Abstract
By controlling and limiting inflammatory conditions, naturally occurring regulatory T cells (nTregs), defined as circulating CD4+CD25brightFoxP3+ cells, play critical roles in maintaining tolerance and preventing autoimmunity, and thus have tremendous potential for adoptive immunotherapy. Because they represent a scanty subset of the CD4+ T lymphocyte subset, several approaches have been developed to isolate and expand ex vivo polyclonal Tregs. However, one limitation of the functional analyses performed on these cultured Tregs is the incomplete characterization of their tissue trafficking properties. As this aspect provides crucial information for their therapeutic effects, we have here explored the chemokine receptor expression profile and function of Tregs cultured ex vivo with validated expansion protocols. Our data show that ex vivo cultured Tregs retained the expression of CCR7 but dramatically down regulated CCR5 as compared to freshly isolated Tregs. The differential chemokine receptors expression pattern corroborated with their respective steady state messenger RNA expression and also their migration towards specific chemokines. Our analyses suggest that ex vivo cultured Tregs may display impaired or suboptimal migration to the inflamed tissues releasing RANTES and MIP-1α chemokines.
Keywords: Tregs, Rapalog, Chemokine
INTRODUCTION
CD4+CD25+FoxP3+ regulatory T cells (Tregs) play a crucial role in controlling and limiting the inflammatory milieu1 through suppression of cytokine production and inhibition of T-cell proliferation2. Transfer of T cells depleted of the CD4+CD25+ subset into neonatal thymectomized mice results in the onset of systemic autoimmune diseases, such as colitis, gastritis, insulin-dependent autoimmune diabetes, and thyroiditis3. These inflammatory conditions are then strongly attenuated when CD4+CD25+ Tregs are co-transferred with T effector (Teff) cells3,4. Several evidences also correlate the emergence of human diseases such as multiple sclerosis, rheumatoid arthritis, type 1 diabetes, inflammatory bowel disease (IBD) and autoimmune lymphoproliferative syndrome with either decreased frequencies or defective suppressive function of Tregs5,6.
The central role of Tregs in modulating inflammatory processes has led to a quest for novel therapies based on the adoptive transfer of these cells to treat a variety of immunologic disorders, ranging from autoimmunity to transplantation, allergy and asthma7. However, the clinical translation of the promising results obtained in preclinical models8 requires the selection and ex vivo expansion of large numbers of Tregs since this T-cell subset represents less than 5% of the CD4+ T cells circulating in the peripheral blood of healthy subjects9.
Circulating naturally occurring Tregs (nTregs) can be isolated by using a two-step magnetic cell-separation that takes advantage of their constitutive co-expression of the CD25 and CD4 cell surface markers10. These CD25bightCD4+ cells can then be expanded ex vivo by stimulation through their T-cell receptor (TCR) using a variety of tools, including monoclonal antibodies or coated Xcyte beads11, irradiated CD4+CD25– feeder cells12 and cytokines such as interleukin-2 (IL-2)13. T-cell products obtained using these methodologies usually retain immunosuppressive capacity, but are frequently contaminated with Teff cells expressing CD25 and expanding in response to TCR stimulation and IL-2. To overcome this limitation, rapamycin analogues (rapalogs) have been added to these cultures to inhibit the growth of contaminating Teff cells while preferentially promoting Treg expansion14.
Although the isolation and expansion of functional inhibitory Tregs is feasible, therapeutic strategies employing Tregs have to take into account that these cells not only require potent suppressive function but also need appropriate tissue trafficking to enable contact with their target cells. A fraction of expanded Tregs must retain the expression of molecules such as CD62L and CCR7 that are crucial for their migration to the lymph nodes draining inflamed tissues where they can block the activation and expansion of reactive or autoreactive Teff cells15. However, Tregs must also migrate directly to the inflammation sites to locally contain the inflammation16. Since distinct chemokine axis are involved in regulating the recruitment of T lymphocytes and Tregs to maintain tissue homeostasis17, we have investigated the chemokine receptor profile of freshly isolated nTregs and compared it to that of ex vivo cultured Tregs, in order to discover if and how culture conditions affect this expression pattern. This analysis is vital, as modifications in Treg-migration properties would have important implications for their clinical use.
MATERIALS AND METHODS
Blood samples
Peripheral blood was obtained from buffy coat preparations derived from 9 healthy volunteer donors (Gulf Coast Regional Blood Center, Houston, TX).
Cell isolation and in vitro expansion protocols
Peripheral blood mononuclear cells (PBMCs) were separated by density-gradient centrifugation over Lymphoprep (AXIS-SHIELD PoC AS, Oslo, Norway).
eTregs expansion
(Fig. 1A): Naturally occurring Treg cells (nTregs) (CD4+CD25bright) were isolated from PBMCs using positive selection, after labeling cells with CD25 magnetic beads (2 µL/107 cells; Miltenyi Biotec Inc., Auburn, CA), followed by selection of CD4+ cells with CD4 MicroBeads (20 µL/107 cells; Miltenyi) as previously described18. Suppl. Fig. 1A and Suppl Table 1 illustrate the gating used to identify the CD25bright population during our purification step. The Mean Fluorescence Intensity (MFI) used to denote CD25bright cells was 99–110. The average purity of CD4+CD25bright T cells was 97% ± 3.4% (n = 9). After selection, CD4+CD25bright (106 cells/mL) were cultured in complete medium consisting of RPMI1640 (Hyclone, South Lorgan, UT), 10% AB-human serum (Valley Biomedical, Winchester, VA, US), 2 mM L-glutamine (BioWhittaker Inc., Walkersville, MD) and penicillin-streptamycin (BioWhittaker Inc., Walkersville, MD), in the presence of β-mercaptoethanol (Invitrogen, Carlsbad, CA). On Day 0, the purified CD4+CD25bright T cells were activated in 24-well plates coated with anti-CD3 antibody (OKT3, Orthoclone, Cilag Ag Int., Zug, Switzerland) (1 µg/mL) and anti-CD28 mAb (1 µg/mL) (BD Biosciences PharMingen, San Diego, CA) in complete medium ± Temsirolimus (LC Laboratories, Woburn, MA) at a final concentration of 1 µg/mL. On Days 7 and 14, the cells were harvested, counted, and restimulated with anti-CD3, anti-CD28, ± Temsirolimus, plus rIL-2 (50 IU/mL) (Proleukin; Chiron, Emeryville, CA). During the experiment, the cells were restimulated only when cell concentration was ≥ 5×106/mL. At the end of 3 weeks (Day 21), cells were harvested, counted and used for experiments. The expanded Tregs obtained through this protocol were named as eTregs to signify that they have been in vitro expanded from nTregs.
Figure 1. Ex vivo culture and functional characterization of freshly isolated and ex vivo expanded Tregs.
(A) Naturally occurring Tregs (nTregs; D4+CD25bright) and (B) CD4+ T cells were purified from PBMCs as schematically represented and cultured to obtain eTreg and rTreg, respectively. (C) Flow cytometry dot plots of nTregs, eTregs and rTregs from 1 representative donor, stained with anti-CD4, anti-CD25 and anti-FoxP3 fluorochrome-conjugated mAbs. (D) The suppressive activities of nTregs, eTregs, rTregs, and control CD25low/neg4+ T cells were evaluated using a CFSE-based assay in which PBMCs labeled with CFSE were stimulated with irradiated allogeneic feeders and OKT3 in the presence or absence of Tregs. Upper panels illustrate the proliferation of T cells using a CFSE-assay in the absence or in the presence of the different Tregs population and for a representative experiment, confirming the inhibitory activity of nTregs, eTregs and rTregs. The lower graph illustrates the percentage of suppression of T cells in the presence of the different Tregs (n = 9 donors). Data show mean ± SD.
rTregs expansion
(Fig. 1B): CD4+ T cells were purified from PBMCs by negative selection using the untouched CD4+ T-cell isolation kit (Miltenyi), according to the manufacturer’s instructions. The average purity of CD4+ T cells was 92% ± 4.2% (n = 9). Purified CD4+ T cells were cultured in complete medium at 106 cells/mL. The in vitro expansion protocol was adapted from a previously described protocol19, with slight modification. Briefly, on Day 0, the purified CD4+ T cells were activated in 24-well plates coated with anti-OKT3 (1 µg/mL) and anti-CD28 mAb (1 µg/mL) in completeRPMI1640 ± Rapamycin (Sigma, St. Louis, MO) at a final concentration of 100 nm. On Days 7 and 14, the cells were harvested, counted, and restimulated with anti-OKT3, anti-CD28, ± Rapamycin, and recombinant rIL-2 (50 IU/mL). During the experiment, the cells were passaged only when the medium was exhausted (turned yellow) or cell concentration was ≥ 5 × 106/mL. At the end of 3 weeks (Day 21), cells were harvested, counted and used for experiments. The expanded Tregs obtained through this protocol are named as rTregs to signify that in vitro expanded in the presence of Rapamycin.
Phenotypic analysis
Expression of cell surface molecules was determined by flow cytometry using a FACSCalibur system (BD Biosciences), equipped with the filter set for quadruple fluorescence signals. The respective mAbs conjugated with phycoerythrin (PE), fluorescein isothiocyanate (FITC), allophycocyanin (PerCP) and/or peridinin chlorophyll proteins (APC) were used: CD3, CD4, CD25, CCR1, CCR3, CCR4, CCR5, CCR7, CCR9, CXCR3 and CXCR4. Staining for FoxP3 (eBioscience Inc, San Diego, CA) was performed following manufacturer’s instruction. Samples were analyzed using CellQuest Pro software (BD Biosciences). At least 10,000 positive events were measured for surface staining and 50,000 events were collected for FoxP3 samples.
Suppression assay
The assay was performed as described previously20. Briefly, PBMCs were labeled with 1.5 µM carboxyfluorescein succinimidyl ester (CFSE) (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions. PBMCs were then stimulated with irradiated (40 Gy) allogeneic PBMCs (at a 4:1 effector/feeders ratio) and 0.5 µg/mL OKT3. To assess their suppressive capacity, Tregs were added to the culture (at a 1:1 or 1:2 ratio). As positive control, we used freshly isolated naturally occurring Treg cells (nTregs). On day 7 of culture, cells were labeled with PE-, PerCP-, or APC-conjugated CD4 and CD8 mAbs and CFSE dilution was analyzed using a FACSCalibur to assess cell proliferation. Suppression was expressed as the % of inhibition of T cells proliferation in the presence of the different Treg-subsets.
Transwell migration assay
Transwell migration assay was performed using the 5-µm pore 24-transwell plates (Corning Life Sciences, Acton, MA), as previously described20. The chemoattractant ligands (R&D system) were added to the lower chamber, whereas 51Cr (100 µCi) labeled Tregs were loaded in the upper chamber. 51Cr labeled cells, loaded directly into the lower chamber, served as the positive control, whereas lower chambers without any recombinant ligands were used as negative controls. Plates were incubated at 37°C, 5% CO2 for 4 hours before being assayed for migration. Post-incubation, the transwell inserts were disposed and the cells from the lower compartment were recovered and lysed with Triton-X (Sigma-Aldrich). Chromium release was quantified in the lysates using a gamma counter (PerkinElmer Life and Analytical Sciences, Waltham, MA). The percentage of migrating cells was calculated as follows: 100×[cpm from experimental supernatant (cells migrated in the lower chamber) - cpm in the presence of media only (random migration) / cpm of positive control - cpm random migration]. Specificity of the observation was proved by blocking migration of Tregs by addition of the respective blocking antibodies (all from R&D Systems) or isotype controls (R&D Systems) to the lower chamber before the chemotactic assay.
RNA Isolation and Quantitative Real Time PCR (qRT-PCR) Analysis
Total RNA was isolated by extraction with Trizol (Invitrogen, Carlsbad, CA) and treated with DNase I (Invitrogen, Carlsbad, CA), following the manufacturer’s protocols. RNAs were quantitated and checked for integrity and quality by 1% non-denaturing agarose (Sigma, St. Louis, MO) gel. 1 µg of total RNA was subjected to reverse transcription using a QuantiTect reverse transcription reagent kit (Qiagen, Valencia, CA). PCR amplification was carried out using iQ SYBR Green Supermix (Bio-Rad, Hercules, CA) and a C1000 Thermal Cycler (Bio-Rad CFX96 Real-Time System). Primers were designed so as to produce different amplicon sizes for each target gene. Before being used in real-time PCR, each primer pair was authenticated by standard reverse transcription-PCR and by checking the amplicons for the right size. The results were expressed after normalization to β-Actin expression levels from three different experiments. Data has been represented as means ± S.D. The primer sequences for the different genes were as follows:
CCR1 forward (F), 5′-TGGAACCAGAGAGAAGCCGGGATG-3′;
CCR1 reverse (R), 5′-AAGGCAAACACGGCGTGGAC-3′;
CCR5 (F), 5′-TGTGTAGTGGGATGAGCAGAGAACA-3′;
CCR5 (R), 5′-AGGCGGGCTGCGATTTGCTT-3′;
CCR7 (F), 5′-CCTTCTGGGCCTACAGCGCG-3′;
CCR7 (R), 5′-TGGTGGTGGTCTCGGCCTCC-3′;
CCR9 (F), 5′-ACACCCACAGACTTCACAAGCCCTA-3′;
CCR9 (R), 5′-TCATGGCCTGGGCAATGGCAAT-3′.
Statistical analysis
All in vitro experiments were summarized as mean ± SD. Student’s t test was used to determine the statistical significant differences between samples, with p < 0.05 indicating a significant difference.
RESULTS AND DISCUSSION
Rapalog-selection enriches functional Tregs
For the current study, we generated Tregs according to two different approaches, as detailed in the Material and Methods section and Fig. 1A and B. For the first approach, the starting population was represented by naturally occurring CD4+CD25bright cells (nTregs) isolated from the PBMCs of nine healthy donors, using immunomagnetic selection. Hereon, Tregs expanded using this protocol will be designated as eTregs (Fig. 1A). For the second approach, the starting cell population was instead represented by CD4+ T cells, also obtained after immunomagnetic selection as previously described19. Hereon, Tregs expanded using this approach will be designated as rTregs (Fig. 1B). First, we identified the rapalog that provided the best expansion rate while preserving suppressive capacity. As shown in Suppl. Fig 1B, eTreg expanded best in the presence of Temsirolimus, while rTregs had superior expansion in the presence of rapamycin. Next, IL-2 concentrations were determined by using a dose response curve to evaluate the change in expansion rate. We found that an increase in IL-2 dose was paralleled by an expansion of the cells number (Suppl Fig. 1C; top panel). However, cells that were expanded with > 100 IU/mL of IL-2 had significantly reduced suppressive functionality (Suppl Fig. 1C; middle panel) and FoxP3 expression (Suppl Fig. 1C; bottom panel). Similarly, titration experiments with different concentrations of Temsirolimus were performed to determine the optimum concentration of the drugs providing expansion without compromising functionality or viability (Suppl Fig. 2). Of note, for rapamycin we used previously validated concentrations19.
At the end of the third week of culture, eTregs and rTregs expanded 5 ± 0.6 and 8 ± 0.7, respectively. Phenotypic analyses after 3 weeks of culture showed that 68% ± 4.3% of eTregs and 41% ± 0.3% of rTregs expressed FoxP3 as compared to 72% ± 0.3% of freshly isolated nTregs (Fig. 1C). In contrast, cells expanded using the same protocol in the absence of rapalogs, lost FoxP3 expression by 3 weeks of culture (4% ± 3%), suggesting that in the absence of rapalog Teff cells preferentially expand (data not shown). The expression of FoxP3 by both eTregs and rTregs paralleled their inhibitory function, as assessed in vitro using a CFSE-based suppression assay (Fig. 1D). As expected, proliferation of T lymphocytes in response to OKT3 antibody and allogeneic feeder cells (90% ± 2.5%) was significantly reduced when freshly isolated nTregs were added to the culture (16.4% ± 5.5%, p<0.01), accounting for a suppression of 88.8% ± 1.5% for CD8+ cells (p < 0.0001). Similarly, proliferation of T cells was reduced when either eTregs or rTregs were added to the cultures (16.7% ± 6.5% and 16.8% ± 5.8%, respectively, p<0.01), accounting for a suppression of 86.3% ± 3% and 87% ± 2.3% for CD8+ cells , respectively (p < 0.0001). In contrast, proliferation of T cells was not significantly changed in the presence of control T cells grown without rapalogs (CD25low/neg4+) (85.1% ± 4.3%), accounting for a suppression of 9% ± 2.8% (p = NS). Of note, we observes similar suppressions irrespective of the Treg:Teff ratio used in the experiment (Suppl Fig. 3).
Ex vivo expanded Tregs show specific pattern of chemokine receptor expression
The tissue homing of Tregs is discretely regulated, which in turn dictate where the in vivo suppressive functions are exerted. Hence, we next determined the chemokine receptor expression profile of eTregs and rTregs as compared to freshly isolated nTregs, with the goals of characterizing their homing capacity and predicting their potential trafficking upon adoptive transfer in vivo. As shown in Fig. 2A and 2B ex vivo expanded Tregs upregulated or downregulated the expression of specific chemokine receptors when compared to freshly isolated nTregs. We found that CXCR4 was upregulated in both eTregs (19.3% ± 4.7%) and rTregs (21.5% ± 1.7%) as compared to nTregs (6.6% ± 1.5%) (p < 0.05). In contrast, CCR4 and CCR5 were both significantly downregulated in eTregs (36.3% ± 2.3% and 0.1% ± 0.1%, respectively) and rTregs (15.6% ± 0.7% and 2.3% ± 0.5%, respectively) as compared to nTregs (63% ± 7.2% and 56% ± 6.5%, respectively) (p < 0.05). No significant differences were observed in receptors such as CXCR3 (eTregs 11.3% ± 1.5%, rTregs 18.6% ± 1.6%, and nTregs 26.6% ± 2.0%, respectively), CCR1, CCR3 and CCR9 (<1% in all three subsets). As previously described21 CCR7 expression (17% ± 4.3% on freshly isolated nTregs) was retained by rTregs (11.5% ± 1.7%) and upregulated by eTregs (37.6% ± 6.4%) (p < 0.05).
Figure 2. Expression profile of chemokine receptors by differentially cultured Tregs.
(A) Analysis of chemokine receptors in freshly isolated nTregs and ex vivo cultured eTregs and rTregs. Shown are flow cytometry dot plots for nTregs, eTregs and rTregs, stained with anti-CXCR4, CCR4, CCR5, CXCR3, CCR1, CCR3, CCR9 and CCR7 fluorochrome-conjugated mAb from 1 representative donor. The percentages shown represent the double positive cells (chemokine receptor and CD25+). (B) Comparative phenotypic analysis of chemokine receptors expression in freshly isolated nTregs and ex vivo cultured eTregs and rTregs. Bars (white for nTregs; black for eTregs and gray for rTregs) represent mean ± SD of 5 different donors.
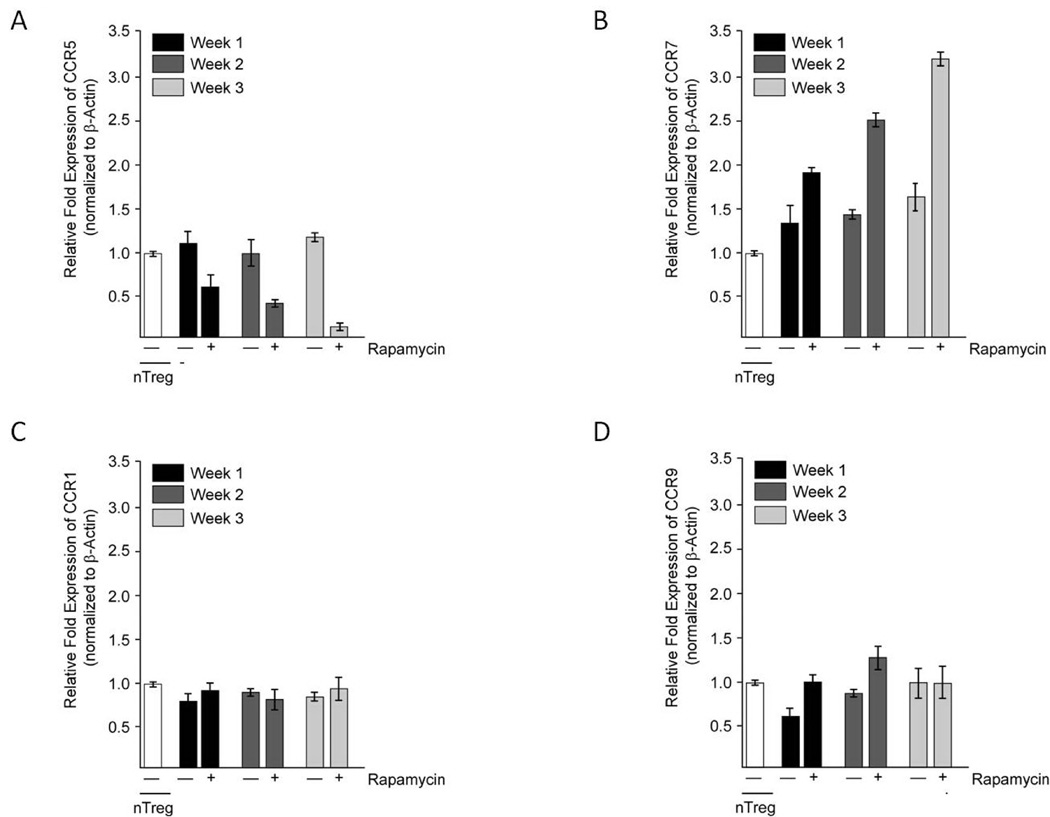
We next investigated if the observed downregulation of CCR5 expression in eTregs (Fig. 3A) and rTregs (data not shown) was associated with corresponding downregulation of its messenger RNA. As shown in Fig. 3A, CCR5 mRNA levels were significantly reduced (85% ± 0.01% reduction) at the end of week 3 of culture in rapalogs (p < 0.05). This indicates that the downregulation of CCR5 expression occurs either at the transcriptional level or at the posttranscriptional level through increased CCR5 mRNA decay or decreased CCR5 mRNA half-life. We also performed qRT-PCR studies to monitor the mRNA expression of CCR7, CCR1, and CCR9 (Fig. 3B, C, and D, respectively) in nTregs cultured in the presence of rapalogs as compared to nTregs cultured without the rapalog. CCR7 mRNA expression was 3.2 ± 0.03 fold higher at the end of week 3 of rapalogs treatment (p <0.05). This finding is in line with the slightly enhanced CCR7 expression in eTregs (37.6% ± 6.4%) compared to nTregs (17.0% ± 4.3%) (Fig. 2). As expected, based on phenotypic analyses, the levels of CCR1 and CCR9 mRNA were low in both rapalog treated and control cells (data not shown). In summary, the overall chemokine receptor expression profile, except for CCR7, in both eTregs and rTregs was similar and showed some critical differences with that of nTregs.
Figure 3. qRT-PCR analyses of chemokine receptors expression by eTregs.
Total mRNAs, isolated from eTregs treated with rapalogs and nTregs maintained without rapalog selection for the indicated times, were subjected to qRT-PCR to assess steady state mRNA expression levels of CCR5 (A), CCR7 (B), CCR1 (C) and CCR9 (D). The values (mean ± SD) shown are representative of relative amount of test mRNA normalized to β-Actin from three different experiments.
Chemokine receptor expression by Tregs correlates with their migratory capacity
To determine if the expression of specific chemokine receptors correlated with different functional migration capacity of Tregs, we tested with a transwell migration assay their response to specific ligands and compared it to that of freshly isolated nTregs (Fig. 4).
Figure 4. Differential migration capacity of ex vivo cultured Tregs.
The migration of nTregs (white bars), eTregs (black bars), and rTregs (gray bars) towards (A) ELC (CCL19), (B) Eotaxin (CCL11), (C) TECK (CCL25), (D) I-TAC (CXCL11), (E) SDF-1 (CXCL2), (F) TARC (CCL17), (G) RANTES (CCL5) and (H) MIP-1α (CCL3) was evaluated using a transwell migration assay. Specificity of the migration assay was confirmed using blocking antibodies and isotype controls. The data are represented as mean ± SD for Tregs isolated or expanded from 9 healthy donors.
Expression of CCR7, which plays a crucial role for the homing of T lymphocytes to lymph nodes21, was not only retained by rTregs and eTregs (Fig 2A and B), but was also functional. Indeed, freshly isolated and cultured Tregs migrated towards ELC (CCL19) gradient proportionally to their receptor expression (nTregs 11.6% ± 1.7%, rTregs 11.3% ± 0.7% and eTregs 75.4% ± 12.8%) (Fig. 4A). As expected, and anticipated by the lack of expression of both CCR3 and CCR9 receptors (Fig. 2 and 3D), we did not observe significant migration of neither freshly isolated Tregs nor cultured Tregs in response to Eotaxin (CCL11) (nTregs 1.2% ± 0.5%, eTregs 1.4% ± 0.4% and rTregs 5.9% ± 1.3%) (Fig. 4B) and TECK (CCL25) (nTreg 3.9% ± 0.9%, eTregs 3.1% ± 0.4% and rTregs 3.2% ± 1.1%) (Fig. 4C). Modest migration was observed in response to I-TAC (CXCL11) (nTreg 14.7% ± 2.6%, eTregs 7.1% ± 1.4% and rTregs 21.8% ± 2.7%) (Fig. 4D) and SDF-1 (CXCL12) (nTregs 4.4% ± 1%; eTregs 12.1% ± 1.7%, rTregs 20.5% ± 2.9% and) (Fig. 4E) and again this paralleled the modest expression of CXCR3 and CXCR4 by these cells (Fig. 2). In fact, the relative increase in migration in response to SDF-1 corroborated with the relative increase in CXCR4 expression in rTregs and eTregs. In sharp contrast, the migration of cultured Tregs against TARC (CCL17), RANTES (CCL5) and MIP-1α (CCL3) was reduced as compared to nTregs. The migration in response to TARC was significantly reduced in rTregs (12.8% ± 02.5%) as compared to eTregs (42.3% ± 9.5%) and nTregs (71.3% ± 10.2%) (Fig. 4F), reflecting the low expression of CCR4 by the former. More dramatically, while nTregs efficiently migrated in response to RANTES (63.7% ± 6.4%) (Fig. 4G) and MIP-1α (50.2% ± 7.3%) (Fig. 4H), both eTregs and rTregs completely lost their capacity to migrate toward these chemokines (RANTES: eTregs 1.8% ± 0.4% and rTregs 4.4% ± 1.6%; MIP-1α: eTregs 2.5% ± 0.7% and rTregs 2.7% ± 0.7%) (Fig. 4G and 4H), as these cells lacked the expression of both CCR5 and CCR1. Although freshly isolated nTregs lacked the expression of CCR1, (Fig. 2) their efficient migration towards MIP-1α can perhaps be explained by the fact that MIP-1α is also a ligand for CCR522, which is expressed by nTregs, but not by the cultured Tregs. Migration evaluated in all these assays was specific as it was significantly prevented by incubation with specific mAbs but not by the isotype controls.
The emergence of Tregs as an essential component of immune homeostasis provides a potential therapeutic opportunity for active immune regulation and induction of long-term tolerance. The optimization of culture conditions to expand T-cell subsets with regulatory function establishes a paradigm for the efficient generation of these cells to implement clinical trials based on the adoptive transfer of Tregs. While Tregs cultured in vitro retain efficient inhibitory function, to our knowledge this report is the first one that discerns the chemokine receptor expression profile of ex vivo cultured Tregs and defines their migration capacity using in vitro assays. These data, in addition to functional inhibitory capacity, are crucial in determining the potential therapeutic effects of these cells.
Freshly isolated nTregs express CCR7, which is considered a crucial chemokine to drive T cell homing to peripheral lymph nodes21. Our current analysis demonstrates that Tregs cultured ex vivo in presence of rapalogs and generated starting from CD4+CD25bright cells (eTregs) or CD4+ cells (rTregs) preserved the expression of CCR7. This indicates that these cells should be able to migrate to lymph nodes after adoptive transfer in vivo and thus inhibit the priming of Teff cells and autoreactive T cells21.
Freshly isolated Tregs are also characterized by the expression of CCR5, which determines their capacity to migrate toward RANTES and MIP-1α gradients, chemokines often present in inflamed tissues22. In sharp contrast, we observed that ex vivo cultured Tregs lacked CCR5 receptor expression and had impaired migration toward both RANTES and MIP-1α as assessed by specific in vitro migration assays. This observation may have relevant implications for the use of these cultured Tregs in autoimmune diseases characterized by inflamed tissues in which RANTES and MIP-1α are abundantly secreted16,22. Cultured Tregs may poorly migrate to these tissues and thus be ineffective in controlling chronic inflammation at the local site. It has indeed been demonstrated that CCR5 downregulation in Tregs prevents their migration to the intestine in an IBD disease model, with consequent significant increase in intestinal inflammation16. Our data suggest that the defective migration towards RANTES and MIP-1α gradients can be partially compensated by the expression of CXCR3, which is the receptor for I-TAC, a chemokine typically released in inflamed tissues17. However, since the expression of CXCR3 is moderate in cultured Tregs we anticipate that this will provide only a marginal migration towards I-TAC.
It has been previously shown that expression and migration towards chemokine receptor is tightly coupled to IL-2 treatment23,24. However, activation of fully responsive T lymphocytes through TCR/CD3 complex and the CD28 antigen had an inverse effect on chemokine effect and IL-2 responsiveness24. Receptor expression and consequent migration were both downregulated in cultured Treg cells even after IL-2 treatment25. Cumulatively, it is believed that the chemokine receptor expression is transcriptionally regulated, as our data also shows, whereas translation of the receptor transcripts and consequent ability to migrate to chemokine gradient depend on IL-2. It will be interesting to further delineate the transcription machinery and its regulation by rapalogs as it will provide additional handles to regulate specific chemokine receptor expression in the ex vivo expanded Tregs and usefully manipulate their migratory behavior as required under specific conditions. In conclusion, our analysis of chemokine receptor expression and function in cultured Tregs provides valuable information to predict their potential clinical activity in specific autoimmune diseases or off-target suppressive effects upon adoptive transfer. In addition, as previously described for antitumor specific cytotoxic T lymphocytes20, our data suggest that manipulation of cultured Tregs by transgenic expression of appropriate chemokine receptors may be useful to optimize their tissue migration.
Supplementary Material
Supplementary Figure 1. (A) Illustration of the gating used to identify the CD25bright population during our purification step. Representative FACS staining with CD4 and CD25 of peripheral blood mononuclear cells from one representative donor to identify the CD4+CD25bright, CD4+CD25Intermediate, and CD4+CD25Low T cells and CD4+CD25Negative subpopulations. (B) Identification of the rapalog that provides the best expansion rate while preserving suppressive capacity. Relative expansion of eTregs (left panel) and rTregs (right panel) obtained by using either Temsirolimus or Rapamycin after 3 weeks of culture. Data show mean ± SD (n = 9 donors for eTregs and rTregs with Temsirolimus and rapamycin, respectively; n = 3 donors for eTregs and rTregs with rapamycin and temsirolimus, respectively). (C) Determination of optimal IL-2 concentration for maximal and functional expansion. Upper panel shows the expansion of cultured Tregs obtained using different concentrations of IL-2. Middle panel shows the percentage of suppression activities for eTregs ex vivo expanded with different doses of IL-2, and nTregs as assessed using a CFSE-based suppression assay. Data show mean ± SD (n = 3 donors). Lower panel shows the expression of CD25, CD4 and FoxP3 by the expanded cells in the presence of different concentration of IL-2.
Supplementary Figure 2. (A) No significant difference in suppression activity was observed with increased concentration of Temsirolimus. Percentage of suppression activities for eTregs ex vivo expanded with different doses of Temsirolimus and nTregs as assessed using a CFSE-based suppression assay (B) Cell viability decreased at concentrations greater than 1.5 µg/mL. Cell expansion after 3 weeks of culture of eTregs obtained using different concentrations of Temsirolimus. Data show mean ± SD (n = 5 donors).
Supplementary Figure 3. Similar suppressions are observed irrespective of the Treg:Teff ratio used in the experiment. Percentage of suppression activities of nTregs and ex vivo expanded Tregs assessed using a CFSE-based suppression assay in which PBMCs labeled with CFSE were stimulated with irradiated allogeneic feeders and OKT3 in the presence of Tregs at 2 different ratios of Treg:Teff. Data show mean ± SD (n = 5 donors).
Acknowledgement
This work is supported in part by RO1 CA142636 NIH-NCI and by RO1CA131027 from NIH-NCI. We are grateful to Reshma Kulkarni for the phenotypic analysis and Rongfu Wang from Baylor College of Medicine for critical review of the manuscript.
Abbreviations
- Rapalog
Rapamycin analogue
- nTregs
Naturally occurring CD4+CD25BrightFoxp3+ Tregs
- eTregs
Ex vivo expanded Tregs from nTregs
- rTregs
Ex vivo expanded Tregs from CD4+ T cell
- Teff
Effector T cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: All authors have declared there are no financial conflicts of interest in regards to this work.
References
- 1.Brusko TM, Putnam AL, Bluestone JA. Human regulatory T cells, role in autoimmune disease and therapeutic opportunities. Immunol Rev. 2008;223:371–390. doi: 10.1111/j.1600-065X.2008.00637.x. [DOI] [PubMed] [Google Scholar]
- 2.Zeiser R, Leveson-Gower DB, Zambricki EA, et al. Differential impact of mammalian target of rapamycin inhibition on CD4+CD25+Foxp3+ regulatory T cells compared with conventional CD4+ T cells. Blood. 2008;111:453–462. doi: 10.1182/blood-2007-06-094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakaguchi S, Takahashi T, Yamazaki S, et al. Immunologic self tolerance maintained by T-cell-mediated control of self-reactive T cells, implications for autoimmunity and tumor immunity. Microbes Infect. 2001;3:911–918. doi: 10.1016/s1286-4579(01)01452-6. [DOI] [PubMed] [Google Scholar]
- 4.Baecher-Allan C, Hafler DA. Suppressor T cells in human diseases. J Exp Med. 2004;200:273–276. doi: 10.1084/jem.20040812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lan RY, Ansari AA, Lian ZX, et al. Regulatory T cells, development, function and role in autoimmunity. Autoimmun Rev. 2005;4:351–363. doi: 10.1016/j.autrev.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Kang SM, Tang Q, Bluestone JA. CD4+CD25+ regulatory T cells in transplantation, progress, challenges and prospects. Am J Transplant. 2007;7:1457–1463. doi: 10.1111/j.1600-6143.2007.01829.x. [DOI] [PubMed] [Google Scholar]
- 7.Bluestone JA, Tang Q. Therapeutic vaccination using CD4+CD25+ antigen-specific regulatory T cells. Proc Natl Acad Sci U S A. 2004;101(Suppl 2):14622–14626. doi: 10.1073/pnas.0405234101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eghtesad S, Morel PA, Clemens PR. The companions, regulatory T cells and gene therapy. Immunology. 2009;127:1–7. doi: 10.1111/j.1365-2567.2009.03069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Z-Z, Ansell SM. The Role of Treg Cells in the Cancer Immunological Response. Am J Immunol. 2009;5:17–28. [Google Scholar]
- 10.Bresatz S, Sadlon T, Millard D, et al. Isolation, propagation and characterization of cord blood derived CD4+ CD25+ regulatory T cells. J Immunol Methods. 2007;327:53–62. doi: 10.1016/j.jim.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Earle KE, Tang Q, Zhou X, et al. In vitro expanded human CD4+CD25+ regulatory T cells suppress effector T cell proliferation. Clin Immunol. 2005;115:3–9. doi: 10.1016/j.clim.2005.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Godfrey WR, Ge YG, Spoden DJ, et al. In vitro-expanded human CD4(+)CD25(+) T-regulatory cells can markedly inhibit allogeneic dendritic cell-stimulated MLR cultures. Blood. 2004;104:453–461. doi: 10.1182/blood-2004-01-0151. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann P, Eder R, Kunz-Schughart LA, et al. Large-scale in vitro expansion of polyclonal human CD4(+)CD25high regulatory T cells. Blood. 2004;104:895–903. doi: 10.1182/blood-2004-01-0086. [DOI] [PubMed] [Google Scholar]
- 14.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 15.Mahnke K, Ring S, Bedke T, et al. Interaction of regulatory T cells with antigen-presenting cells in health and disease. Chem Immunol Allergy. 2008;94:29–39. doi: 10.1159/000154854. [DOI] [PubMed] [Google Scholar]
- 16.Kang SG, Piniecki RJ, Hogenesch H, et al. Identification of a chemokine network that recruits FoxP3(+) regulatory T cells into chronically inflamed intestine. Gastroenterology. 2007;132:966–981. doi: 10.1053/j.gastro.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Ebert LM, Schaerli P, Moser B. Chemokine-mediated control of T cell traffic in lymphoid and peripheral tissues. Mol Immunol. 2005;42:799–809. doi: 10.1016/j.molimm.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 18.Porter CM, Bloom ET. Human CD4+CD25+ regulatory T cells suppress anti-porcine xenogeneic responses. Am J Transplant. 2005;5:2052–2057. doi: 10.1111/j.1600-6143.2005.00972.x. [DOI] [PubMed] [Google Scholar]
- 19.Battaglia M, Stabilini A, Migliavacca B, et al. Rapamycin promotes expansion of functional CD4+CD25+Foxp3+ regulatory T cells of both healthy subjects and Type 1 diabetic patients. J Immunol. 2006;177:8338–8347. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 20.Di Stasi A, De Angelis B, Rooney CM, et al. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood. 2009;113:6392–6402. doi: 10.1182/blood-2009-03-209650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirao M, Onai N, Hiroishi K, et al. CC Chemokine Receptor-7 on Dendritic Cells is Induced after Interaction with Apoptotic Tumor Cells, Critical Role in Migration from the Tumor Site to Draining Lymph Nodes. Cancer Res. 2000;60:2209–2217. [PubMed] [Google Scholar]
- 22.Wang CR, Liu MF. Regulation of CCR5 expression and MIP-1α production in CD4+ T cells from patients with rheumatoid arthritis. Clin Exp Immunol. 2003;132:371–378. doi: 10.1046/j.1365-2249.2003.02126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olson TS, Ley K. Chemokines and chemokine receptors in leukocyte trafficking. Am J Physiol Regul Integr Comp Physiol. 2002;283:R7–R28. doi: 10.1152/ajpregu.00738.2001. [DOI] [PubMed] [Google Scholar]
- 24.Loetscher P, Seitz M, Baggiolini M, Moser B. Interleukin-2 regulates CC chemokine receptor expression and chemotactic responsiveness in T lymphocytes. J Exp Med. 1996;184:569–577. doi: 10.1084/jem.184.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wuest TY, Willette-Brown J, Durum SK, Hurwitz AA. The influence of IL-2 family cytokines on activation and function of naturally occurring regulatory T cells. J Leuko Biol. 2008;84:973–980. doi: 10.1189/jlb.1107778. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. (A) Illustration of the gating used to identify the CD25bright population during our purification step. Representative FACS staining with CD4 and CD25 of peripheral blood mononuclear cells from one representative donor to identify the CD4+CD25bright, CD4+CD25Intermediate, and CD4+CD25Low T cells and CD4+CD25Negative subpopulations. (B) Identification of the rapalog that provides the best expansion rate while preserving suppressive capacity. Relative expansion of eTregs (left panel) and rTregs (right panel) obtained by using either Temsirolimus or Rapamycin after 3 weeks of culture. Data show mean ± SD (n = 9 donors for eTregs and rTregs with Temsirolimus and rapamycin, respectively; n = 3 donors for eTregs and rTregs with rapamycin and temsirolimus, respectively). (C) Determination of optimal IL-2 concentration for maximal and functional expansion. Upper panel shows the expansion of cultured Tregs obtained using different concentrations of IL-2. Middle panel shows the percentage of suppression activities for eTregs ex vivo expanded with different doses of IL-2, and nTregs as assessed using a CFSE-based suppression assay. Data show mean ± SD (n = 3 donors). Lower panel shows the expression of CD25, CD4 and FoxP3 by the expanded cells in the presence of different concentration of IL-2.
Supplementary Figure 2. (A) No significant difference in suppression activity was observed with increased concentration of Temsirolimus. Percentage of suppression activities for eTregs ex vivo expanded with different doses of Temsirolimus and nTregs as assessed using a CFSE-based suppression assay (B) Cell viability decreased at concentrations greater than 1.5 µg/mL. Cell expansion after 3 weeks of culture of eTregs obtained using different concentrations of Temsirolimus. Data show mean ± SD (n = 5 donors).
Supplementary Figure 3. Similar suppressions are observed irrespective of the Treg:Teff ratio used in the experiment. Percentage of suppression activities of nTregs and ex vivo expanded Tregs assessed using a CFSE-based suppression assay in which PBMCs labeled with CFSE were stimulated with irradiated allogeneic feeders and OKT3 in the presence of Tregs at 2 different ratios of Treg:Teff. Data show mean ± SD (n = 5 donors).