information to an old memory can be viewed simply as new learning based upon evoked memories, which would be expected to necessitate consolidation mechanisms, rather than true memory updating [5]. In a study designed to address directly the functional role of memory reconsolidation, I similarly capitalised on the doubly dissociable mechanisms of consolidation and reconsolidation in hippocampal contextual fear memories. A simple form of memory updating, namely strengthening through a further learning episode, was dependent selectively upon reconsolidation mechanisms [20]. Therefore, memory reconsolidation may well prove to be the mechanism by which memories are updated through further experience, though it remains to be determined whether reconsolidation plays a similar functional role in other forms of memory updating, such as memory weakening or changes in memory content.
Box 1. Some behavioural tasks commonly used in reconsolidation studies.
Fear conditioning
Rodents are trained that exposure to a discrete stimulus (e.g. a tone) predicts the occurrence of an aversive footshock, in a manner dependent upon the basolateral amygdala. Re-exposure to the tone subsequently elicits the typical freezing response, whereby no movement other than breathing can be detected [4]. Contextual fear conditioning, a task dependent upon the hippocampus, assesses the fear acquired by the environmental context. Thus conditioning can take place in the absence of any discrete stimuli (subjects being shocked upon exposure to the context alone) [22]. Variants of this procedure are used to condition fear in other species from the medaka fish [48] to humans [32].
Inhibitory avoidance
Rodents are trained that if they step down off a platform onto a grid floor, they receive an aversive footshock. Subsequently, subjects with intact memory will display a long latency to step down again off the platform. Inhibitory avoidance memories depend upon the hippocampus, but the basolateral amygdala also plays a role [23, 24].
Spatial memories
Typically tested with the use of a water maze, in which rodents are trained to learn the location of a submerged invisible platform, the location of which remains constant relative to the wider environment over several days of learning. The standard task can be modified to a delayed non-mapping to sample version, in which the location of the platform changes on a daily basis, with several training trials per day. The reconsolidation of spatial memory in this latter version of the task depends upon the hippocampus [36].
Taste memories
One way in which memories for taste can be assessed is via an attenuation of neophobia paradigm. New tastants such as saccharin generate a neophobic response of reduced consumption, which declines over several days. This attenuation of neophobia reflects an increasing memory for the taste, which is dependent upon the insular cortex in rats [43]. Taste aversion memories can also be assessed, whereby a tastant is associated with gastric malaise (typically induced with lithium chloride), thus inducing a subsequent reduction in consumption. Such conditioned taste aversion memories are also dependent upon the insular cortex [43, 53].
Aversive memories in Chasmagnathus
In the crab Chasmagnathus, reconsolidation has been studied in an aversive context-signal memory procedure [51]. Repeated exposure to a visual danger stimulus (an opaque rectangle that passes overhead) results in a change in behaviour from escape to freezing. This change depends upon an association between the experimental context and the visual danger signal.
Human episodic memory
List-learning procedures have been used to assess reconsolidation in human episodic memory [13]. Subjects are instructed to memorise a list of objects, and on a subsequent day are primed to recall the learning episode before memorising a second list. The reconsolidation effect is manifested as a significant number of intrusions from list 2 to list 1 in a subsequent memory test. Thus list 1 has been updated to include some list 2 items.
As a result of this recent functional support for the memory-updating role of reconsolidation, it is pertinent to discuss whether the wider literature on the reconsolidation phenomenon is similarly consistent. In particular, does the process lend itself to the modification of memories, and how can negative findings be accommodated into a hypothesis that places reconsolidation in a central role for memory persistence?
Reconsolidation is a special process and a special state
Much of the research into memory reconsolidation has taken a comparative view, capitalising upon the wealth of knowledge already gained about consolidation, and the degree to which the molecular substrates of the two processes overlaps is of importance. Indeed, although the mechanisms of memory reconsolidation largely recapitulate those of initial consolidation, there are notable dissociations between the two (See Alberini, 2005 [6] for a comprehensive review). In particular, there is evidence that reconsolidation recruits specific mechanisms that are not critically involved in consolidation. The reconsolidation, but not consolidation, of discrete fear memories is vulnerable to β-adrenergic receptor blockade [21]. Moreover, the cellular mechanisms of memory consolidation and reconsolidation for both contextual fear [22] and inhibitory avoidance [23, 24] are doubly dissociable. Such double dissociations rule out simple quantitative or non-specific factors, such as time or the absence of the highly motivating footshock reinforcer, as being the cause of differences between the mechamisms of consolidation and reconsolidation. Therefore, reconsolidation is a neurobiologically-distinct memory process, which is beginning to be associated with specific cellular mechanisms, such as the expression of the immediate-early gene zif268 [22, 25].
The existence of a reconsolidation process is largely revealed by its absence. Typically, when amnesia for a memory that is one or more days old is induced in a manner that is dependent upon reactivation of that memory through retrieval, reconsolidation is said to have been impaired [4, 17]. However, in common with other cognitive functions, experimental treatments targeting memory reconsolidation can also result in subsequent improvements [26-30]. Gain-of-function findings are of particular importance in refuting non-specific accounts of amnesia. Moreover, the ability to improve a memory through post-retrieval processing suggests a potentially adaptive function for the reconsolidation process. Rather than simply being process that restabilises a memory following its retrieval, it represents a special state, providing an opportunity for renewed memory plasticity and modulation [11]. Notably, the aforementioned memory-enhancing interventions include naturalistic phenomena such as water deprivation and the administration of glucose [28, 29]. Therefore, the capacity to modify (e.g. strengthen) a previously-acquired memory in a potentially adaptive manner is not limited to exogenous pharmacological treatment, but is likely also to be relevant to naturalistic situations of memory updating.
Reconsolidation is common, but is it ubiquitous?
An understanding of when reconsolidation does and does not occur is of primary importance in assigning potential functional roles to the process. Reconsolidation has come to be viewed as an almost universal memory process, with reactivation-dependent amnesia indicative of memory reconsolidation having been observed across a variety of species, from C. elegans [31] to humans [12, 13, 32] (see [10] for a comprehensive review). Moreover, the majority of studies have been conducted in rodents, in which it has been possible to investigate the reconsolidation of many different memory types across a number of neural loci. Briefly, both aversive [4, 22] and appetitive [33, 34] memories, as well as non-emotional object recognition [15, 35] and spatial [36, 37] memories undergo reconsolidation in rodents. However, despite this apparent commonality of reconsolidation across species and paradigms, it cannot currently be claimed to be a universal process.
Two studies stand out as failing to observe reactivation-dependent amnesia, and hence memory reconsolidation [38, 39]. Biedenkapp and Rudy [38] showed that a pure unemotional contextual memory, unlike contextual fear memories, does not appear to undergo reconsolidation. Giving rats an electric footshock immediately upon entering a novel environment, and before a contextual representation can be adequately encoded, results in no fear memory being conditioned to that context. This immediate shock deficit can be overcome by pre-exposing the rats to the environment, thereby making it familiar. Therefore, an intact pure contextual memory can be assessed by its capacity to overcome the immediate shock deficit. Intra-hippocampal administration of the protein synthesis inhibitor anisomycin failed to impair the contextual memory when given immediately after re-exposure to the familiar context. Therefore, there is no evidence to suggest that pure contextual memories undergo reconsolidation. Similarly, it is unclear whether instrumental memories for food-reinforced lever pressing are also subject to reconsolidation processes. Hernandez & Kelley [39] failed to find evidence that systemic protein synthesis inhibition following instrumental memory retrieval impaired subsequence performance, a finding that is consistent with several studies in appetitive instrumental tasks, in which the pavlovian memories but not the instrumental components were impaired by post-retrieval manipulations [25, 40-42]. Thus any theory of memory reconsolidation must account either for why contextual and instrumental memories do not undergo reconsolidation, or detail the reasons why the above studies failed to find positive evidence for a reconsolidation process.
While it is possible that certain types of memories may not undergo any reconsolidation process, the conclusion that reconsolidation is not ubiquitous is not entirely compelling. Certainly, one may make the case that pure contextual or spatial memories might benefit from being invulnerable to change, and Hernandez & Kelley [39] also suggest that it would be adaptive for instrumental memories not to reconsolidate once they are well-learned and proficient. However, they acknowledge that instrumental memories may reconsolidate earlier on in learning [39]. These sentiments are consistent with the notion that memory updating governs the reconsolidation phenomenon. A well-learned instrumental memory no longer benefits from further training, and hence updating mechanisms are not engaged by additional behavioural sessions. A strong prediction from this view is that weakly trained instrumental memories should be subject to modification and hence reactivation-dependent amnesia (though the ability of both goal-directed and habitual processes to support instrumental responding presents a further challenge to the interpretation of instrumental memory studies; thus apparent normal responding may mask an amnestic effect on either the goal-directed or habitual memory).
The same interpretation can also be applied to the contextual memory paradigm of Biedenkapp & Rudy [38]. The contextual pre-exposure that forms the basis for the contextual memory consisted of 6 separate exposures to the context, totalling 8 min 20 s, in a single day. Whether this can be expected to result in a well-learned contextual memory (i.e. near-asymptotic levels of learning) is not clear, but it may be equally predicted that a reduction in the level of pre-exposure would render the contextual memory subject to updating/strengthening processes and hence vulnerable to reactivation-dependent amnesia. Indeed, the strength of prior training has been shown previously to be an important factor determining vulnerability to reactivation-dependent amnesia. In both a taste memory attenuation of neophobia paradigm and a spatial memory task, protein synthesis inhibition only effected amnesia when applied early on in training, and not once the memories were learned to asymptotic levels of performance [43, 44]. Therefore, it may not be unexpected that the studies on relatively well-learned contextual and instrumental memories failed to observe evidence for reconsolidation.
The importance of the studies by Biedenkapp and Rudy [38] and Hernandez and Kelley [39] lies in the fact that the reconsolidation of memories for context and instrumental responding remains unsubstantiated. While this can be taken to imply that reconsolidation is not ubiquitous [38], the above reasoning suggests instead that reconsolidation may be a universal property of memories, given the necessity for all memories to be updated to maintain their relevance. Such universality should not be taken to imply that reconsolidation occurs under all circumstances, merely that all memory systems should display reconsolidation phenomena under appropriate conditions governed by the requirement for memory updating.
There are several other negative findings in relation to memory reconsolidation, or specifically reactivation-dependent amnesia [e.g. 45, 46]. However, these can be contrasted with positive results in similar paradigms. For example, while Cammarota et al [45] failed to observe amnesia in an inhibitory avoidance task following protein synthesis inhibition in the basolateral amygdala, Milekic et al. [24] did observed a reconsolidation impairment when inhibiting the synthesis in the basolateral amygdala of the specific protein C/EBPβ. It remains unclear whether parametric differences can account for such contrasting results, and so it is possible that the negative findings may be related to boundary conditions.
Boundary conditions on reconsolidation
Even in paradigms with well-established demonstrations of reactivation-dependent amnesia, there are conditions under which reconsolidation does not take place. Therefore, there exist certain boundary conditions, which for the purposes of this review, are considered simply to be a description of the boundaries around which reconsolidation may or may not be observed. Boundary conditions are important in the context of the current discussion as it will be argued that the engagement of reconsolidation mechanisms is critically dependent upon whether a memory is being updated. Hence memory updating may provide the underlying explanation for boundary conditions on reconsolidation.
Other than the impact of memory strength, several boundary conditions exist. The first among these is temporal in nature. In inhibitory avoidance in rats [47], as well as in fear conditioning in the medaka fish [48], 14-day old memories did not display reactivation-dependent amnesia, whereas younger memories did show evidence of reconsolidating. However, this is by no means a universal finding, with contextual fear and appetitive cocaine-related memories reconsolidating up to a month after learning [42, 49]. Nevertheless, it remains possible that all memories do display an age-dependent sensitivity to reconsolidation impairment, but with different timecourses not yet revealed by the current literature. Alternatively, given that there is an interaction between memory age and duration of stimulus re-exposure required successfully to reactivate a contextual fear memory [50], it is also possible that all memories undergo reconsolidation regardless of their age, but that previous studies have failed to use sufficiently intense memory reactivation conditions for older memories. However, if the age of a memory does indeed represent a limit on the engagement of reconsolidation mechanisms, this might speculatively fit in with an updating hypothesis. Perhaps the passage of time, under certain circumstances, results in new experiences being more likely to be encoded separately from the original memory. A prediction of this view would be that updating an old memory should engage consolidation-specific mechanisms (e.g. BDNF in the hippocampus for contextual fear memories [22]). Moreover, selective interference with these mechanisms should affect only the new updating information, thus resulting not in amnesia, as would be expected if reconsolidation mechanisms were being engaged and disrupted, but in a failure to modify the memory.
The issue of whether a new experience updates an existing memory or triggers new memory formation may also underlie the established constraint that extinction places on reconsolidation. Memory reactivation protocols typically involve short extinction sessions. However, lengthier non-reinforced stimulus exposure reverses the impact of amnestic treatment. Thus for contextual fear memories, protein synthesis impairs reconsolidation to reduce fear when the context re-exposure is short, but disrupts extinction to maintain high levels of fear when the duration of context re-exposure is more prolonged [50]. This pattern of result has been replicated in cued fear memories [27] as well as in contextual aversive learning in the crab Chasmagnathus [51], though it appears that extinction does not always prevent reconsolidation from taking place [52]. It is not simply the level of extinction training, but its relation to initial learning, that governs the interaction between reconsolidation and extinction. Protein synthesis inhibition during the same reactivation/extinction parameters produced opposing effects when the strength of initial training on a conditioned taste aversion task was varied [53]. This has previously been conceptualised as a trace dominance process, whereby the dominant trace engaged by reactivation/extinction is that which is impacted upon by experimental treatment [53]. However, rather than appealing to competition between traces, the extent of extinction training relative to conditioning may determine whether or not a new inhibitory memory is formed. This argument states that if stimulus exposure is sufficient to engage extinction learning, this would not concomitantly modify the original excitatory memory. More limited exposure, by contrast, would trigger memory updating in the absence of new inhibitory learning. Perhaps in support of this interpretation is the recent finding in Chasmagnathus that the transcription factor NF-κB reflects a molecular switch between reconsolidation and extinction [54]. Inhibiting NF-κB both impairs reconsolidation [55] and enhances extinction [54] under the appropriate conditions. Consequently, short memory reactivation induces a functional upregulation of NF-κB, whereas more prolonged extinction results in a functional inhibition. If we make the assumption that NF-κB activity is reflective of a reconsolidation/updating process, the extinction-induced inhibition would be consistent with a suppression of memory updating in favour of new extinction learning.
A further boundary condition on memory reconsolidation has recently been termed the “predictability of the reactivation stimulus” [10]. This reflects the findings emerging primarily from the Chasmagnathus literature that a mismatch between expected and actual events during reactivation triggers reconsolidation. Pedreira et al. [56] found that reconsolidation only took place, and thus could only be disrupted, when the predictive context terminated in the unexpected absence of the aversive outcome. It is not simply that memory reactivation must differ in some manner to conditioning, as there are numerous instances where reconsolidation impairments have been observed when the reactivation session is operationally identical to training (e.g. using reinforced reactivation procedures in fear conditioning [48, 57], and in many [35, 58], but not all [59] studies of object recognition memories). Instead, reconsolidation is triggered by a violation of expectation based upon prior learning, whether such a violation is qualitative (the outcome not occurring at all) or quantitative (the magnitude of the outcome not being fully predicted). It is predicted, then, that more extended initial training of fear or object memories will render those memories resistant to reconsolidation impairments with the use of reactivation sessions that are identical to training. This interpretation, therefore, partially reduces to the prior discussion of memory strength, in that incompletely, but not fully, learned memories are subject to reconsolidation because of the requirement for memory updating in order to optimise further the predictive accuracy of the memory.
Reconsolidation and memory updating: relation to existing theories
The prior sections argue that currently-identified boundary conditions on memory reconsolidation, as well as prior failures to demonstrate reconsolidation in contextual and instrumental memories, are consistent with a hypothesis emphasising the memory-updating properties of the reactivation experience. Thus reconsolidation may be viewed as a fundamental process in the ongoing modification and storage of memories.
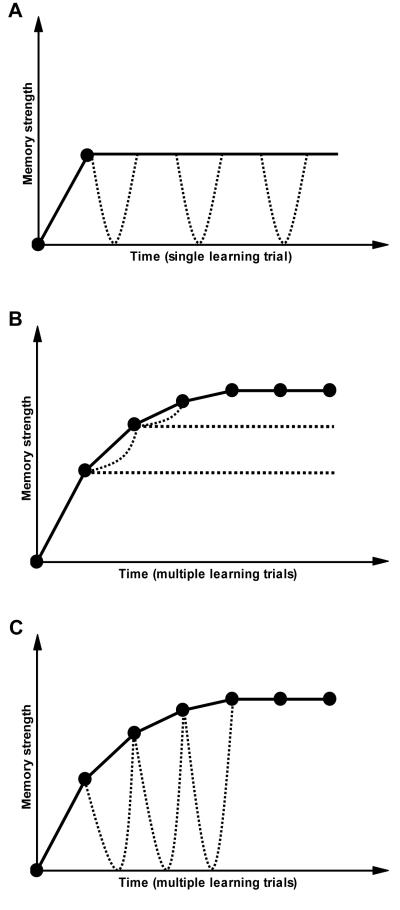
Several positions have been advanced regarding the role of reconsolidation in wider memory processes. Two of these [6, 8] have used the apparent temporal boundary condition to argue that reconsolidation plays a part in an extended process of memory stabilisation. Specifically, Alberini [6] suggests that repeated reactivations (which may be implicit during sleep) gradually increase memory stability as part of a lengthy consolidation process, such that when sufficient time has elapsed, a memory can no longer be disrupted, but can be added to or modified. Dudai & Eisenberg [8] similarly integrate reconsolidation within a “lingering consolidation” process, whereby the reactivation and reconsolidation cycle progressively stabilises a memory (Fig. 1A). In contrast to these emphases on reconsolidation enhancing memory stability, the present focus on memory updating does not require that reconsolidation has an endogenous part to play in the ongoing processing of a memory that requires no further modification. Indeed, the reverse may be suggested, in that a memory will persist in a stable and fixed form only if reconsolidation is not engaged, precisely because reconsolidation is the mechanistic instantiation of memory updating. Thus reconsolidation only plays a part in enhancing memory stability if such enhancement is dependent upon modification of the memory.
Fig. 1.
Schematic representation of the different hypotheses regarding the function of memory reconsolidation. Filled circles connected by solid lines depict idealised data points on a learning curve (i.e. behaviour observed). Dotted lines represent the hypothesised action of reconsolidation, with the lowest edge showing the impact of disrupting the reconsolidation process. A, reconsolidation may serve progressively to stabilise an acquired memory. As part of a longer consolidation process, memory reactivation and reconsolidation likely occur repeatedly in order to stabilise further the memory, with no necessity for qualitative or quantitative change to the memory. What the figure does not depict is the temporal limitation on this process, in that reconsolidation only occurs for a certain period following acquisition. This hypothesis presumably does not distinguish between memories acquired through a single trial (as shown), or over several trials, and does not propose a specific role for reconsolidation in memory strengthening/modification. B, reconsolidation may be a consolidation of updating information. A major purpose of reconsolidation is to enable the integration of updating information without suffering the deleterious impact of interference. Reconsolidation does not invoke the complete destabilisation of the previously-consolidated memory, and so disruption of reconsolidation entails little or no loss to the existing memory (for clarity, the figure shows only the theorised reconsolidation process on the 2nd & 3rd trials; reconsolidation itself mediates the strengthening of the memory [curved dotted lines], in the absence of which the memory remains relatively constant in strength [straight dotted lines]). C, memory updating involves destabilisation and reconsolidation. In order to strengthen a memory, that memory must be fully destabilised such that the updating information can be added or integrated into a modified unitary memory trace. Thus each updating of the memory involves destabilisation that places the memory at risk of disruption if the reconsolidation process is impaired. Equally, memories only reconsolidate when the memory is being updated (i.e. not when fully learned).
Rather than focussing on parametric factors in the constraint of reconsolidation, Morris et al. [36] argue for a mode-based explanation. Namely it may be the dual activation of retrieval and encoding states that drives reconsolidation processes. This model is well suited to account for situations in which new experiences result in profound changes to the memory; a change in the location of an escape platform in a water maze being the example used for the delayed non-mapping to place task [36]. However, it is not clear either how it may be adapted to conditions of more minor memory modifications (such as strength), or whether the activation of an “encoding mode” is sufficient to trigger reconsolidation. For example, extinction training clearly involves memory retrieval as well as new memory encoding, but under such circumstances reconsolidation is not obviously engaged [27, 50, 51, 53]. Moreover, the mode requirement appears to be an additional, rather than alternative, boundary condition to those discussed previously. A hypothesis based upon memory updating, in contrast, both incorporates the principles of the dual state proposal (in that a requirement for updating depends upon the same conditions as those proposed to engage an encoding state), and can potentially account for other boundary conditions.
Boundary conditions on memory reconsolidation also influence a further hypothesis of memory updating that is superficially similar to that advanced here. Rodriguez-Ortiz & Bermudez-Rattoni [60] conceive of reconsolidation as an “updating consolidation” mechanism (Fig. 1B). As well as showing that fully-learned memories are not subject to reactivation-dependent amnesia, these authors observed in both spatial and taste memories that when learning had reached near-asymptotic levels, only partial amnesia resulted from reactivation and protein synthesis inhibition [43, 44]. This partial amnesia is inferred to reflect only the partial destabilisation of the existing memory trace to enable updating. As such, this idea is not dissimilar to Alberini’s suggestion that old memories can be updated, but not disrupted [6]. Moreover, Rodriguez-Ortiz & Bermudez-Rattoni suggest that reconsolidation-associated response decrements do not reflect memory loss for the original consolidated memory, but rather result from a failure to integrate new learning, leading to interference. However, such an interpretation cannot account for my recent results in contextual fear memories [20]. If reconsolidation impairments result from new learning interfering with the stable old memory trace, disruption of the new learning itself should result in an unchanged memory. This is not what was observed when the consolidation-specific protein BDNF was knocked down in the hippocampus during memory strengthening/updating. Instead, while knocking down BDNF had no impact on memory strengthening, the modification of the old memory was completely dependent upon the reconsolidation-selective upregulation of zif268. Furthermore, interfering with memory destabilisation both protected against the disruptive effects of protein synthesis inhibition and fixed the memory at the same strength in spite of further learning [20]. Therefore, memory updating requires the destabilisation of the original memory in order in integrate new information. As a result, impairment of the restabilisation process (e.g. through protein synthesis inhibition) affects not only the new information, but also the reactivated memory, thus leading to amnesia (Fig. 1C).
A strong interpretation of the current memory updating hypothesis presented is that reconsolidation, as process that restabilises retrieved memories, is actually an epiphenomenon. In fact, the hallmark reactivation-dependent amnesia is revealed only through artificial interference with the memory updating process. Few naturalistic phenomena have been shown to disrupt memories following their reactivation. While stress can impair retrieved memories [15, 16], mild arousal can be memory enhancing [61]. Moreover, interference-based procedures only produce amnesia in an operational sense. Such impairments (e.g. intrusions of items between lists in human episodic memory [13]) can equally be viewed as normal and potentially adaptive updating in an artificial setting. Therefore, based upon current evidence, there is little risk entailed in a system that is proposed to update memories through making the existing memory trace labile. As such, any behavioural experience that has the effect of updating an existing memory, subject to the conditions discussed previously, renders the trace vulnerable to disruption precisely because this is the mechanism by which the memory is updated to remain adaptively relevant. Interruption of the updating process results in amnesia, operationally defined as a reactivation-dependent memory impairment, thereby revealing the reconsolidation phenomenon. Thus, reconsolidation does not simply restabilise a reactivated memory, but instead is revealed through the experimental targeting of the updating process. Perhaps, just as consolidation can be defined as the post-acquisition stabilisation of a memory, reconsolidation should be viewed as the mechanistic implementation of memory updating rather than a process in its own right.
Reconsolidation integrated into learning theory
Given that an updating hypothesis places reconsolidation in a central role in memory persistence, it should be possible to integrate reconsolidation into existing theories of learning and memory. As a salient example, I will focus briefly on only one such area; the role of surprise in learning.
Rescorla & Wagner [62] formalised the role of surprise in their model of learning that places critical emphasis on prediction error. That is, the amount of learning generated on a particular trial depends upon, among other parameters, the discrepancy between the total asymptotic amount of learning the outcome is able to support, and the amount of learning already acquired by the training stimuli. Therefore, learning only occurs when there is informational value present within a given trial. This restriction of learning as a function of informational value is overtly similar to the position advanced here in relation to the engagement of reconsolidation only when memories require updating. Thus following the very first training trial, reconsolidation might be argued to be the primary mechanism by which learning takes place, as memory modifications, including those apparent in a standard learning curve, engage the updating mechanism that reconsolidation is proposed to subserve. Such a view anticipates that the existence of a prediction error signal may be a critical pre-requisite for reconsolidation to be triggered. Little is known about the mechanisms of memory reactivation, but they include signalling at NMDA and CB1 cannabinoid receptors and L-type voltage-gated calcium channels (LGVCCs) [63, 64], as well as synaptic protein degradation [65]. Perhaps the source of the signal transduced by these mechanisms is the aforementioned prediction error. This would provide, therefore, a neurobiological mechanism by which the principle of reconsolidation being governed by updating operates. To give one concrete example, the absence of prediction error when learning reaches asymptotic levels also means that the memory is not destabilised and hence reconsolidation is not engaged, thereby accounting for the observed boundary condition of memory strength.
Reconsolidation updates memory storage
It has been fiercely debated whether experimentally-induced consolidation and reconsolidation impairments reflect a storage deficit or an inability to retrieve the memory normally [66]. While there is no conclusive evidence to favour either view, the retrieval account is inconsistent with the proposed action of reconsolidation to modify the strength and even content of a memory. An initial novel finding, making use of positive predictions of the storage view, strongly indicates that consolidation deficits do indeed reflect storage impairments [67], but as yet has not been extended to reconsolidation settings. However, there are instances of reconsolidation impairments being phenomenologically different to consolidation deficits, particularly with respect to recovery from amnesia [68, 69], though the significance of such differences remains to be determined. A clear prediction of the proposed view is that reactivation-dependent amnesia should reflect an impairment in memory storage rather than retrieval, and this will be a critical test of the memory updating hypothesis of reconsolidation.
Conclusion
Memory reconsolidation is a rapidly expanding field of research, which is becoming widely accepted as a fundamental process in long-term memory. While advances are continually being made in terms of the pharmacological and cellular mechanisms of reconsolidation, these do not address the fundamental question of the role of memory reconsolidation in memory persistence. In this review, I have proposed that the function of memory reconsolidation may be to mediate the updating of a memory in order to maintain its adaptive relevance. This memory updating hypothesis of reconsolidation has the potential to account for when reconsolidation does and does not take place. Indeed, I suggest both that reconsolidation is in fact a universal property of memories and that it is engaged specifically under conditions of memory updating. A major question that emerges is that of how memory reactivation is determined at the mechanistic level depending on whether a memory should be updated.
References
- 1.Bartlett FC. Remembering: A study in experimental and social psychology. MacMillan; 1932. [Google Scholar]
- 2.Schacter DL, et al. The cognitive neuroscience of constructive memory. Annu Rev Psychol. 1998;49:289–318. doi: 10.1146/annurev.psych.49.1.289. [DOI] [PubMed] [Google Scholar]
- 3.McGaugh JL. Time-dependent processes in memory storage. Science. 1966;153:1351–1358. doi: 10.1126/science.153.3742.1351. [DOI] [PubMed] [Google Scholar]
- 4.Nader K, et al. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- 5.Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci. 2007;8:262–275. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- 6.Alberini CM. Mechanisms of memory stabilization: are consolidation and reconsolidation similar or distinct processes? Trends Neurosci. 2005;28:51–56. doi: 10.1016/j.tins.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Nader K. Memory traces unbound. Trends Neurosci. 2003;26:65–72. doi: 10.1016/S0166-2236(02)00042-5. [DOI] [PubMed] [Google Scholar]
- 8.Dudai Y, Eisenberg M. Rites of passage of the engram: reconsolidation and the lingering consolidation hypothesis. Neuron. 2004;44:93–100. doi: 10.1016/j.neuron.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Sara SJ. Retrieval and reconsolidation: Toward a neurobiology of remembering. Learn Mem. 2000;7:73–84. doi: 10.1101/lm.7.2.73. [DOI] [PubMed] [Google Scholar]
- 10.Nader K, Hardt O. A Single Standard For Memory: The Case For Reconsolidation. Nature Reviews Neuroscience. 2009;10:224–234. doi: 10.1038/nrn2590. [DOI] [PubMed] [Google Scholar]
- 11.Dudai Y. Post-activation state: a critical rite of passage of memories. In: Bontempi B, et al., editors. Memories: Molecules and Circuits. Springer-Verlag; 2007. pp. 69–82. [Google Scholar]
- 12.Walker MP, et al. Dissociable stages of human memory consolidation and reconsolidation. Nature. 2003;425:616–620. doi: 10.1038/nature01930. [DOI] [PubMed] [Google Scholar]
- 13.Hupbach A, et al. Reconsolidation of episodic memories: A subtle reminder triggers integration of new information. Learn Mem. 2007;14:47–53. doi: 10.1101/lm.365707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon WC, Feldman DT. Reactivation-induced interference in a short-term retention paradigm. Learn Motiv. 1978;9:164–178. [Google Scholar]
- 15.Maroun M, Akirav I. Arousal and stress effects on consolidation and reconsolidation of recognition memory. Neuropsychopharmacology. 2008;33:394–405. doi: 10.1038/sj.npp.1301401. [DOI] [PubMed] [Google Scholar]
- 16.Wang XY, et al. Stress impairs reconsolidation of drug memory via glucocorticoid receptors in the basolateral amygdala. J Neurosci. 2008;28:5602–5610. doi: 10.1523/JNEUROSCI.0750-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- 18.Dudai Y. Reconsolidation: the advantage of being refocused. Curr Opin Neurobiol. 2006;16:174–178. doi: 10.1016/j.conb.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Tronel S, et al. Linking new information to a reactivated memory requires consolidation and not reconsolidation mechanisms. PLoS Biol. 2005;3:e293. doi: 10.1371/journal.pbio.0030293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JLC. Memory reconsolidation mediates the strengthening of memories by additional learning. Nat Neurosci. 2008;11:1264–1266. doi: 10.1038/nn.2205. [DOI] [PubMed] [Google Scholar]
- 21.Debiec J, LeDoux JE. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience. 2004;129:267–272. doi: 10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 22.Lee JLC, et al. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science. 2004;304:839–843. doi: 10.1126/science.1095760. [DOI] [PubMed] [Google Scholar]
- 23.Taubenfeld SM, et al. The consolidation of new but not reactivated memory requires hippocampal C/EBP beta. Nat Neurosci. 2001;4:813–818. doi: 10.1038/90520. [DOI] [PubMed] [Google Scholar]
- 24.Milekic MH, et al. Temporal requirement of C/EBPbeta in the amygdala following reactivation but not acquisition of inhibitory avoidance. Learn Mem. 2007;14:504–511. doi: 10.1101/lm.598307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JLC, et al. Disrupting reconsolidation of drug memories reduces cocaine seeking behavior. Neuron. 2005;47:795–801. doi: 10.1016/j.neuron.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Tronson NC, et al. Bidirectional behavioral plasticity of memory reconsolidation depends on amygdalar protein kinase A. Nat Neurosci. 2006;9:167–169. doi: 10.1038/nn1628. [DOI] [PubMed] [Google Scholar]
- 27.Lee JLC, et al. Reconsolidation and extinction of conditioned fear: inhibition and potentiation. J Neurosci. 2006;26:10051–10056. doi: 10.1523/JNEUROSCI.2466-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frenkel L, et al. Memory strengthening by a real-life episode during reconsolidation: an outcome of water deprivation via brain angiotensin II. Eur J Neurosci. 2005;22:1757–1766. doi: 10.1111/j.1460-9568.2005.04373.x. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez WA, et al. Effects of glucose and fructose on recently reactivated and recently acquired memories. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:1285–1317. doi: 10.1016/s0278-5846(99)00063-9. [DOI] [PubMed] [Google Scholar]
- 30.Blaiss CA, Janak PH. Post-training and post-reactivation administration of amphetamine enhances morphine conditioned place preference. Behav Brain Res. 2006 doi: 10.1016/j.bbr.2006.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rose JK, Rankin CH. Blocking memory reconsolidation reverses memory-associated changes in glutamate receptor expression. J Neurosci. 2006;26:11582–11587. doi: 10.1523/JNEUROSCI.2049-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kindt M, et al. Beyond extinction: erasing human fear responses and preventing the return of fear. Nat Neurosci. 2009;12:256–258. doi: 10.1038/nn.2271. [DOI] [PubMed] [Google Scholar]
- 33.Lee JLC, Everitt BJ. Reactivation-dependent amnesia in pavlovian approach and instrumental transfer. Learn Mem. 2008;15:597–602. doi: 10.1101/lm.1029808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee JLC, Everitt BJ. Appetitive memory reconsolidation depends upon NMDA receptor-mediated neurotransmission. Neurobiol Learn Mem. 2008;90:147–154. doi: 10.1016/j.nlm.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Kelly A, et al. Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase in hippocampal circuitry is required for consolidation and reconsolidation of recognition memory. J Neurosci. 2003;23:5354–5360. doi: 10.1523/JNEUROSCI.23-12-05354.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morris RG, et al. Memory Reconsolidation: Sensitivity of Spatial Memory to Inhibition of Protein Synthesis in Dorsal Hippocampus during Encoding and Retrieval. Neuron. 2006;50:479–489. doi: 10.1016/j.neuron.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 37.Rossato JI, et al. Retrieval induces hippocampal-dependent reconsolidation of spatial memory. Learn Mem. 2006;13:431–440. doi: 10.1101/lm.315206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biedenkapp JC, Rudy JW. Context memories and reactivation: constraints on the reconsolidation hypothesis. Behav Neurosci. 2004;118:956–964. doi: 10.1037/0735-7044.118.5.956. [DOI] [PubMed] [Google Scholar]
- 39.Hernandez PJ, Kelley AE. Long-term memory for instrumental responses does not undergo protein synthesis-dependent reconsolidation upon retrieval. Learn Mem. 2004;11:748–754. doi: 10.1101/lm.84904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Milton AL, et al. Reconsolidation of appetitive memories for both natural and drug reinforcement is dependent on b-adrenergic receptors. Learn Mem. 2008;15:88–92. doi: 10.1101/lm.825008. [DOI] [PubMed] [Google Scholar]
- 41.Milton AL, et al. Intra-amygdala and systemic antagonism of NMDA receptors prevents the reconsolidation of drug-associated memory and impairs subsequently both novel and previously acquired drug-seeking behaviors. J Neurosci. 2008;28:8230–8237. doi: 10.1523/JNEUROSCI.1723-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee JLC, et al. Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J Neurosci. 2006;26:5881–5887. doi: 10.1523/JNEUROSCI.0323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodriguez-Ortiz CJ, et al. Protein synthesis underlies post-retrieval memory consolidation to a restricted degree only when updated information is obtained. Learn Mem. 2005;12:533–537. doi: 10.1101/lm.94505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodriguez-Ortiz CJ, et al. Intrahippocampal anisomycin infusions disrupt previously consolidated spatial memory only when memory is updated. Neurobiol Learn Mem. 2008;89:352–359. doi: 10.1016/j.nlm.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 45.Cammarota M, et al. Retrieval does not induce reconsolidation of inhibitory avoidance memory. Learn Mem. 2004;11:572–578. doi: 10.1101/lm.76804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yim AJ, et al. Protein synthesis inhibition in the basolateral amygdala following retrieval does not impair expression of morphine-associated conditioned place preference. Behav Brain Res. 2006;171:162–169. doi: 10.1016/j.bbr.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 47.Milekic MH, Alberini CM. Temporally Graded Requirement for Protein Synthesis following Memory Reactivation. Neuron. 2002;36:521–525. doi: 10.1016/s0896-6273(02)00976-5. [DOI] [PubMed] [Google Scholar]
- 48.Eisenberg M, Dudai Y. Reconsolidation of fresh, remote, and extinguished fear memory in medaka: old fears don’t die. Eur J Neurosci. 2004;20:3397–3403. doi: 10.1111/j.1460-9568.2004.03818.x. [DOI] [PubMed] [Google Scholar]
- 49.Debiec J, et al. Cellular and systems reconsolidation in the hippocampus. Neuron. 2002;36:527–538. doi: 10.1016/s0896-6273(02)01001-2. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki A, et al. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pedreira ME, Maldonado H. Protein synthesis subserves reconsolidation or extinction depending on reminder duration. Neuron. 2003;38:863–869. doi: 10.1016/s0896-6273(03)00352-0. [DOI] [PubMed] [Google Scholar]
- 52.Duvarci S, et al. Extinction is not a sufficient condition to prevent fear memories from undergoing reconsolidation in the basolateral amygdala. Eur J Neurosci. 2006;24:249–260. doi: 10.1111/j.1460-9568.2006.04907.x. [DOI] [PubMed] [Google Scholar]
- 53.Eisenberg M, et al. Stability of retrieved memory: Inverse correlation with trace dominance. Science. 2003;301:1102–1104. doi: 10.1126/science.1086881. [DOI] [PubMed] [Google Scholar]
- 54.Merlo E, Romano A. Memory extinction entails the inhibition of the transcription factor NF-kappaB. PLoS ONE. 2008;3:e3687. doi: 10.1371/journal.pone.0003687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Merlo E, et al. Activation of the transcription factor NF-kappaB by retrieval is required for long-term memory reconsolidation. Learn Mem. 2005;12:23–29. doi: 10.1101/lm.82705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pedreira ME, et al. Mismatch between what is expected and what actually occurs triggers memory reconsolidation or extinction. Learn Mem. 2004;11:579–585. doi: 10.1101/lm.76904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duvarci S, Nader K. Characterization of fear memory reconsolidation. J Neurosci. 2004;24:9269–9275. doi: 10.1523/JNEUROSCI.2971-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Akirav I, Maroun M. Ventromedial prefrontal cortex is obligatory for consolidation and reconsolidation of object recognition memory. Cereb Cortex. 2006;16:1759–1765. doi: 10.1093/cercor/bhj114. [DOI] [PubMed] [Google Scholar]
- 59.Rossato JI, et al. On the role of hippocampal protein synthesis in the consolidation and reconsolidation of object recognition memory. Learn Mem. 2007;14:36–46. doi: 10.1101/lm.422607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rodriguez-Ortiz CJ, Bermudez-Rattoni F. Memory reconsolidation or updating consolidation? In: Bermudez-Rattoni F, editor. Neural plasticity and memory: From genes to brain imaging. Taylor and Francis Group; 2007. pp. 209–224. [PubMed] [Google Scholar]
- 61.McGaugh JL. Stress hormones and amygdala regulate long-term memory storage. Int. J. Psychol. 2000;35:405–405. [Google Scholar]
- 62.Rescorla RA, Wagner AR. A Theory of Pavlovian Conditioning: Variations in the Effectiveness of Reinforcement and Nonreinforcement. In: Prokasy AHBWF, editor. Classical Conditioning II: Current Research and Theory. Appleton-Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- 63.Suzuki A, et al. Activation of LVGCCs and CB1 receptors required for destabilization of reactivated contextual fear memories. Learn Mem. 2008;15:426–433. doi: 10.1101/lm.888808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ben Mamou C, et al. NMDA receptors are critical for unleashing consolidated auditory fear memories. Nat Neurosci. 2006;9:1237–1239. doi: 10.1038/nn1778. [DOI] [PubMed] [Google Scholar]
- 65.Lee SH, et al. Synaptic protein degradation underlies destabilization of retrieved fear memory. Science. 2008;319:1253–1256. doi: 10.1126/science.1150541. [DOI] [PubMed] [Google Scholar]
- 66.Nader K, Wang SH. Fading in. Learn Mem. 2006;13:530–535. doi: 10.1101/lm.350906. [DOI] [PubMed] [Google Scholar]
- 67.Hardt O, et al. Storage or retrieval deficit: the yin and yang of amnesia. Learn Mem. doi: 10.1101/lm.1267409. (in press) [DOI] [PubMed] [Google Scholar]
- 68.Lattal KM, Abel T. Behavioral impairments caused by injections of the protein synthesis inhibitor anisomycin after contextual retrieval reverse with time. Proc Natl Acad Sci U S A. 2004;101:4667–4672. doi: 10.1073/pnas.0306546101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Power AE, et al. Anisomycin infused into the hippocampus fails to block “reconsolidation” but impairs extinction: The role of re-exposure duration. Learn Mem. 2006;13:27–34. doi: 10.1101/lm.91206. [DOI] [PMC free article] [PubMed] [Google Scholar]