Abstract
ORF40 (named fatE) in the Vibrio anguillarum pJM1 plasmid encoding anguibactin iron transport systems is a homologue of ATPase genes involved in ferric-siderophore transport. Mutation of fatE did not affect ferric-anguibactin transport indicating that there must be other ATPase gene(s) in addition to fatE. By searching the genomic sequence of V. anguillarum 775(pJM1) we identified a homologue of fatE named fvtE on chromosome 2. It is of interest that in this locus we also identified homologues of fatB, fatC and fatD that we named fvtB, fvtC and fvtD, respectively. The fvtE mutant still showed ferric-anguibactin transport while the double fatE and fvtE mutation completely abolished the ferric-anguibactin transport indicating that fatE and fvtE are functional ATPase homologues for ferric-anguibactin transport. Furthermore, we demonstrate that fvtB, fvtC, fvtD and fvtE are essential for ferric-vanchrobactin and ferric-enterobactin transport.
INTRODUCTION
Vibrio anguillarum is a part of the natural flora in the aquatic environment, and some strains cause vibriosis, a terminal hemorrhagic septicemia in marine as well as fresh water fish and invertebrates (Toranzo & Barja, 1990; Aguirre-Guzmán et al., 2004; Paillard et al., 2004; Toranzo et al., 2005). 23 serotypes of V. anguillarum have been reported so far, and serotypes O1, O2 and O3 are mainly causative agents of vibriosis (Sorensen & Larsen, 1986; Toranzo & Barja, 1990; Larsen et al., 1994; Grisez & Ollevier, 1995; Tiainen et al., 1997; Pedersen et al., 1999). Many serotype O1 strains carry the pJM1-type plasmids harboring the genes involved in the siderophore anguibactin transport system that is an essential virulence factor for V. anguillarum (Crosa, 1980; Crosa & Walsh, 2002; Di Lorenzo et al., 2003; Wu et al., 2004). V. anguillarum biosynthesizes inside the cell a small molecular peptide iron chelator anguibactin, and secretes it to the external environment. Then, the iron-bound anguibactin, ferric-anguibactin, is transported back into the cell to utilize iron for survival under the iron limiting conditions that can be found in marine environments and inside hosts (Crosa, 1980; Actis et al., 2011; Naka & Crosa, 2011b). It has been shown that the ferric-anguibactin is transported to the periplasmic space of V. anguillarum via the specific outer membrane receptor FatA (Lopez & Crosa, 2007). In this step, the ExbB2-ExbD2-TonB2-TtpC complex is required to transduce the energy generated from proton motive force to the FatA protein to change its conformation and enable the transport of ferric-siderophore into the periplasmic space (Stork et al., 2004; Stork et al., 2007; Kuehl & Crosa, 2010; Kustusch et al., 2011).
In Gram-negative bacteria, ferric-siderophores pass through the cytoplasmic membrane using ABC transport systems that rely on ATPases or MSF type siderophore transporters that depend on the proton motive force (Crosa & Walsh, 2002; Cuiv et al., 2004; Raymond & Dertz, 2004; Winkelmann, 2004; Hannauer et al., 2010; Reimmann, 2012). We have shown that ferric-anguibactin is transported across the cytoplasmic membrane using the ABC transport system including the periplasmic binding protein FatB and cytoplasmic membrane proteins FatC and FatD (Actis et al., 1995; Naka et al., 2010). However, the gene encoding an ATPase that should be part of the ABC transporter for ferric-anguibactin was still unknown, although there is a homologue (ORF40) of ATPase genes in the pJM1 plasmid (Di Lorenzo et al., 2003).
In addition to anguibactin, V. anguillarum 775 (pJM1) can transport exogenous siderophores such as vanchrobactin and enterobactin (Naka et al., 2008; Balado et al., 2009; Naka & Crosa, 2011a). One of them, vanchrobactin, is a chromosomally encoded siderophore produced by natural pJM1-less V. anguillarum O1 strains and other serotype strains (Lemos et al., 1988; Conchas et al., 1991; Balado et al., 2006; Soengas et al., 2006; Balado et al., 2008). We previously showed that outer membrane proteins, FvtA and FetA, are involved in the transport of these exogenous siderophores in V. anguillarum 775(pJM1) (Naka & Crosa, 2011a). However, genes encoding the ABC transporter system(s) for ferric-vanchrobactin and ferric-enterobactin are still unknown. In this work, we characterized two ABC transport systems involved in ferric-siderophore uptake in V. anguillarum 775(pJM1): FatBCDE encoded on the pJM1 plasmid and FvtBCDE encoded in the chromosome are involved in ferric-anguibactin and ferric-vanchrobactin/enterobactin transport, respectively. The two ABC transport systems are specific for their respective siderophores except that both FatE and FvtE are functional for ferric-anguibactin transport.
MATERIALS AND METHODS
Bacterial strains, primers and media
Strains and plasmids used in the present study are listed in Table 1. The primers used in this study are listed in Table 2. E. coli strains were grown in LB broth with appropriate antibiotics. V. anguillarum strains were grown in Trypticase Soy Broth supplemented with 1.5 % NaCl (TSBS) with appropriate antibiotics [for E. coli: ampicillin (Amp) 100 μg/ml, kanamycin (Km) 50 μg/ml, chloramphenicol (Cm) 30 μg/ml and trimethoprim (Tp) 100 μg/ml and for V. anguillarum: Km 250 μg/ml, Cm 10 μg/ml, Tp 10 μg/ml and rifampicin (Rif) 100 μg/ml]. The authenticity of V. anguillarum strains was confirmed by oxidase test, pJM1 plasmid extraction and colony PCR using V. anguillarum specific primers. All strains were stored at −80 °C as glycerol stocks (TSBS supplemented with 30 % glycerol), and strains were streaked from the stock vials for each experiment.
Table 1.
Strains and plasmids used in this study
| Strains and plasmids | Characteristics | Reference or source |
|---|---|---|
| V. anguillarum strains | ||
| 775(pJM1) | Wild type, Washington (serotype O1, pJM1), isolated from coho salmon (Oncorhynchus kisutch) | (Crosa, 1980) |
| H775-3 | Plasmidless derivative of 775(pJM1) | (Crosa, 1980) |
| HNVA-10 | H775-3ΔfvtA::Km | This study |
| 775(pJM1)-pMMB | 775(pJM1) harboring pMMB208 | (Naka et al., 2008) |
| CC9-16 | 775 (pJM1) derivative of anguibactin deficient, anguibactin transport system proficient | (Walter et al., 1983) |
| HNVA-11 | CC9-16ΔfatE::Tp | This study |
| HNVA-12 | CC9-16ΔfvtE::Km | This study |
| HNVA-13 | CC9-16ΔfatE::TpΔfvtE::Km | This study |
| HNVA-14 | CC9-16ΔfvtB | This study |
| HNVA-15 | CC9-16ΔfvtC | This study |
| HNVA-16 | CC9-16ΔfvtD | This study |
| 96F-pMMB | vanchrobactin producer (serotype O1, plasmidless) harboring pMMB208 | (Naka et al., 2008) |
| E. coli strains | ||
| DH5α | F−, φ80lacZΔM15, endA1, recA1, hsdR17, (rK−mK+), supE44, thi-1, gyrA96, relA1, Δ(lacZYA-argF)U169, λ− | Laboratory stock |
| S17-1λpir | λ-pir lysogen; thi pro hsdR hsdM+recA RP4 2-Tc::Mu-Km::Tn7(Tpr Smr) | (Simon et al., 1983) |
| Plasmids | ||
| pCR2.1 | Ampr, Kmr, PCR cloning vector | Invitrogen |
| pGEM-T Easy | A vector for the cloning of PCR products with blue/white screening, Apr | Promega |
| pBlue-Km-SmaI | Source of the Km resistance cassette with SmaI recognition sites in both sides | (Naka et al., 2012) |
| p34E-Tp | Source of the Tp resistance cassette with SmaI recognition sites in both sides | (DeShazer & Woods, 1996) |
| pDM4 | Suicide plasmid sacB gene, R6K origin, Cmr | (Milton et al., 1996) |
| pHN15 | pDM4 harboring ΔfatE::Tp of V. anguillarum 775(pJM1) | This study |
| pHN16 | pDM4 harboring ΔfvtE::Km of V. anguillarum 775(pJM1) | This study |
| pHN17 | pDM4 harboring ΔfvtB of V. anguillarum 775(pJM1) | This study |
| pHN17 | pDM4 harboring ΔfvtC of V. anguillarum 775(pJM1) | This study |
| pHN17 | pDM4 harboring ΔfvtD of V. anguillarum 775(pJM1) | This study |
| pMMB208 | A broad-host-range expression vector; Cmr IncQ lacIq Ptac; polylinker from M13mp19 | (Morales et al., 1991) |
| pHN18 | pMMB208 harboring V. anguillarum 75(pJM1) fatE | This study |
| pHN19 | pMMB208 harboring V. anguillarum 775(pJM1) fvtE | This study |
| pHN20 | pMMB208 harboring V. anguillarum 775(pJM1) fvtB | This study |
| pHN21 | pMMB208 harboring V. anguillarum 775(pJM1) fvtC | This study |
| pHN22 | pMMB208 harboring V. anguillarum 775(pJM1) fvtD | This study |
Construction and complementation of mutants
The two flanking regions of the target genes to be mutated were PCR-amplified, and fragments thus obtained were combined by using SOE PCR (Senanayake & Brian, 1995). Then, the PCR products were ligated into pCR2.1. To mutate fatE and fvtE genes, the Kmr gene [the SmaI fragment from pBlue-Km-SmaI (Naka et al., 2012)] and Tpr gene [the SmaI fragment from p34E-Tp (DeShazer & Woods, 1996)] were respectively ligated in the Eco47III sites that are located in the middle of the deletion fragments. The fragments were subcloned into the suicide vector pDM4, transformed into E. coli S17-1 λpir and conjugated into V. anguillarum as described before (Naka et al., 2008). Transconjugants that show resistance to Cm (from the pDM4 plasmid) and Rif (resistance from V. anguillarum) were selected. The colonies obtained were inoculated into TSBS without antibiotics, cultured overnight, and plated on TSAS with 15% sucrose to select 2nd recombinants (for fatE and fvtE mutants, Km and Tp were added, respectively). The colonies obtained were checked for Cm sensitivity (loss of pDM4), and the mutations were confirmed by colony PCR using primers constructed outside and inside the target genes. To complement the mutants, primers with appropriate restriction enzyme sites were used to PCR-amplify the target genes with upstream regions including the Shine-Dalgarno sequence and the start codon. The fragments thus obtained were ligated into pGEM-T Easy and subcloned into pMMB208. The plasmids were transformed into E. coli S17-1λpir and conjugated into V. anguillarum.
Bioassay
Bioassay (cross feeding assay) was performed as described before (Tolmasky et al., 1988). Briefly, 2x CM9 medium supplemented with 20 μg/ml Cm, 1 mM IPTG, 40 μM EDDA and an overnight culture of V. anguillarum strains (5 μl/ml) grown in CM9 medium, was mixed 1:1 with 3.0 % melted agar adjusted to ~50 °C. After solidification, siderophore producing bacteria grown in CM9 was spotted on the plates, and the existence of a growth halo around the spots was recorded after 24 and 48 hours incubation at 25 °C.
Growth experiments
Overnight cultures of V. anguillarum strains in TSBS were inoculated 1:100 in CM9 broth and incubated overnight reaching an OD600 of 2.3 – 2.8. After incubation, the OD600 values of each strain were adjusted to 1 that corresponds to ~1.4 × 107 cells/ml, and 50 μl of the cultures were inoculated into 5 ml CM9 broth with or without ferric ammonium citrate (10μg/ml) or iron chelater EDDA (0, 0.1, 0.5, 1 and 5μM). The OD600 values were measured after 24 hours incubation at 25 °C. For streptonigrin experiments, 1 μg/ml streptonigrin (Sigma) dissolved in 10 mM Tris-HCl, pH 7.5 (1 mg/ml) was added in CM9, and the OD600 values were measured after 24 hours incubation at 25 °C.
RESULTS
Identification of fatE and fvtE encoding ATP binding proteins
The pJM1 plasmid encodes a homologue of ATP binding proteins of ABC transport systems (ORF40) (Di Lorenzo et al., 2003). We first constructed a deletion mutant of ORF40 named fatE to test whether this gene is essential for ferric-anguibactin transport. The result of cross-feeding assays in Table 3 shows that the deletion of fatE affects neither ferric-anguibactin nor ferric-vanchrobactin/enterobactin transport. The fact that the pJM1 cured strain, H775-3, still transports ferric-vanchrobactin/enterobactin indicates that other gene(s) encoding an ATP binding protein for ferric-vachrobactin/enterobactin must exist in the chromosome of this strain, and possibly also functional for ferric-anguibactin transport. The whole genome sequencing of V. anguillarum strain 775(pJM1) revealed that there is an ABC binding protein homologue, fvtE that exhibits 84% similarity (65% identity) with the fatE gene (Naka et al., 2011). We also found that the locus containing the fvtE gene also carries fvtB, fvtD and fvtC potentially encoding ABC transporters of ferric-siderophore (Fig. 1). We then mutated the fvtE gene in strain H775-3. The H775-3ΔfvtE::Km showed a defect in ferric-vanchrobactin/enterobactin transport while the fvtE mutant complemented in trans recovers the transport phenotype indicating that the fvtE gene is necessary for the ferric-vanchrobactin/enterobactin transport in H775-3 (Table 3). The fvtE mutation in strain 775(pJM1) (CC9-16ΔfvtE::Km) also caused the defect in ferric-vanchrobactin/enterobactin uptake but still could take up anguibactin. The double fatE and fvtE mutant in 775(pJM1) (CC9-16ΔfatE::TpΔfvtE::Km) transports neither ferric-anguibactin nor ferric-vanchrobactin/enterobactin (Table 3). Taken together, these results indicate that only fvtE is indispensable for ferric-vanchrobactin/enterobactin transport while both fatE and fvtE are functional for ferric-anguibactin transport. We also found that the overexpression of fatE in the double fatE and fvtE mutant resulted in weak ferric-vanchrobactin/enterobactin transport only at 48 hours but not at 24 hours incubation. However, this could be an artifact due to overexpression of fatE from the Ptac promoter of pMMB208.
Table 3.
Bioassay to assess whether fatE or fvtE is necessary for ferric siderophore transport
| Indicator strains | anguibactin | Iron sources | FAC | |
|---|---|---|---|---|
| vanchrobactin | enterobactin | |||
| H775-3 | − | + | + | + |
| H775-3ΔfvtE::Km | − | − | − | + |
| CC9-16 (pMMB208) | + | + | + | + |
| CC9-16ΔfatE::Tp (pMMB208) | + | + | + | + |
| CC9-16ΔfvtE::Km (pMMB208) | + | − | − | + |
| CC9-16ΔfatE::TpΔfvtE::Km (pMMB208) | − | − | − | + |
| CC9-16ΔfatE::TpΔfvtE::Km (pMMB208-fatE) | + | −* | −* | + |
| CC9-16ΔfatE::TpΔfvtE::Km (pMMB208-fvtE) | + | + | + | + |
A 50 µl aliquot of an overnight culture of indicator strains in CM9 broth was mixed with 20 ml of melted CM9 1.5% agar (adjusted to ~50°C) supplemented with 20 µM EDDA, 500 µM IPTG and µ0 mg ml−1 Cm. After the agar became solid, 5 μl of V. anguillarum 775(pJM1)(pMMB208) overnight culture as a source of anguibactin, 5μl of V. anguillarum 96F(pMMB208) overnight culture as a source of vanchrobactin, 1μl of 1 mg ml−1 purified enterobactin from EMC microcollections GmbH, and 1 μl of 1 mg ml−1 ferric ammonium citrate (FAC) were spotted on each plate. The existence of growth halos around the spots were recorded after 24 hours incubation at 25 °C.
+, growth;
−, no growth;
−*, a weak halo was observed after 48 hours incubation, possibly due to overexpression of fatE from the Ptac promoter in pMMB208.
H775-3, pJM1-cured strain of 775(pJM1); CC9-16, 775(pJM1) derivative of a Tn1 insertion mutant able to utilize ferric-anguibactin complexes but unable to synthesize anguibactin (Walter et al., 1983).
Fig 1. Two ABC transporter clusters identified in V. anguillarum 775(pJM1).
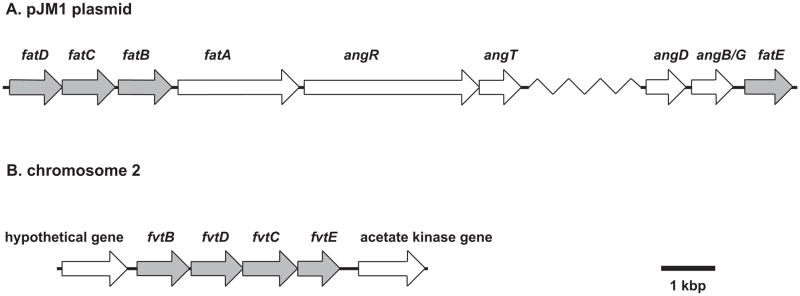
Schematic maps of the fatDCB-fatE locus on the pJM1 plasmid (panel A) and of the fvtBDCE on the chromosome 2 (panel B).
Growth effects of fvtE and fatE mutations under iron limiting conditions
We tested whether the mutation in fatE or fvtE actually affect the growth of the 775(pJM1) strain in various iron conditions. As shown in Fig. 2, under iron limitation, each fatE or fvtE mutant exhibited a similar growth rate to the wild type strain, while the double fatE and fvtE mutant showed less growth as compared with the wild type. Growth was restored close to the wild type strain level when the double fatE fvtE mutant was complemented with either fatE or fvtE in trans. On the other hand, we did not observe clear growth difference between any of the strains tested under iron rich conditions. These results indicate that fatE and fvtE are indeed important for the growth of V. anguillarum 775(pJM1) to survive under iron limiting conditions.
Fig 2. FatE and FvtE are important for the survival of V. anguillarum 775(pJM1) under various iron conditions.
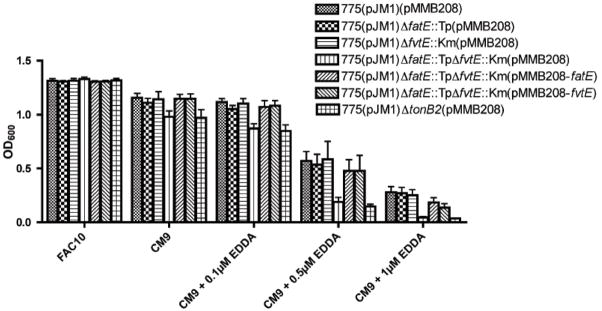
Overnight culture of V. anguillarum in CM9 was adjusted to an OD600 of 1.0, and 50 μl of the culture was inoculated into 5 ml CM9 broth either with or without addition of ferric ammonium citrate (10 μg/ml) or iron chelator EDDA (0, 0.1, 0.5, 1 and 5μM). The growth of each strain (OD600) was measured after 24 hours incubation at 25 °C. Experiments were repeated five times.
Streptonigrin survival test
Streptonigirin is an antibiotic that works when the iron concentration inside bacterial cells is high, thus we can compare the internal iron content by culturing the bacteria with and without this antibiotic. The wild type strain in which the internal iron concentration is high, showed much lower growth rate as compared with the tonB2 mutant in which internal iron concentration is low (Fig. 3). The double fatE and fvtE mutant grew at a higher rate than each single mutant while the double mutant complemented with either fatE or fvtE showed a comparable growth rate to the single mutants or similar growth to the wild type (Fig. 3). These results indicate that the iron concentration inside the double mutant is lower than in the wild type and each single mutant, and both FatE and FvtE are able to increase the internal iron concentration.
Fig 3. The double fatE fvtE mu\tant contains less iron inside the cell.
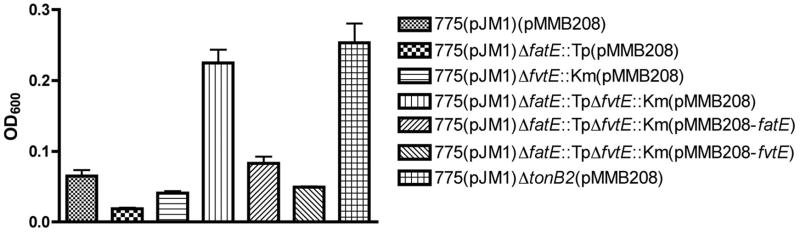
Overnight culture of V. anguillarum strain in CM9 was adjusted to an OD600 of 1.0, and 50 μl of the culture was inoculated into 5 ml CM9 broth supplemented with 1 μg/ml streptonigrin, and the growth of each strain (OD600) was measured after 24 hours incubation at 25 °C. Experiments were repeated at least three times.
Characterization of fvtB, fvtC and fvtD
The genome sequencing of V. anguillarum 775(pJM1) revealed that fvtE is located in the cluster containing genes potentially encoding ferric-siderophore ABC transporter proteins (Fig. 1). fvtB, fvtC and fvtD are homologues of fatB (31 % identity and 54 % similarity in amino acid sequence), fatC (38 % identity and 68 % similarity in amino acid sequence) and fatD (32 % identity and 57 % similarity in amino acid sequence), respectively. Since fvtE is involved in ferric-vanchrobactin and ferric-enterobactin transport as described above, we were interested in analyzing the requirement of fvtB, fvtC and fvtD for the ferric-siderophore transport. Our bioassay results indicate that the mutation in each gene abolishes the ferric-vanchrobactin or ferric-enterobactin transport while ferric-anguibactin transport is not affected with these mutations (Table 4). The ferric-vanchrobactin or ferric-enterobactin transport was recovered when these mutations were complemented in trans with each wild type gene (Table 4). Taken together, fvtB, fvtC and fvtD are specifically involved in ferric-vanchrobactin or ferric-enterobactin transport.
Table 4.
Bioassay to assess whether fvtBCD are necessary for ferric siderophore transport
| Indicator strains | anguibactin | Iron sources | FAC | |
|---|---|---|---|---|
| vanchrobactin | enterobactin | |||
| CC9-16 (pMMB208) | + | + | + | + |
| CC9-16ΔfvtB (pMMB208) | + | − | − | + |
| CC9-16ΔfvtB (pMMB208-fvtB) | + | + | + | + |
| CC9-16ΔfvtC (pMMB208) | + | − | − | + |
| CC9-16ΔfvtC (pMMB208-fvtC) | + | + | + | + |
| CC9-16ΔfvtD (pMMB208) | + | − | − | + |
| CC9-16ΔfvtD (pMMB208-fvtD) | + | + | + | + |
A 50 µl aliquot of an overnight culture of indicator strains in CM9 broth was mixed with 20 ml of melted CM9 1.5% agar (adjusted to ~40°C) supplemented with 20 µM EDDA, 500 µM IPTG and 10 µg ml−1 Cm. After the agar became solid, 5 μl of V. anguillarum 775(pJM1)(pMMB208) overnight culture as a source of anguibactin, 5μl of V. anguillarum 96F(pMMB208) overnight culture as a source of vanchrobactin, 1μl of 1 mg ml−1 purified enterobactin from EMC microcollections GmbH, and 1 μl of 1 mg ml−1 ferric ammonium citrate (FAC) were spotted on each plate. The existence of growth halos around the spots were recorded after 24 hours incubation at 25 °C.
+, growth
−, no growth.
CC9-16, 775(pJM1) derivative of a Tn1 insertion mutant able to utilize ferric-anguibactin complexes but unable to synthesize anguibactin (Walter et al., 1983).
CONCLUSIONS
In this work, we have identified two genes, fatE and fvtE, encoding ATP binding proteins involved in ferric-anguibactin transport. fatE is specific to ferric-anguibactin transport while fvtE is functional for both ferric-anguibactin and ferric-vanchrobactin/enterobactin transport. Furthermore, we identified homologues of ABC transport proteins for ferric-vanchrobactin/enterobactin. FvtB is a homologue of a periplasmic binding protein while fvtC and fvtD are homologues of cytoplasmic membrane proteins. We showed that fvtB, fvtC and fvtD are essential for ferric-vancrhobactin/enterobactin transport but not for ferric-anguibactin transport. Our previous report showed that fatB encoding a periplasmic binding protein, and fatC and fatD encoding cytoplasmic membrane proteins are only functional for ferric-anguibactin transport but not for ferric-vanchrobactin/enterobactin transport (Naka et al., 2010). Taken together, we conclude that two ferric-siderophore ABC transporters, pJM1-encoded FatDCB-FatE and chromosome 2-encoded FvtBDCE, play a role in ferric-anguibactin and ferric-vanchrobactin/enterobactin, respectively, and FvtE is also functional for ferric-anguibactin transport. These results are quite different from the finding in Vibrio cholerae. In V. cholerae, it has been shown that two sets of ABC transport systems encoded by vctPDGC and viuPDGC are functionally redundant for the transport of both, the endogenous siderophore vibriobactin and the exogenous siderophore enterobactin, recognized by different specific outer membrane receptors (Wyckoff et al., 1999; Mey et al., 2002). fvtB, fvtD, fvtC and fvtE are homologues of vctP, vctD, vctG and vctC, respectively, and the genetic organization of V. anguillarum fvtBDCE and V. cholerae vctPDGC is very similar. The region encompassing vctPDGC that includes the hly region containing the hemolysin gene hlyA and lipase genes lipAB, was proposed to be a pathogencity island encoding products capable of damaging host cells and/or involved in nutrient acquisition (Ogierman et al., 1997). The homologous locus of the V. cholerae hlyA region has been identified in V. anguillarum (Rock & Nelson, 2006). Our analysis of genomic data of V. anguillarum 775(pJM1) indicates that the fvtBDCE cluster is also located adjacent to the V. anguillarum hly region. Based on these facts, we hypothesize that fvtBDCE in V. anguillarum and vctPDGC in V. choleare might have originated from the same ancestral ABC transporter while fatBCD-fatE in V. anguillarum and viuPDGC in V. cholerae could be horizontally acquired after these bacteria were separated from the common ancestor. We previously proposed that the pJM1 plasmid was possibly acquired by V. anguillarum to obtain a stronger anguibactin siderophore system by replacing the chromosomal-encoded siderophore vanchrobactin (Naka et al., 2008). In addition to pJM1-encoded FatE, the existence of another functional ATP binding protein, FvtE, for ferric-anguibactin transport could probably easily have facilitated the acquisition of the pJM1 plasmid during evolution ensuring the ability to take up ferric-anguibactin.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health grants AI19018 GM64600 to J.H.C. We thank Lidia M. Crosa, Ph.D., for reviewing the manuscript.
Footnotes
The authors have no conflict of interest to declare.
References
- Actis LA, Tolmasky ME, Crosa JH. Vibriosis. In: Woo PTK, Bruno DW, editors. Vibriosis. Oxfordshire, UK: CABI International; 2011. pp. 570–605. [Google Scholar]
- Actis LA, Tolmasky ME, Crosa LM, Crosa JH. Characterization and regulation of the expression of FatB, an iron transport protein encoded by the pJM1 virulence plasmid. Mol Microbiol. 1995;17:197–204. doi: 10.1111/j.1365-2958.1995.mmi_17010197.x. [DOI] [PubMed] [Google Scholar]
- Aguirre-Guzmán G, Mejia Ruíz H, Ascencio F. A review of extracellular virulence product of Vibrio species important in diseases of cultivated shrimp. Aquaculture Research. 2004;35:1395–1404. [Google Scholar]
- Balado M, Osorio CR, Lemos ML. A gene cluster involved in the biosynthesis of vanchrobactin, a chromosome-encoded siderophore produced by Vibrio anguillarum. Microbiology. 2006;152:3517–3528. doi: 10.1099/mic.0.29298-0. [DOI] [PubMed] [Google Scholar]
- Balado M, Osorio CR, Lemos ML. Biosynthetic and regulatory elements involved in the production of the siderophore vanchrobactin in Vibrio anguillarum. Microbiology. 2008;154:1400–1413. doi: 10.1099/mic.0.2008/016618-0. [DOI] [PubMed] [Google Scholar]
- Balado M, Osorio CR, Lemos ML. FvtA is the receptor for the siderophore vanchrobactin in Vibrio anguillarum: utility as a route of entry for vanchrobactin analogues. Appl Environ Microbiol. 2009;75:2775–2783. doi: 10.1128/AEM.02897-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conchas RF, Lemos ML, Barja JL, Toranzo AE. Distribution of plasmid- and chromosome-mediated iron uptake systems in Vibrio anguillarum strains of different origins. Appl Environ Microbiol. 1991;57:2956–2962. doi: 10.1128/aem.57.10.2956-2962.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa JH. A plasmid associated with virulence in the marine fish pathogen Vibrio anguillarum specifies an iron-sequestering system. Nature. 1980;284:566–568. doi: 10.1038/284566a0. [DOI] [PubMed] [Google Scholar]
- Crosa JH, Walsh CT. Genetics and assembly line enzymology of siderophore biosynthesis in bacteria. Microbiol Mol Biol Rev. 2002;66:223–249. doi: 10.1128/MMBR.66.2.223-249.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuiv PO, Clarke P, Lynch D, O'Connell M. Identification of rhtX and fptX, novel genes encoding proteins that show homology and function in the utilization of the siderophores rhizobactin 1021 by Sinorhizobium meliloti and pyochelin by Pseudomonas aeruginosa, respectively. J Bacteriol. 2004;186:2996–3005. doi: 10.1128/JB.186.10.2996-3005.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeShazer D, Woods DE. Broad-host-range cloning and cassette vectors based on the R388 trimethoprim resistance gene. Biotechniques. 1996;20:762–764. doi: 10.2144/96205bm05. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo M, Stork M, Tolmasky ME, Actis LA, Farrell D, Welch TJ, Crosa LM, Wertheimer AM, Chen Q, Salinas P, Waldbeser L, Crosa JH. Complete sequence of virulence plasmid pJM1 from the marine fish pathogen Vibrio anguillarum strain 775. J Bacteriol. 2003;185:5822–5830. doi: 10.1128/JB.185.19.5822-5830.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisez L, Ollevier F. Comparative Serology of the Marine Fish Pathogen Vibrio anguillarum. Appl Environ Microbiol. 1995;61:4367–4373. doi: 10.1128/aem.61.12.4367-4373.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannauer M, Barda Y, Mislin GL, Shanzer A, Schalk IJ. The ferrichrome uptake pathway in Pseudomonas aeruginosa involves an iron release mechanism with acylation of the siderophore and recycling of the modified desferrichrome. J Bacteriol. 2010;192:1212–1220. doi: 10.1128/JB.01539-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehl CJ, Crosa JH. The TonB energy transduction systems in Vibrio species. Future Microbiol. 2010;5:1403–1412. doi: 10.2217/fmb.10.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kustusch RJ, Kuehl CJ, Crosa JH. Power plays: iron transport and energy transduction in pathogenic vibrios. Biometals. 2011;24:559–566. doi: 10.1007/s10534-011-9437-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen JL, Pedersen K, Dalsgaard I. Vibrio anguillarum serovars associated with vibriosis in fish. Journal of Fish Diseases. 1994;17:259–267. [Google Scholar]
- Lemos ML, Salinas P, Toranzo AE, Barja JL, Crosa JH. Chromosome-mediated iron uptake system in pathogenic strains of Vibrio anguillarum. J Bacteriol. 1988;170:1920–1925. doi: 10.1128/jb.170.4.1920-1925.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez CS, Crosa JH. Characterization of ferric-anguibactin transport in Vibrio anguillarum. Biometals. 2007;20:393–403. doi: 10.1007/s10534-007-9084-9. [DOI] [PubMed] [Google Scholar]
- Mey AR, Wyckoff EE, Oglesby AG, Rab E, Taylor RK, Payne SM. Identification of the Vibrio cholerae enterobactin receptors VctA and IrgA: IrgA is not required for virulence. Infect Immun. 2002;70:3419–3426. doi: 10.1128/IAI.70.7.3419-3426.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton DL, O'Toole R, Horstedt P, Wolf-Watz H. Flagellin A is essential for the virulence of Vibrio anguillarum. J Bacteriol. 1996;178:1310–1319. doi: 10.1128/jb.178.5.1310-1319.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales VM, Backman A, Bagdasarian M. A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene. 1991;97:39–47. doi: 10.1016/0378-1119(91)90007-x. [DOI] [PubMed] [Google Scholar]
- Naka H, Chen Q, Mitoma Y, Nakamura Y, McIntosh-Tolle D, Gammie AE, Tolmasky ME, Crosa JH. Two replication regions in the pJM1 virulence plasmid of the marine pathogen Vibrio anguillarum. Plasmid. 2012;67:95–101. doi: 10.1016/j.plasmid.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka H, Crosa JH. Identification and characterization of a novel outer membrane protein receptor FetA for ferric enterobactin transport in Vibrio anguillarum 775 (pJM1) Biometals. 2011a doi: 10.1007/s10534-011-9488-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka H, Crosa JH. Genetic Determinants of Virulence in the Marine Fish Pathogen Vibrio anguillarum. Fish Pathol. 2011b;46:1–10. doi: 10.3147/jsfp.46.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka H, Dias GM, Thompson CC, Dubay C, Thompson FL, Crosa JH. Complete Genome Sequence of the Marine Fish Pathogen Vibrio anguillarum Harboring the pJM1 Virulence Plasmid and Genomic Comparison with Other Virulent Strains of V. anguillarum and V. ordalii. Infect Immun. 2011;79:2889–2900. doi: 10.1128/IAI.05138-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka H, Lopez CS, Crosa JH. Reactivation of the vanchrobactin siderophore system of Vibrio anguillarum by removal of a chromosomal insertion sequence originated in plasmid pJM1 encoding the anguibactin siderophore system. Environ Microbiol. 2008;10:265–277. doi: 10.1111/j.1462-2920.2007.01450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka H, Lopez CS, Crosa JH. Role of the pJM1 plasmid-encoded transport proteins FatB, C and D in ferric anguibactin uptake in the fish pathogen Vibrio anguillarum. Environ Microbiol Rep. 2010;2:104–111. doi: 10.1111/j.1758-2229.2009.00110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogierman MA, Fallarino A, Riess T, Williams SG, Attridge SR, Manning PA. Characterization of the Vibrio cholerae El Tor lipase operon lipAB and a protease gene downstream of the hly region. J Bacteriol. 1997;179:7072–7080. doi: 10.1128/jb.179.22.7072-7080.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillard C, Le Roux F, Borrego JJ. Bacterial disease in marine bivalves, a review of recent studies: trends and evolution. Aquatic Living Resources. 2004;17:477–498. [Google Scholar]
- Pedersen K, Grisez L, van Houdt R, Tiainen T, Ollevier F, Larsen JL. Extended serotyping scheme for Vibrio anguillarum with the definition and characterization of seven provisional O-serogroups. Curr Microbiol. 1999;38:183–189. doi: 10.1007/pl00006784. [DOI] [PubMed] [Google Scholar]
- Raymond KN, Dertz EA. In: Iron Transport in Bacteria. Crosa JH, Mey AR, Payne SM, editors. 2004. pp. 3–17. [Google Scholar]
- Reimmann C. Inner-membrane transporters for the siderophores pyochelin in Pseudomonas aeruginosa and enantio-pyochelin in Pseudomonas fluorescens display different enantioselectivities. Microbiology. 2012;158:1317–1324. doi: 10.1099/mic.0.057430-0. [DOI] [PubMed] [Google Scholar]
- Rock JL, Nelson DR. Identification and characterization of a hemolysin gene cluster in Vibrio anguillarum. Infect Immun. 2006;74:2777–2786. doi: 10.1128/IAI.74.5.2777-2786.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senanayake SD, Brian DA. Precise large deletions by the PCR-based overlap extension method. Mol Biotechnol. 1995;4:13–15. doi: 10.1007/BF02907467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R, Priefer U, Puhler A. A broad host range mobilization system in vivo genetic engineering transposon mutagenesis in gram negative bacteria. Bio/Technology. 1983;1:787–796. [Google Scholar]
- Soengas RG, Anta C, Espada A, Paz V, Ares IR, Balado M, Rodríguez J, Lemos ML, Jiménez C. Structural characterization of vanchrobactin, a new catechol siderophore produced by the fish pathogen Vibrio anguillarum serotype O2. Tetrahedron letters. 2006;47:7113–7116. [Google Scholar]
- Sorensen UB, Larsen JL. Serotyping of Vibrio anguillarum. Appl Environ Microbiol. 1986;51:593–597. doi: 10.1128/aem.51.3.593-597.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork M, Di Lorenzo M, Mourino S, Osorio CR, Lemos ML, Crosa JH. Two tonB systems function in iron transport in Vibrio anguillarum, but only one is essential for virulence. Infect Immun. 2004;72:7326–7329. doi: 10.1128/IAI.72.12.7326-7329.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork M, Otto BR, Crosa JH. A novel protein, TtpC, is a required component of the TonB2 complex for specific iron transport in the pathogens Vibrio anguillarum and Vibrio cholerae. J Bacteriol. 2007;189:1803–1815. doi: 10.1128/JB.00451-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiainen T, Pedersen K, Larsen JL. Vibrio anguillarum serogroup O3 and V. anguillarum-like serogroup O3 cross-reactive species—comparison and characterization. Journal of applied microbiology. 1997;82:211–218. [PubMed] [Google Scholar]
- Tolmasky ME, Actis LA, Crosa JH. Genetic analysis of the iron uptake region of the Vibrio anguillarum plasmid pJM1: molecular cloning of genetic determinants encoding a novel trans activator of siderophore biosynthesis. J Bacteriol. 1988;170:1913–1919. doi: 10.1128/jb.170.4.1913-1919.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toranzo AE, Barja JL. A review of the taxonomy and seroepizootiology of Vibrio anguillarum, with special reference to aquaculture in the Northwest of Spain. Diseases of aquatic organisms. 1990;9:73–82. [Google Scholar]
- Toranzo AE, Magariños B, Romalde JL. A review of the main bacterial fish diseases in mariculture systems. Aquaculture. 2005;246:37–61. [Google Scholar]
- Walter MA, Potter SA, Crosa JH. Iron uptake system medicated by Vibrio anguillarum plasmid pJM1. J Bacteriol. 1983;156:880–887. doi: 10.1128/jb.156.2.880-887.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelmann G. Iron Transport in Bacteria. 2004. Ecology of siderophores; pp. 437–450. [Google Scholar]
- Wu H, Ma Y, Zhang Y, Zhang H. Complete sequence of virulence plasmid pEIB1 from the marine fish pathogen Vibrio anguillarum strain MVM425 and location of its replication region. J Appl Microbiol. 2004;97:1021–1028. doi: 10.1111/j.1365-2672.2004.02387.x. [DOI] [PubMed] [Google Scholar]
- Wyckoff EE, Valle AM, Smith SL, Payne SM. A multifunctional ATP-binding cassette transporter system from Vibrio cholerae transports vibriobactin and enterobactin. J Bacteriol. 1999;181:7588–7596. doi: 10.1128/jb.181.24.7588-7596.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


