Abstract
The collectins have been shown to play a role in host defense against influenza A virus (IAV) and other significant viral pathogens (e.g., HIV). The ficolins are a related group of innate immune proteins that are present at relatively high concentrations in serum but also in respiratory secretions; however, there has been very little study of the role of ficolins in viral infection. In this study we demonstrate that purified recombinant human H-ficolin and H-ficolin in human serum and bronchoalveolar lavage fluid (BALF) bind to IAV and inhibit viral infectivity and hemagglutination activity in vitro. Removal of ficolins from human serum or BALF reduces their antiviral activity. Inhibition of IAV did not involve the calcium-dependent lectin activity of H-ficolin. We demonstrate that H-ficolin is sialylated and that removal of sialic acid abrogrates IAV inhibition, while addition of the neuraminidase inhibitor oseltamivir potentiates neutralization, HA inhibition and viral aggregation caused by H-ficolin. Pandemic and mouse adapted strains of IAV are generally not inhibited by the collectins surfactant protein D (SP-D) or mannose binding lectin (MBL) due to paucity of glycan attachments on the hemagglutinin of these strains. In contrast, H-ficolin inhibited both the mouse adapted PR-8 H1N1 strain and a pandemic H1N1 strain from 2009. H-ficolin also fixed complement to a surface coated with IAV. These findings suggest that H-ficolin contributes to host defense against IAV.
INTRODUCTION
The collectins surfactant protein D (SP-D), surfactant protein A (SP-A) and mannose-binding lectin (MBL), have been shown to contribute to innate defense against influenza A virus (IAV) infection. The ficolins resemble MBL in their overall structure, calcium-dependent binding to pathogens, and their ability to fix complement in an antibody independent manner (1). In humans there are three different ficolin forms (H-, L-, and M-ficolin) and one form of MBL (2). H-ficolin has a shorter collagen domain than the other two ficolins. H-ficolin exists in blood at a mean level of ~20μg/ml [reported ranges from 8–80μg/ml; (3, 4)]which greatly exceeds that of the other ficolins (L-ficolin: 3.4μg/ml, M-ficolin: 1.4μg/ml) or MBL (1.1μg/ml) (5). H-ficolin is also produced by alveolar type II cells and ciliated bronchial epithelial cells in the lung and has been demonstrated to be present in BALF (6) although the concentration in BALF has not been determined. Subjects have been described who are homozygous for a truncated version of H-ficolin, and essential absence of this protein in serum (7, 8). The major clinical manifestation of the adult patient was recurrent respiratory infections while the other two patients were neonates with necrotizing enterocolitis. It is likely therefore that H ficolin plays a role in innate immunity.
The interactions of ficolins with bacteria have been well studied but there are limited data regarding ficolin interactions with viruses. L-ficolin has been shown to bind to envelope proteins of hepatitis C virus and to fix complement on HCV infected hepatocytes (9). Recently L-ficolin was also shown to inhibit influenza A virus (IAV) in vitro and in mice (10). Porcine ficolin has been shown to neutralize porcine reproductive and respiratory syndrome virus (PRRSV) (11). In both of these cases the antiviral effect related to recognition of N-linked glycans on the viral envelope proteins by the ficolin. Recent studies have also shown that chimeric proteins containing the N-terminal domains of ficolins and the carbohydrate recognition domain of MBL strongly inhibit Ebola virus and IAV (12, 13); however, in this case the reaction is mediated by the binding of the carbohydrate recognition domain of MBL to virus associated carbohydrates. The recognition domain of the ficolins differs from that of the collectins (which are C type lectins). It has some homology to domains of fibrinogen, and is thus named a fibrinogen-like domain. Ficolins recognize acetylated compounds (both N-acetylated sugars and other acetylated molecules) whereas MBL and other collectins preferentially bind to terminal carbohydrate groups with horizontal OH groups at the 3 and 4 position, e.g., mannose rich glycans on pathogens (14).
In this paper we focused mainly on H-ficolin due to its probable role in respiratory infections. We demonstrate that H-ficolin neutralizes various strains of IAV through a distinct mechanism that does not involve their calcium-dependent lectin activity. This feature allows the ficolins to inhibit viral strains not inhibited well by collectins.
MATERIALS AND METHODS
Virus Preparations
Philippines 82/H3N2 (Phil82) strain was kindly provided by Dr. E. Margot Anders (Univ. of Melbourne, Melbourne, Australia). The PR-8 (1934 H1N1) strain was graciously provided by Jon Abramson (Wake Forest University, Winston-Salem, North Carolina). These IAV strains were grown in the chorioallantoic fluid of ten day old chicken eggs and purified on a discontinuous sucrose gradient as previously described (15). The virus were dialyzed against PBS to remove sucrose, aliquoted and stored at −80°C until needed. Post thawing the viral stocks contained ~5×108 infectious focus forming units/ml. The California 2009 H1N1 strain was derived by reverse genetics and grown in MDCK cells.
Protein Preparations and other reagents
Recombinant H-ficolin was produced and purified as described (4). In addition, H-ficolin was purified from human serum in complex with the MBL-associated serine protein (MASP2) as described (4). L-ficolin was purified as described (14). M-ficolin was produced and purified as described (16). Pentraxin-3 was purchased from Abcam (ab85335). Recombinant human MBL was a kind gift of Dr Kazue Takahashi (Mass. General Hospital, Boston, MA) and human alveolar proteinosis derived SP-A was a kind gift of Dr. Frank McCormack (University of Cincinnati School of Medicine). Oseltamivir was provided by Roche Inc.
Measurement of H-ficolin levels in human serum and bronchoalveolar lavage fluid (BALF)
Human serum and BALF was obtained from healthy volunteer donors under approval from the Institutional Review Board of the Boston University School of Medicine. The degree of dilution of BALF from the two donors was similar since the urea concentrations of these samples was 430 and 490 μg/ml prior to concentration. The level of H-ficolin in the serum or BALF was obtained using a commercial ELISA kit designed for this purpose (Hycult Biotechnology). Each sample was measured at various dilutions and the average of results from these dilutions was calculated. A standard curve was used to give H-ficolin levels. To deplete ficolin from serum or BALF the fluids were incubated with N-Acetyl-D-Galactosamine-Agarose (Sigma, Catalog Number-A2787) overnight at 4°C as described (17). Ficolin bound to N-Acetyl-D-Galactosamine-Agarose was removed by centrifugation at 3500g for 10 min. Effective removal of H-ficolin was confirmed by ELISA.
Binding of H-Ficolin to IAV
Binding of H-ficolin was assessed by solid phase ELISA. Plates were coated with 10 μg/ml IAV in coating buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6) overnight at 4°C with PBS containing 2.5% (w/v) BSA (fraction V, fatty acid free, and low endotoxin, A8806, Sigma-Aldrich) as background control. Following washing three times with PBS with 2mM calcium and magnesium (PBS++),the plates were blocked with PBS++ containing 2.5% BSA for 3 h. These coated plates were then incubated with ficolin and then washed with PBS++ with 0.02% Tween 20, followed by addition of an antibody against H-ficolin (Santa Cruz catalog #SC55202) diluted in the same buffer. Incubation of IAV with ficolins was performed in PBS++. Bound anti-ficolin Ab was detected with HRP-labeled goat anti-rabbit Abs followed by incubation with tetramethylbenzidine as a substrate (Bio-Rad). The reaction was stopped using 1 N sulfuric acid. The OD was measured on an ELISA plate reader at 450 nm wavelength. Each individual data point was performed in duplicate. Background nonspecific binding was assessed by coating plates with fatty acid free BSA but no IAV. Binding to BSA was subtracted from the ficolin binding values.
Assessment of ficolins by Western blot or glycan blot
Human serum (30μl of a 1:100 dilution per well) or BALF (30μl of concentrated fluid per well) was subjected to SDS-PAGE followed by transfer to nitrocellulose and treatment with anti-H-ficolin antibody. For glycan blotting, 10μl of recombinant H-ficolin or MBL was added directly to nitrocellulose and labeled SNA or MAA lectins (Roche Applied Science) were added. Binding was detected by use of Anti-Digoxigenin-AP as per manufacturer’s recommendation. SNA and MAA lectins detect α(2,3)- and α(2,6)-linked sialic acids, respectively.
Hemagglutination (HA) inhibition assay
HA inhibition was measured by serially diluting ficolins or other host defense protein preparations in round bottom 96 well plates (Serocluster U-Vinyl plates; Costar, Cambridge, MA) using PBS++ as a diluent (18). After adding 25 μl of IAV, giving a final concentration of 40 HA units per ml or 4 HA units/well, the IAV/protein mixture was incubated for 15 min at room temperature, followed by addition of 50 μl of a type O human erythrocyte suspension. The minimum concentration of protein required to fully inhibit the hemagglutinating activity of the viral suspension was determined by noting the highest dilution of protein that still inhibited hemagglutination. Inhibition of HA activity in a given well is demonstrated by the absence of the formation of an erythrocyte pellet. If no inhibition of HA activity was observed at the highest protein concentration used then the value is expressed as > the maximal protein concentration.
Measurement of viral aggregation
Viral aggregation was measured by assessing light absorbance by stirred suspensions of IAV. This was done using a Perkin Elmer Lambda 35 UV/Vis spectrophotometer at 350nm. In addition, viral aggregation was assessed using electron microscopy (EM) as described (19).
Fluorescent focus assay of IAV infectivity
MDCK cell monolayers were prepared in 96 well plates and grown to confluency. These layers were then infected with diluted IAV preparations for 45 min at 37°C in PBS and tested for presence of IAV infected cells after 7 h using a monoclonal antibody directed against the influenza A viral nucleoprotein (provided by Dr. Nancy Cox, CDC, Atlanta, GA) as previously described (20). IAV was pre-incubated for 30 min at 37°C with ficolins or control buffer, followed by addition of these viral samples to the MDCK cells.
Measurement of viral RNA
RNA for the viral M protein was measured using real time PCR. A549 cells were infected with Phil 82 virus strain incubated for 30 min at 37°C with or without various doses of ficolin. RNA extraction was done at 45 min and 24 hrs post infection using Magmax viral RNA isolation kit (Applied Biosytems, Carlsbad, California) as per manufacturer’s instructions. Both lysed cells and cell supernatant were used for extraction. Viral RNA was also extracted from different concentrations of virus with known infectious units/ml (as defined by the fluorescent focus assay - see above) which was used as standard series. RNA was reverse transcribed using TaqMan® reverse transcription reagents (Applied Biosytems, Carlsbad, California). The reaction mix and the cycle conditions were as per manufacturer’s instructions. For real time PCR, primers specific for IAV M protein (Forward AGA CCA ATC CTG TCA CCT CTGA and Reverse: CTG CAG TCC TCG CTC ACT) were used. The primers and TaqMan®-labelled probes with non-fluorescent minor groove binder (MGB) moieties were designed manually using the Primer Express software version 3.0 (Applied Biosystems, Carlsbad, California) and were also synthesized by Applied Biosystems. The assay sequences were examined for specificity by nucleotide BLAST. The experiment was performed in a 7500 Real time PCR system (Applied Biosytems, Carlsbad, California) using volume of 20 μl containing 2 μl of template cDNA, 0.9 μM primer 0.25 μM of 6-FAM™ dye-labeled TaqMan® MGB probe (6-FAM-ATT TGT GTT CAC GCT CAC CGT G-MGB), and 1X TaqMan® Universal PCR master mix (Applied Biosytems, Carlsbad, California). Thermal cycling proceeded at 50 °C for 2 min, 95 °C for 10 min followed by 40 cycles of 95°C for 15 s, 60 °C for 1 min and 72 °C for 30 s.
Confocal microscopy
MDCK cells were pre-incubated with the PR-8 strain of IAV, H-ficolin(10μg/ml), or H-ficolin and oseltamivir(10μg/ml) for 45 min, followed by washing and fixation using 1% Paraformaldehyde. WGA orogon Green 488 (4μg/ml) and DAPI 350 were used to stain the cell membrane and nucleus respectively. The virus was Alexa Flour 594 labeled. Confocal pictures were taken at Zeiss LSM510 (LSEB) on 100x resolution.
Complement fixation assay
Fixation of complement component C4 on to ELISA plates coated with IAV or acetylated BSA was tested as described (4) using the H-ficolin/MASP-2 complex purified from human serum. Briefly, 96 well plates were coated overnight in sodium bicarbonate buffer with either 5ug/ml acetylated BSA (Sigma, Cat#B2518) or 10ug/ml Phil82 IAV at 4°C. The next morning, plates were washed three times with wash buffer (10mM Tris, pH 7.4, 120 mM NaCL, 10 mM CaCl2, 0.05% tween 20) and blocked with blocking buffer (0.1% BSA in 10mM Tris, pH7.4, 120mM NaCl, 10mM CaCl2) for 2 hours, shaking at 37°C. Plates were then washed three times. H-Ficolin-MASP was combined with C4 complement, in BBS buffer (4mM barbital buffer, pH 7.5, 145mM NaCl2, 2mM CaCl2, 1mM MgCl2) and added to the plate for 1.5 hours. Plates were then washed and labeled with rabbit anti-human C4c (1hour at 37°C) followed by peroxidase labeled streptavidin (Kirkegaard & Perry Laboratories, Cat#14-30-00). Plates were washed three times between each antibody and HRP levels were detecting using 1-Step ELISA (Fisher Scientific). Plates were read at 450nm with a wavelength correction of 540nm on a POLARstar OPTIMA plate reader (BMG Labtech).
Statistics
Statistical comparisons were made using Student’s paired, two-tailed t test or ANOVA with post hoc test (Tukey’s). ANOVA was used for multiple comparisons to a single control.
RESULTS
Human H-ficolin bind to IAV
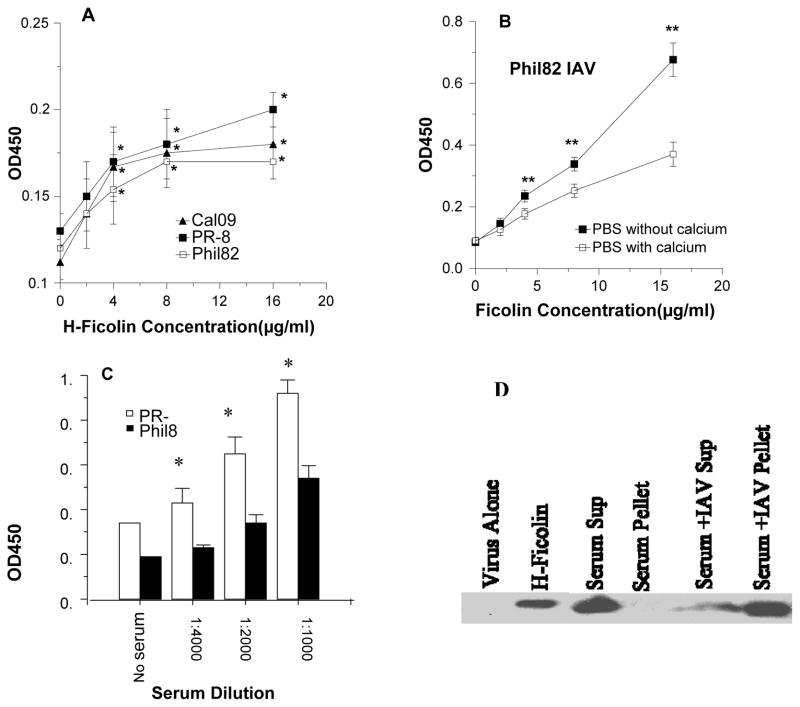
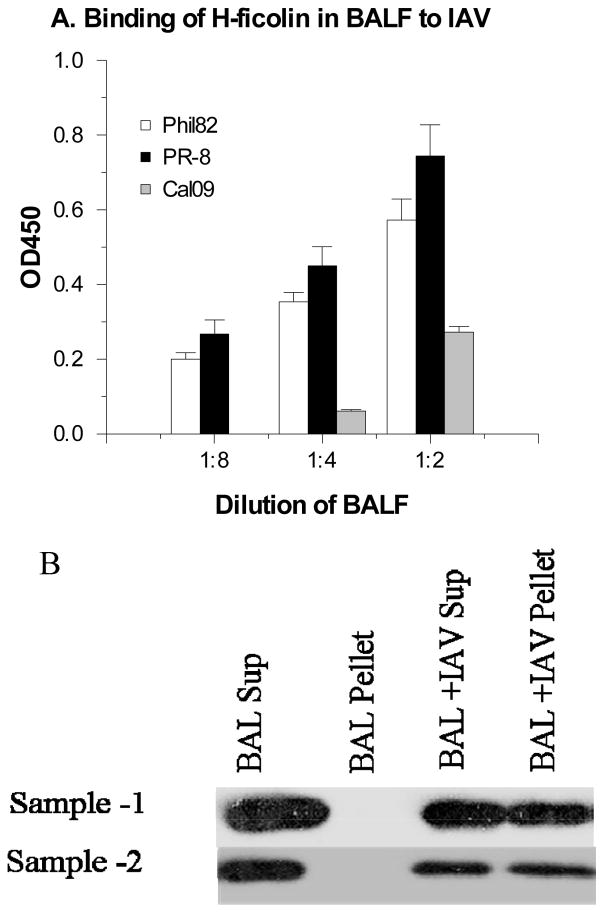
We first tested the ability of recombinant human H-ficolin to bind to strains of IAV. Figure 1A shows that recombinant H-ficolin bound to the Phil82, PR-8 and Cal09 strains in a dose dependent manner. Unexpectedly, binding of H-ficolin to IAV was increased in buffer lacking calcium and magnesium (Figure 1B). Figure 1C demonstrates that H-ficolin present in human serum also bound to IAV (PR-8 and Phil82 strains used in this case). Human bronchoalveolar lavage fluid (BALF) has also been reported to contain H-ficolin (6). We measured the level of H-ficolin in BALF using ELISA. The mean level of H-ficolin obtained in BALF samples from two healthy volunteers was 102±2 ng/ml. This BALF was concentrated 5 fold prior to assay. Presumably the concentration of H-ficolin in alveolar lining fluid substantially exceeds the levels present in BALF. As shown in figure 2A, H-ficolin in BALF also bound to IAV as measured ELISA. As an additional means of testing binding of H-ficolin in serum and BALF to IAV, we pre-incubated IAV (PR-8 strain) with human serum or BALF, followed by centrifugation of the fluids for 15 min at 13,000g. We have previously shown that that collectins induce viral aggregation such that centrifugation of collectin-IAV mixtures in this manner results in pelleting of the virus (21). This procedure resulted in precipitation of H-ficolin out of serum (figure 1D) or BALF (figure 2B). This again confirms that IAV binds to H-ficolin present in human serum and BALF.
Figure 1. Binding of recombinant and human serum H-ficolin to IAV.
In panel A ELISA plates were coated with three IAV strains indicated (California 2009 H1N1, PR-8 1934 H1N1, and Philippines 1982 H3N2) and then incubated with increasing concentrations of recombinant H-ficolin. Background binding to BSA coated plates was subtracted from the values shown. There was significant binding (p<0.05; indicated by *) of H-ficolin to all viruses at all the doses shown. Panel B shows binding of H-ficolin to the PR-8 strain of IAV in presence or absence of calcium and magnesium. Binding was significantly greater in the absence of calcium and magnesium. Panel C shows results of incubation of various dilutions of human serum with similar ELISA plates, followed by antibody detection of bound H-ficolin. Here again there was significant binding of H-ficolin at all dilutions shown, although binding to PR-8 was significantly greater than binding to Phil82. Results are mean±SEM of 4 experiments. Panel D shows a Western blot of H-ficolin in serum. In this experiment the serum was incubated with the PR-8 strain of IAV or in control buffer, followed by centrifugation to pellet the virus. Supernatant and pellet samples (resuspended in the same amount of buffer) were analyzed by Western blot. This experiment is representative of 3 similar experiments.
Figure 2. Binding of H-ficolin in human BAL fluid to IAV.
Binding was demonstrated using two methods. In panel A, BAL fluid was incubated with IAV coated plates and binding of H-ficolin in BAL fluid was detected by ELISA. Binding was significant (p<0.05 for all tested dilutions of BAL fluid). Results are mean±SEM of 4 experiments. In panel B, virus (PR-8 strain) was incubated with BAL fluid for 45 min followed by centrifugation to pellet virus particles. The presence of H-ficolin in the supernatant and pellet was demonstrated by Western blot. Results are representative of 3 experiments.
Human ficolins inhibit infectivity of IAV strains, including Cal09 H1N1
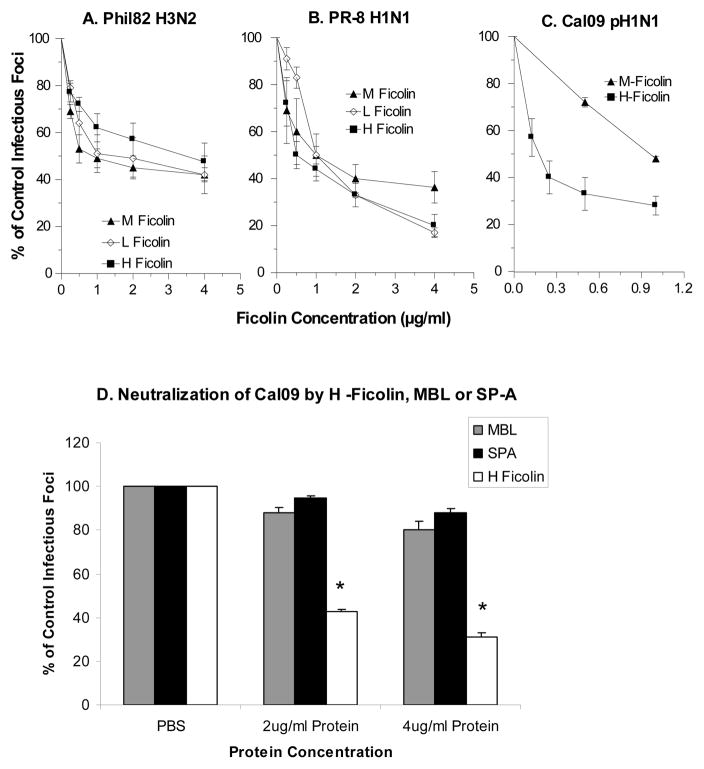
The ability of the ficolins to inhibit infectivity of IAV strains was tested using a fluorescent focus assay detecting expression of viral nucleoprotein in MDCK cells. As shown in figure 3A and B, H-, M-, and L-ficolins inhibited the Phil82 and PR-8 strains of IAV. H-ficolin also had relatively strong inhibitory activity for the Cal09 pandemic H1N1 strain. The Cal09 strain is not inhibited effectively by SP-D or pentraxins (22, 23). We compared the activity of H-ficolin to that of SP-A or MBL in an additional set of experiments. As shown in figure 3D, H-ficolin caused significantly greater inhibition of Cal09 H1N1 than either MBL or SP-A.
Figure 3. Neutralization of IAV strains by ficolins.
Viral neutralization was tested using a fluorescent focus assay and MDCK cells as described. The number of fluorescent (infected) cells was counted after 7 hours of infection. Diluted viral strains were incubated with control buffer (PBS with 2mM calcium and magnesium) or different concentrations of ficolins, MBL, or SP-A, followed by addition of these samples to cells. The multiplicity of infection (MOI) for these experiments was 1. In panels A and B, M-, L-, and H-ficolin were all tested for their ability to inhibit the Phil82 (panel A) or PR-8 (panel B) strains of IAV. In panel C, H-ficolin was tested for its activity against Cal09. All ficolins caused significant inhibition of all IAV strains at the concentrations tested (p<0.05 vs control). Panel D shows an additional set of experiments in which the activity of H-ficolin was compared to that of human SP-A (native protein from BAL) or recombinant human MBL. H-ficolin caused significantly greater inhibition of Cal09 than either SP-A or MBL at the concentrations tested (* indicates significant difference by ANOVA). Results are mean±SEM of 4 experiments.
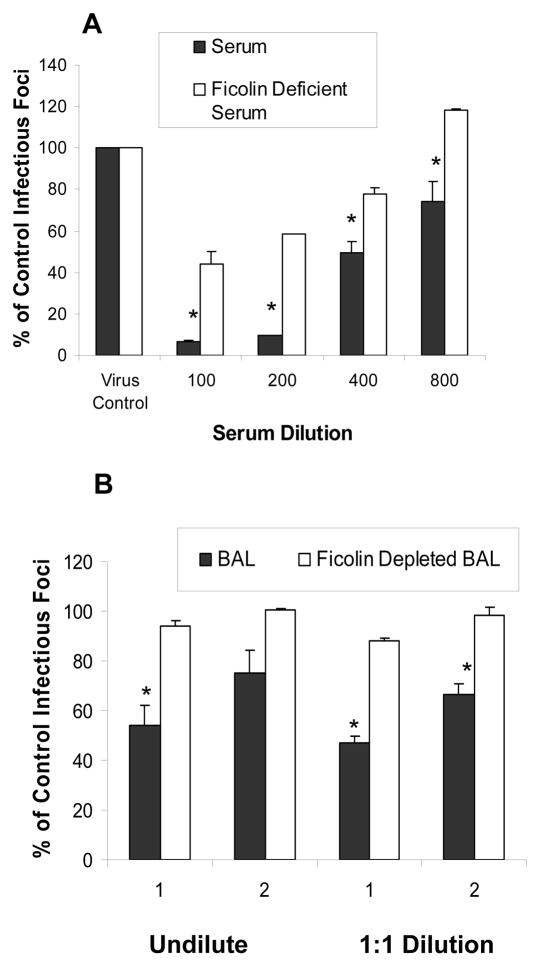
To confirm that native H-ficolin present in serum and BAL fluid contributes to inhibition of IAV we tested neutralizing activity of these fluids before and after removal of H-ficolin through use of N-Acetyl-D-Galactosamine overnight at 4°C as described (17). Ficolin bound to N-Acetyl-D-Galactosamine-Agarose was removed by centrifugation at 3500g for 10 min. H-ficolin levels in the serum used were 70 and 0.46 μg/ml pre- and post incubation with the N-Acetyl-D-Galactosamine-Agarose. As shown in figure 4 removal of ficolin from either serum or BAL fluid significantly reduced antiviral activity. We used the PR-8 virus for these experiments to exclude any possible effect of SP-D or MBL (which do not inhibit this viral strain).
Figure 4. Effect of ficolin depletion on viral neutralizing activity of human serum or BAL fluid.
Normal donor human serum or BAL fluid was incubated with N-Acetyl-D-Galactosamine-Agarose as described to remove ficolins and the serum or BALF pre- or post-depletion were compared for viral neutralizing activity using the infectious focus assay and PR-8. The serum or BALF was pre-incubated with the virus for 45min followed by titration on MDCK cells. Panel A shows results obtained with serum from one donor at the indicated dilutions. Panel B shows results obtained with BALF from two separate donors (labeled 1 and 2). The BALF was used either undiluted or at 1:1 dilution in PBS as indicated. The results are mean±SEM of 3 experiments with each dilution of serum or BALF.
* indicates were depleted BALF or serum had significantly less neutralizing activity than the untreated samples
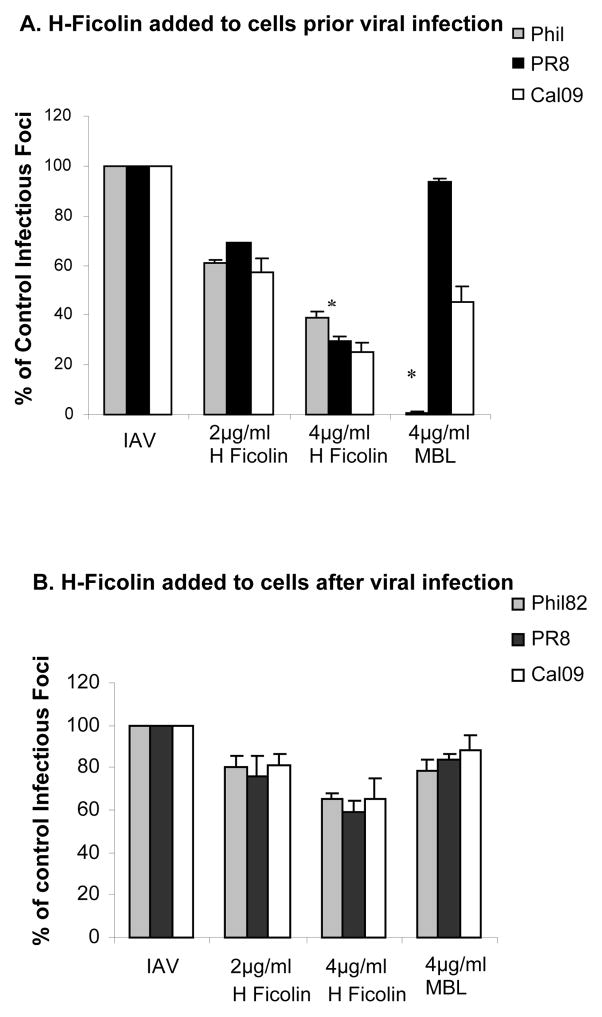
As a first step in determining the mechanism of action of H-ficolin we compared the effects of adding H-ficolin to the cells before or after infection of the cells with the PR-8 virus. Figure 5A shows the effect of adding H-ficolin or MBL to cells for 45min followed by washing off excess protein and infecting the cells with IAV. H-ficolin had similar viral inhibitory activity using this method as when the virus and H-ficolin were pre-incubated in figure 3. Of interest, MBL appeared to be somewhat more effective at inhibiting the Cal09 strain using this method of incubation than when virus was pre-incubated with MBL (compare figure 5A to 3D). We next infected the cells with the virus for 45 min, followed by washing off the remaining virus and then adding H-ficolin or MBL (figure 5B). Using this method there was still some inhibition of infectivity but the effect was reduced compared to either pre-incubating the cells or the virus with ficolin. This suggests that H-ficolin mainly acts through binding to virus and possibly cells prior to viral internalization.
Figure 5. Effect of adding H-ficolin to MDCK cells before or after virus infection on neutralizing activity.
The neutralization assay was performed as in figure 3 except that in panel A the MDCK cells were pre-incubated with the ficolin prior to infection with IAV, and in panel B ficolin was added to MDCK cells after viral infection. (In figure 3 virus was pre-incubated with ficolins prior to infection of MDCK cells). Pre-incubation of MDCK cells with H-ficolin caused comparable inhibition of viral infection with the three indicated viral strains as in figure 3. Results with human MBL are shown for comparison. H-ficolin caused significantly greater inhibition of the PR-8 strain than MBL, while MBL caused significantly greater inhibition of the Phil82 strain than H-ficolin at 4μg/ml (* indicates these differences as assessed by ANOVA). Results in panel B show that addition of H-ficolin or MBL after viral infection for 45 min causes much less inhibition than found in panel A or in figure 3. Note, however, that H-ficolin still caused significant inhibition compared to control using this method (p<0.05 for all viruses at 4μg/ml). Results are mean±SEM of 4 experiments.
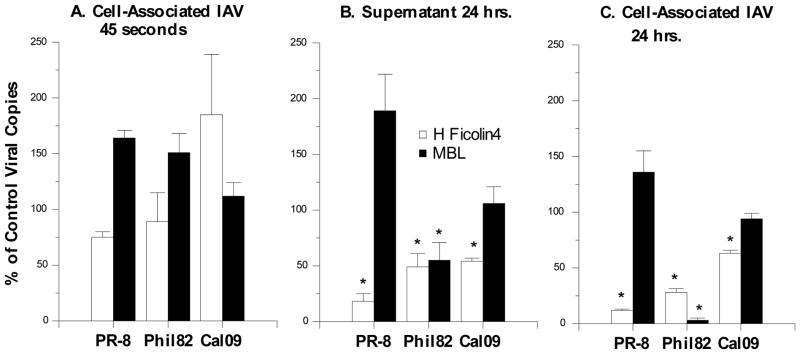
We also used qPCR to assess whether H-ficolin inhibits viral attachment or uptake to A549 cells. To do this we measured the amount of viral copies in homogenates of the cells after a 45 min infection period and extensive washing of cells to remove free virus. As shown in figure 6A, H-ficolin did not reduce the amount of cell associated virus measured after 45 min. However, H-ficolin inhibited synthesis of RNA of the viral M protein in the cells or in the cell free supernatant after 24 hours of infection as shown in figure 6B (for supernatant) or C (for cell associated virus).
Figure 6. Effect of recombinant H-ficolin or MBL on viral uptake and replication in A549 cells as assessed by RT-PCR.
The indicated viral strains were pre-incubated with H-ficolin or MBL followed by infection of A549 cells for 45 min. The MOI used in these experiments was 1 as in the infectious focus assays. Cell associated virus or virus in culture supernatant were then measured using RT-PCR to detect the viral RNA encoding M protein. To assess viral uptake cell associated virus was measured just after completion of viral infection (45 min) in panel A. Neither H-ficolin nor MBL reduced the amount of cell-associated virus at this time point. In contrast, H-ficolin significantly reduced the amount of virus detectable in the culture supernatant (panel B) or cells (panel C) for all viruses tested (* indicates p<0.05 vs control). MBL only caused significant inhibition of the Phil82 strain in panels B and C. Results are mean±SEM of 4 experiments.
H-ficolin inhibits hemagglutination activity of IAV in a non calcium-dependent manner
We tested the ability of H-ficolin to inhibit HA activity caused by two strains of virus, Phil82 H3N2 and PR-8. As shown in Table 1, H-ficolin inhibited Phil82 although the activity was considerably reduced as compared to that of MBL. However, when the assay was carried out in buffer lacking calcium or in buffer containing EDTA, the activity of H-ficolin was strongly enhanced, whereas that of MBL was abrogated. H-ficolin had considerably stronger HA inhibitory activity against the PR-8 strain of IAV than against Phil82 and this activity was again further increased in buffer lacking calcium and magnesium or containing EDTA. As shown in figure 1B, binding of H-ficolin to IAV was also increased in the absence of calcium and magnesium perhaps accounting for the increased HA inhibition. As previously reported, MBL did not inhibit the PR-8 strain under any condition (24). This finding is consistent with the known mechanism of inhibition by MBL which involves calcium-dependent binding to glycans on the viral HA. The PR-8 strain lacks glycan attachments on the head region of the HA while the Phil82 strain has multiple such glycans (25).
Table 1.
Hemagglutination Inhibition by H-Ficolin or MBL
| Viral Strain | Buffer | H Ficolin | Oseltamivir | Oseltamivir + H-ficolin | MBL |
|---|---|---|---|---|---|
| Phil82 H3N2 | PBS + Ca2+and Mg2+ | 2.25±0.5* | 0.001±0 | ||
| PBS no Ca2+and Mg2+ | 0.15±0.004** | >5 | |||
| PBS+EDTA | 0.23±0.02** | >5 | |||
| PR-8 H1N1 | PBS+Ca2+and Mg2+ | 1.17±0.6* | >5 | 0.32±0.25§ | >5 |
| PBS no Ca2+and Mg2+ | 0.55±0.13** | >5 | 0.13±0.11§ | >5 | |
| PBS+EDTA | 0.55±0.22** | >5 | 0.06±0.05§ | >5 |
Results are expressed as mean±SEM in μg/ml of ficolin protein resulting in inhibition of 40 Hemagglutinin units of the indicated strains of IAV. The concentration of oseltamivir added was 10μg/ml. For H ficolin n>4; for MBL n=3 or 4 except in case of Phil82 in PBS with calcium and magnesium where n=2.
significantly reduced compared to control buffer (p<0.05)
significantly reduced compared to control buffer or H-ficolin in PBS with calcium and magnesium
significantly reduced compared to H-ficolin without oseltamivir
Role of ficolin associated sialic acid rich glycan in IAV inhibition
Several innate inhibitors of IAV (e.g., SP-A, pentraxins, gp340) work by presenting a decoy sialic acid rich ligand to which the viral HA binds (26, 27). This process, called γ-inhibition, is not calcium dependent. In our previous studies with other γ-inhibitors of IAV (e.g., mucins, SP-A or gp-340), addition of the neuraminidase inhibitor, oseltamivir, led to potentiation of antiviral activity (28, 29). When H-ficolin was combined with oseltamivir a significant increase in HA inhibitory activity occurred (Table 1). This suggests a role for binding of the viral HA to sialylated glycans on H-ficolin in HA inhibition.
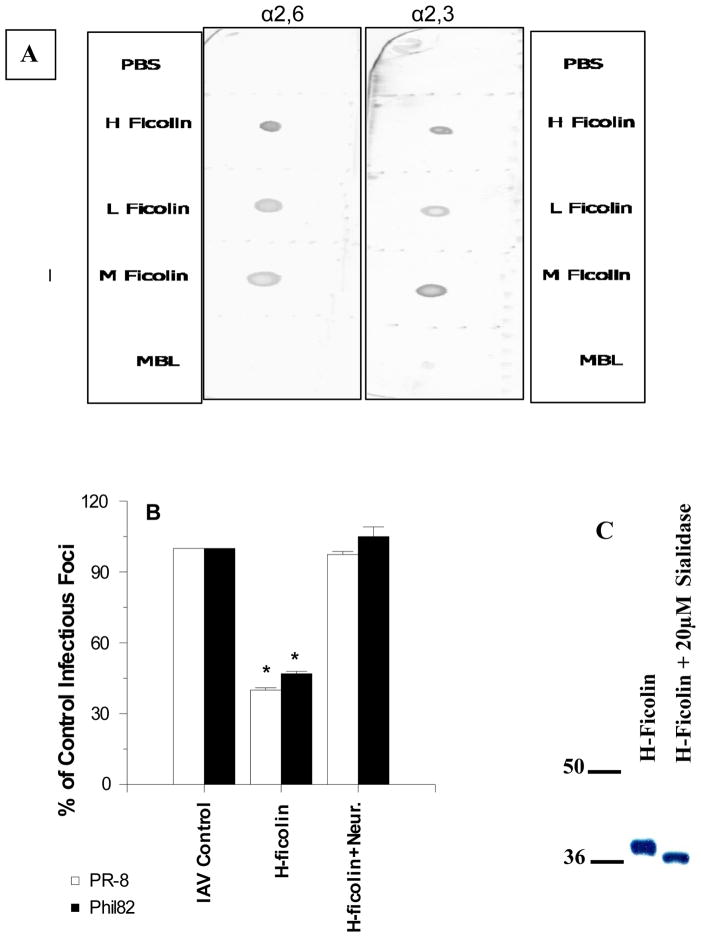
The ficolins have N-linked glycan attachments in their fibrinogen like domain (30). To determine if these are sialylated we did glycan blotting of the ficolins. As shown in figure 7A, all three ficolins showed the presence of α(2,3)- and α(2,6)-linked glycans, whereas MBL (which does not have any N-linked glycan attachment site) did not. To confirm the role for ficolin associated sialic acids in viral inhibition, we treated H-ficolin with neuraminidase and then re-purified the protein. This treatment completely abrogated the HA inhibitory activity of H-ficolin (figure 7B). Neuraminidase treatment caused the expected small decrease in the apparent molecular weight of H-ficolin, but did not result in degradation of the protein (figure 7C).
Figure 7. Presence of α(2,3) or α(2,6)-linked sialic acids on recombinant ficolins and effect of neuraminidase treatment of H-ficolin on antiviral activity.
In panel A, the presence of α(2,3) or α(2,6)-linked sialic acids on recombinant H-, L-, and M-ficolins was assessed by lectin blotting using SNA and MAA as sialic acid detecting molecules as described in methods. For comparison, MBL (which has no N-linked oligosaccharide attachments) was tested as well. In panel B, untreated H-ficolin, neuraminidase treated H-ficolin, or neuraminidase alone was pre-incubated with PR-8 or Phil82 IAV followed by infection of MDCK cells with the virus samples and infectious focus assay. Only untreated H-ficolin significantly inhibited viral infectivity (* indicates p<0.01 vs control). Panel C shows SDS-PAGE of untreated and neuraminidase treated H-ficolin. Results in panel B are mean±SEM of 4 experiments.
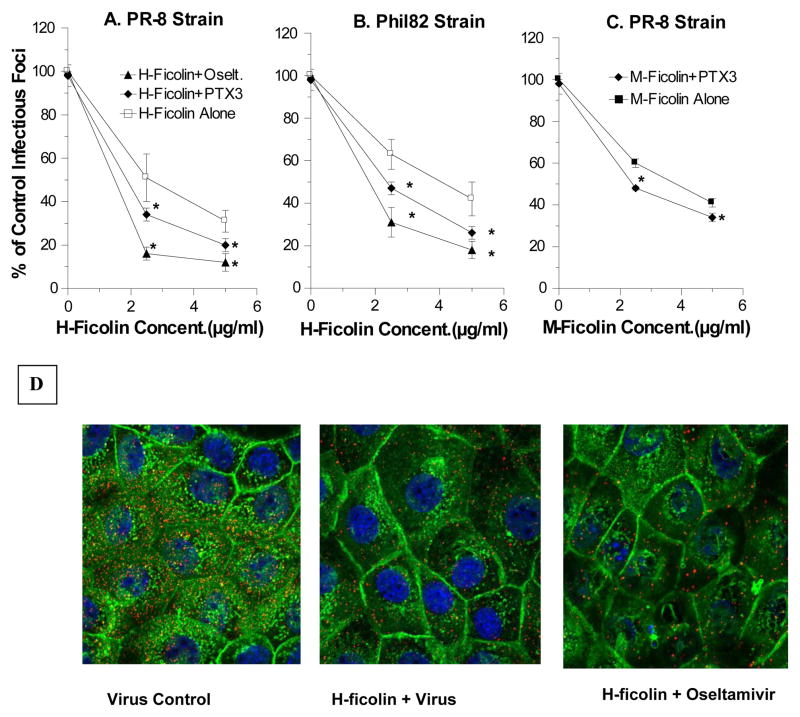
BALF causes inhibition of HA activity of IAV (Table 2). BALF was much less effective at inhibiting HA activity of the PR-8 strain than the Phil82 strain. This reflects the importance of SP-D in inhibiting seasonal strains of IAV as previously reported (22, 25). Note, however, that there is some inhibition of the fully SP-D-resistant PR-8 strain that was strongly potentiated by oseltamivir, suggesting that this inhibition is mediated to a significant extent by γ-inhibitors. The viral neutralizing activity of BALF for Phil82 and PR-8 strains of IAV was also increased in the presence of oseltamivir (figure 8A and B). There are multiple γ-inhibitors in BALF some of which have been shown to inhibit IAV, including pentraxin-3 (PTX3) and gp340 (26, 31). Of interest, recent studies demonstrated specific binding of PTX3 to M- and L-ficolins and synergistic host defense activites between these proteins (32). H-ficolin and PTX3 caused cooperative inhibition of HA activity (Table 2) and infectivity of PR-8 and Phil82 (figure 8A and B). M-ficolin had a similar effect (figure 8C). Hence, it is possible that H-ficolin in BALF contributes to inhibition of IAV through direct effects as well as through interactions with other inhibitors.
Table 2.
HA inhibition by BALF, H-ficolin and pentraxin
| Inhibitor | Viral Strain | |
|---|---|---|
| Phil82 H3N2 | PR-8 H1N1 | |
| BALF | 2.6±0.86 | 12±3.7 |
| BALF+Oseltamivir | 0.66±0.21* | 2.16±0.41* |
| H-Ficolin | 1.8±0.16 | 0.36±0.07 |
| Pentraxin 3 | >10 | >10 |
| H-Ficolin+Pentraxin | 1.09±0* | 0.186±0.04** |
Results are expressed as mean±SEM of 4 experiments in μg/ml of H-ficolin or BALF protein resulting in inhibition of 40 Hemagglutinin units of the indicated strains of IAV. The concentration of oseltamivir added was 10μg/ml. Pentraxin 3 had no independent HA inhibitory activity in this assay up to the maximum concentration tested (i.e., 10 μg/ml). Note that BALF results are expressed as total BALF protein.
indicates where oseltamivir significantly reduced the amount of BALF protein needed to inhibit IAV.
indicates where the effect of the combination of H-ficolin and pentraxin was significantly greater than the effect of either protein alone.
Figure 8. Effect of oseltamivir or PTX-3 on viral neutralizing activity of H-ficolin or on effects of H-ficolin on viral attachment or uptake by A549 cells as assessed with confocal microscopy.
Panel A–C show results of infectious focus assays using H- or M-ficolin alone or combined with either oseltamivir (5μg/ml) or PTX-3 (5μg/ml). Results in panel A–C are mean±SEM of 4 experiments. Addition of both oseltamivir and PTX3 significantly increased neutralizing activity compared to H-ficolin alone at either the 2.5 or 5μg/ml concentration of H-ficolin. This was true both for the Phil82 (panel A) or PR-8 (panel B) strains of IAV. PTX3 or oseltamivir alone did not reduce viral infectivity in this assay. The results for PTX3 are shown at the zero concentration of ficolins in the curves labeled PTX3. PTX3 significantly increased the neutralizing activity of M-ficolin as shown in panel C. Panel D shows confocal microscopic pictures of virus (red) after 45 min incubation with A549 cells (cell membrane green and nucleus blue). The MOI for the confocal experiments was 200 (i.e., higher than in infectious focus and Q-PCR assays). Results are representative of three experiments.
We performed confocal microscopy to directly evaluate how H-ficolin alone or in presence of oscltamivir alters interaction of IAV with A549 cells within 45 minutes of infection. As shown in figure 8B H-ficolin alone caused what appears to be a reduced number of viral particles associated with cells at this time point. As shown in figure 5 qPCR did not show reduced total viral RNA associated with the cells under these conditions. This suggested to us that ficolin might be reducing particle numbers through viral aggregation. It is difficult to assess this at this level of magnification unless the aggregates are large (for instance, SP-D causes formation of large viral aggregates that are easily visible by fluorescent microscopy) [(21) and data not shown]; however, the viral particles appeared somewhat larger in H-ficolin treated samples vs. control. Of interest, when H-ficolin and oseltamivir were used together viral particle sizes appeared larger than with H-ficolin alone (figure 8B right panel).
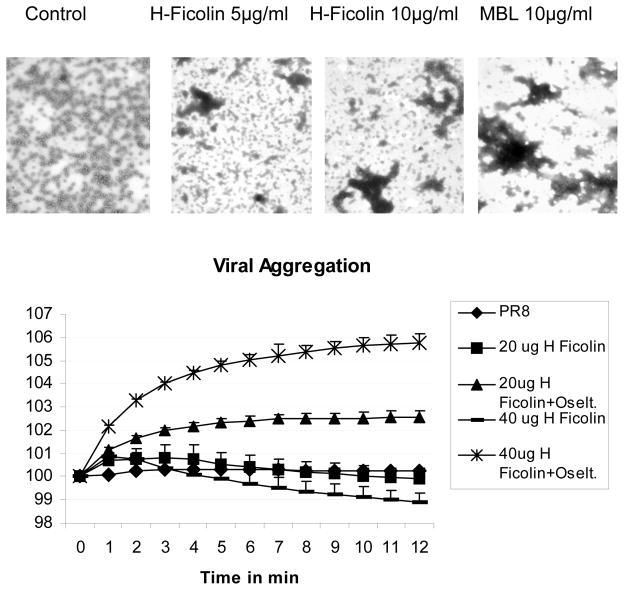
H-ficolin causes viral aggregation
Given the results obtained with confocal microscopy we used more sensitive techniques to assess viral aggregation. H-ficolin induced aggregation of IAV as assessed by electron microscopy or light absorbance assays (figure 9A and B, respectively). On the light transmission assay aggregation induced by H-ficolin alone was subtle (although comparable to that induced by MBL; not shown). As in the case of defensins (19), aggregation caused by H-ficolin was most clearly evident on electron microscopy. Addition of oseltamivir along with H-ficolin dramatically increased aggregation as assessed by light absorbance.
Figure 9. Viral aggregation induced by H-ficolin or MBL.
Panel A shows representative (of 4 experiments) electron microscopic images of PR-8 IAV alone (control) vs. IAV pre-treated with the indicated concentrations of H ficolin or MBL. In these experiments, 10μg/ml of H-ficolin caused a similar degree of viral aggregation as MBL. In panel B the ability of H-ficolin to cause viral aggregation was also tested by light absorbance assay. In this assay increased light transmission results from viral aggregation. This assay is less sensitive for detecting viral aggregation than EM; however, 20μg/ml and 40μg/ml of H-ficolin did cause significant increase in light transmission two minutes after addition to the viral suspension. Addition of oseltamivir (10μg/ml) during incubation of IAV with H-ficolin resulted in much more pronounced and sustained viral aggregation. No aggregation was seen with oseltamivir alone or with the PR-8 virus alone (black diamonds in panel B). Results in panel B are mean±SEM of 4 experiments.
H-ficolin fixes complement to IAV-coated surfaces
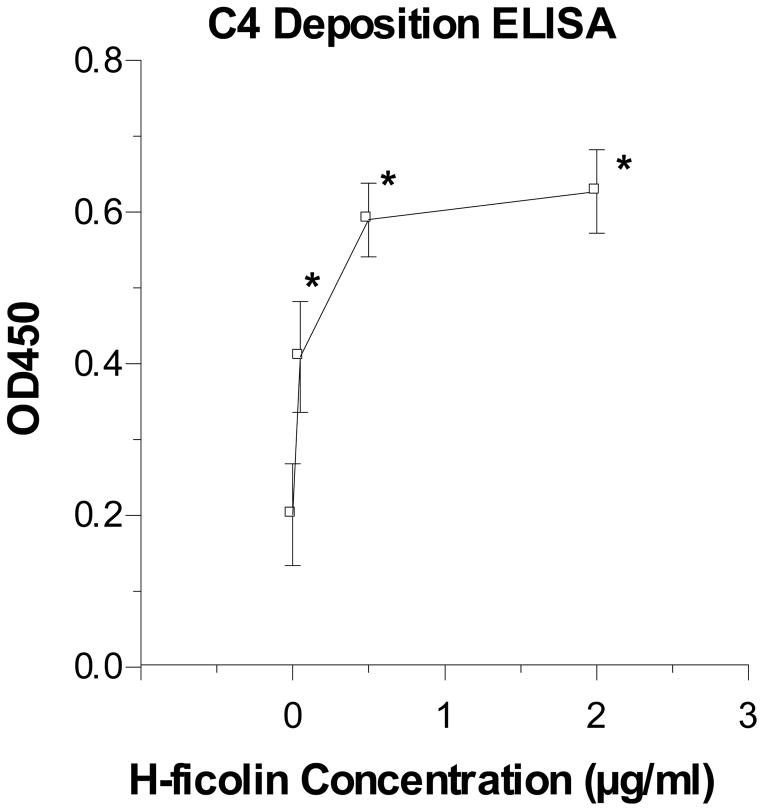
The PR-8 strain of IAV was coated onto the surface of ELISA plates as for the ELISA assay shown in figure 1. These plates were then incubated with H-ficolin/MASP-2 complexes purified from human serum as described (4). As shown in figure 10, there was dose-related deposition of the C4 component of complement onto the virus-coated surface. The extent of complement deposition was less than the amount seen on the acetylated BSA surface which served as the positive control.
Figure 10. H-ficolin/MASP-2 complexes fix complement in presence of IAV.
ELISA plates were coated with IAV as in figure 1. H-ficolin/MASP-2 complexes were purified from human serum and incubated with the surface bound IAV as described in “Materials and Methods”. Complement fixation was detected using antibody to complement component C4. H-ficolin caused dose related deposition of C4 onto the viral surface (n=5; * indicates p<0.05 vs. IAV-coated plates incubated with complement in the absence of H-ficolin. As a positive control 2μg/ml of the H-ficolin/MASP-2 complex was incubated with wells coated with acetylated BSA. This resulted in fixation of complement as reported (mean±SEM OD450 for C4 was 1.2±0.1).
DISCUSSION
In this paper we demonstrate for the first time that H-ficolin has antiviral activity against IAV. We also measure the levels of H-ficolin in BALF for the first time and show that H-ficolin in human serum and BALF bind to IAV. Importantly, H-ficolin effectively inhibited the 2009 pandemic H1N1 strain of IAV which is resistant to other innate inhibitors, including SP-D, MBL and pentraxin. We finally show that H-ficolin/MASP-2 complexes fix complement to IAV-coated surfaces. Overall our findings suggest that H-ficolin may play a role in innate defense against IAV in the airway and could contribute to the rarity of viremia in IAV infection. Pan et al recently demonstrated that M-ficolin inhibits IAV and that treatment of IAV with L-ficolin protects them against effects of IAV infection (10). Our findings differ somewhat from those of Pan et al since they showed that inhibition mediated by L-ficolin was calcium-dependent and presumably mediated by the lectin property of L-ficolin. We cannot readily account for this difference but we carried out extensive experiments to confirm that the inhibition mediated by H-ficolin is not mediated by its lectin property. The mechanism of neutralization caused by IAV was rather that of a γ-inhibitor (i.e., a glycosylated protein that provides a decoy sialylated ligand for binding by the viral HA). Binding and inhibition by γ-inhibitors is not calcium-dependent. Unexpectedly, we found that binding and inhibition by H-ficolin was actually increased in the absence of calcium which differs from findings with PTX3 where absence of calcium did not alter anti-IAV activity (26).
Other important γ-inhibitors of IAV include SP-A, gp-340, mucins, α2-macroglobulin and pentraxins (26, 27, 29, 33). Porcine SP-D is distinctive because it acts as a combined β- and γ-inhibitor (34). The effectiveness of γ-inhibitors depends in part on the extent to which the viral neuraminidase is able to cleave these sialic acids and release the virus from attachment to the inhibitor. As an example, mucins are relatively ineffective as inhibitors of IAV since the sialic acid ligands on mucins are readily cleaved by the viral neuraminidase. As a result, incubation of IAV with the combination of mucins and the neuraminidase inhibitor oseltamivir results in marked increase in viral inhibition compared to that which is achieved with either alone (28, 29). Other γ-inhibitors like SP-A, porcine SP-D, gp-340, pentraxin and ficolins have at least partial resistance to the activity of neuraminidase since they have antiviral activity in the absence of oseltamivir. In the case of SP-A this intrinsic antiviral activity can, however, be further increased by oseltamivir. We now show that oseltamivir increases neutralizing, HA inhibitory and aggregating activity of H-ficolin. With or without oseltamivir, the HA inhibitory and neutralizing activity of H-ficolin alone was much greater than for either mucins or SP-A (e.g., HA inhibitory concentrations for H-ficolin are approximately 5 times lower than those of SP-A) (29). We propose that H-ficolin plays a role in host defense against IAV in vivo (as has been demonstrated for SP-A), and that one of the mechanisms through which oseltamivir is beneficial in treating IAV is through potentiating the activity of γ-inhibitors, including H-ficolin.
SP-D plays the major role in the innate inhibitory activity of BALF for seasonal IAV strains (31, 35). As a result BALF causes much less inhibition of SP-D resistant strains like PR-8. This is important because pandemic IAV strains are generally resistant to inhibition by SP-D as well. There is some level of inhibition of such strains by BALF and this is increased significantly in the presence of oseltamivir as shown in table 2. A significant part of this activity appears to be mediated by ficolins based on our ficolin depletion results. The contribution of H-ficolin to the antiviral activity of BALF could be mediated by H-ficolin acting independently or via cooperative interactions of H-ficolin with other inhibitors. For instance, we show that H- and M-ficolins have cooperative HA inhibitory effects when combined with PTX3. M-ficolin has been shown to bind to PTX3 (whereas H-ficolin does not) (32, 36); however, M-ficolin did not have a significantly greater cooperative interaction with PTX3 than H-ficolin. This suggests that the binding of PTX3 to M-ficolin does not significantly contribute to combined antiviral effects. Since both PTX3 and ficolins resemble C1q and fix complement, further studies of combined effects on complement fixation in the presence of IAV would be of interest (32). In any case we propose that γ-inhibitors provide an important level of innate protection against IAV strains that are able to bypass the action of SP-D or MBL.
The activity of γ-inhibitors for specific viral strains depends on the specific types of sialic acid linkage present on the proteins (34, 37). In general the HA of avian or mouse adapted IAV strains have preferential binding to α(2,3)-linked sialic acids, whereas human strains prefer binding to α(2,6)-linked sialic acids and these preferences coincide with the type of sialic acid linkages found respectively on avian (or mouse) vs. human epithelia targeted by the virus. The collectins (other than SP-A and porcine SP-D) depend for their antiviral activity on the presence of glycans on viral envelope proteins (38). High mannose glycans on the viral HA are most important for inhibition by MBL, SP-D or the bovine serum collectins, conglutinin, CL43 and CL46 (39). Since the activity of γ-inhibitors is not dependent on viral envelope protein glycosylation they have activity against strains that are resistant to MBL or SP-D. In fact, γ-inhibitors appear to have greater activity against some hypoglycosylated viral strains (e.g., PR-8) than against strains like Phil82 that have abundant glycans on the HA (21). This could reflect the fact that addition of glycans to the globular domain of the HA reduces HA binding affinity for sialylated ligands. This appears to be the case for H-ficolin which has substantially greater inhibitory activity for the PR-8 strain. In this case of PR-8 virus, however, it could also depend on the presence of α(2,3)-linked sialic acids on the γ-inhibitor, since PR-8 is highly selective for this type of sialic acid linkage. The ficolins expressed both types of sialic acid linkage and this may account for their ability to inhibit both the human seasonal strain Phil82 and the mouse adapted PR-8 strain.
Known pandemic strains of IAV isolated over the past 100 years all had reduced glycosylation of their HA as compared to seasonal H1N1 and H3N2 strains (22). The ficolins are of particular interest in that they retain activity against the pandemic Cal09 H1N1 strain. This strain is not inhibited by SP-D or MBL effectively and requires significantly increased concentrations of SP-A for inhibition (23). Furthermore, pentraxins also did not inhibit this strain (23). It will be of great interest to determine if ficolins inhibit other pandemic or avian strains (e.g., H5N1).
The ability of collectins and defensins to induce viral aggregation contributes to their viral neutralizing activity. Ficolins resemble the collectins MBL and SP-A on a structural basis due to similarities in their oligomeric structure and collagen domains. In fact, replacement of the N-terminal and collagen domains of MBL with those of L-ficolin results in chimeric molecules that have equal or greater viral aggregating and neutralizing activity than MBL (12, 13). Like the collectins, H-ficolin is able to induce viral aggregation and this could be an important contributor to its antiviral activity in vivo. Aggregation could reduce particle numbers for infection of cells (see figure 8B) and promote viral clearance by phagocytes or mucociliary mechanisms. Further studies will be needed to understand more completely how H-ficolin alters the viral life cycle. We show that viral neutralization by H-ficolin involves interactions with the epithelial cells and or virus prior to internalization and that H-ficolin does not appear to alter the total amount of viral RNA attaching to or being taken up by cells. It is possible that through inducing subtle viral aggregation, or through engagement of distinct cellular receptors, H-ficolin alters processing of the virus after internalization.
We provide here the first measurements of levels of H-ficolin in normal donor BAL fluid. We also show H-ficolin in BALF or serum binds to IAV and that incubation of IAV with these fluids results in the precipitation of H-ficolin from the mixture. Hence, native H-ficolin, as it is found in normal blood or respiratory secretions, binds to IAV. Furthermore, depletion of ficolins from these fluids reduces their antiviral activity. The ficolin depletion procedure we used may have removed any M- or L-ficolin present in serum and BALF along with the H-ficolin. H-ficolin is, however, the most abundant ficolin in serum. Further studies would be needed to determine levels of M- or L-ficolin in BALF although it is likely they are less than H-ficolin. Another important avenue for further investigation will be studies of the role of ficolins in host defense against IAV in vivo in mouse models although mice lack a functional H-ficolin gene so that gene-deletion studies will not be possible (40). As noted in the introduction subjects with H-ficolin deficiency with increased susceptibility to infection has been reported (7, 8). It is tempting to speculate that individuals lacking or with reduced levels of H-ficolin may also be more susceptible to IAV infection. Susceptibility to IAV infection could also result in more frequent bacterial respiratory infections since IAV predisposes to bacterial infection.
Footnotes
This work was supported by NIH Grant AI-83222 (KLH) and Grant HL069031 (KLH), NIH intramural funds (JKT) and Novo Nordic Foundation (ST). This research was funded in part by the intramural research program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
References
- 1.Thiel S. Complement activating soluble pattern recognition molecules with collagen-like regions, mannan-binding lectin, ficolins and associated proteins. Mol Immunol. 2007;44:3875–3888. doi: 10.1016/j.molimm.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Garred P, Borregaard N. The ficolins. J Innate Immun. 2009;2:1–2. doi: 10.1159/000254982. [DOI] [PubMed] [Google Scholar]
- 3.Akaiwa M, Yae Y, Sugimoto R, Suzuki SO, Iwaki T, Izuhara K, Hamasaki N. Hakata antigen, a new member of the ficolin/opsonin p35 family, is a novel human lectin secreted into bronchus/alveolus and bile. J Histochem Cytochem. 1999;47:777–786. doi: 10.1177/002215549904700607. [DOI] [PubMed] [Google Scholar]
- 4.Zacho RM, Jensen L, Terp R, Jensenius JC, Thiel S. Studies of the pattern recognition molecule H-ficolin: specificity and purification. J Biol Chem. 2012;287:8071–8081. doi: 10.1074/jbc.M111.301044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sallenbach S, Thiel S, Aebi C, Otth M, Bigler S, Jensenius JC, Schlapbach LJ, Ammann RA. Serum concentrations of lectin-pathway components in healthy neonates, children and adults: mannan-binding lectin (MBL), M-, L-, and H-ficolin, and MBL-associated serine protease-2 (MASP-2) Pediatr Allergy Immunol. 2011;22:424–430. doi: 10.1111/j.1399-3038.2010.01104.x. [DOI] [PubMed] [Google Scholar]
- 6.Hummelshoj T, Fog LM, Madsen HO, Sim RB, Garred P. Comparative study of the human ficolins reveals unique features of Ficolin-3 (Hakata antigen) Mol Immunol. 2008;45:1623–1632. doi: 10.1016/j.molimm.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Munthe-Fog L, Hummelshoj T, Honore C, Madsen HO, Permin H, Garred P. Immunodeficiency associated with FCN3 mutation and ficolin-3 deficiency. N Engl J Med. 2009;360:2637–2644. doi: 10.1056/NEJMoa0900381. [DOI] [PubMed] [Google Scholar]
- 8.Schlapbach LJ, Thiel S, Kessler U, Ammann RA, Aebi C, Jensenius JC. Congenital H-ficolin deficiency in premature infants with severe necrotising enterocolitis. Gut. 2010;10:1438–1439. doi: 10.1136/gut.2010.226027. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Ali MA, Shi Y, Zhao Y, Luo F, Yu J, Xiang T, Tang J, Li D, Hu Q, Ho W, Zhang X. Specifically binding of L-ficolin to N-glycans of HCV envelope glycoproteins E1 and E2 leads to complement activation. Cell Mol Immunol. 2009;6:235–244. doi: 10.1038/cmi.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan Q, Chen H, Wang F, Jeza VT, Hou W, Zhao Y, Xiang T, Zhu Y, Endo Y, Fujita T, Zhang XL. L-Ficolin Binds to the Glycoproteins Hemagglutinin and Neuraminidase and Inhibits Influenza A Virus Infection Both in vitro and in vivo. J Innate Immun. 2012 doi: 10.1159/000335670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keirstead ND, Lee C, Yoo D, Brooks AS, Hayes MA. Porcine plasma ficolin binds and reduces infectivity of porcine reproductive and respiratory syndrome virus (PRRSV) in vitro. Antiviral Res. 2008;77:28–38. doi: 10.1016/j.antiviral.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang WC, Hartshorn KL, White MR, Moyo P, Michelow IC, Koziel H, Kinane BT, Schmidt EV, Fujita T, Takahashi K. Recombinant chimeric lectins consisting of mannose-binding lectin and L-ficolin are potent inhibitors of influenza A virus compared with mannose-binding lectin. Biochem Pharmacol. 2011;81:388–395. doi: 10.1016/j.bcp.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michelow IC, Dong M, Mungall BA, Yantosca LM, Lear C, Ji X, Karpel M, Rootes CL, Brudner M, Houen G, Eisen DP, Kinane TB, Takahashi K, Stahl GL, Olinger GG, Spear GT, Ezekowitz RA, Schmidt EV. A novel L-ficolin/mannose-binding lectin chimeric molecule with enhanced activity against Ebola virus. J Biol Chem. 2010;285:24729–24739. doi: 10.1074/jbc.M110.106260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krarup A, Thiel S, Hansen A, Fujita T, Jensenius JC. L-ficolin is a pattern recognition molecule specific for acetyl groups. J Biol Chem. 2004;279:47513–47519. doi: 10.1074/jbc.M407161200. [DOI] [PubMed] [Google Scholar]
- 15.Hartshorn KL, Collamer M, Auerbach M, Myers JB, Pavlotsky N, Tauber AI. Effects of influenza A virus on human neutrophil calcium metabolism. J Immunol. 1988;141:1295–1301. [PubMed] [Google Scholar]
- 16.Frederiksen PD, Thiel S, Larsen CB, Jensenius JC. M-ficolin, an innate immune defence molecule, binds patterns of acetyl groups and activates complement. Scand J Immunol. 2005;62:462–473. doi: 10.1111/j.1365-3083.2005.01685.x. [DOI] [PubMed] [Google Scholar]
- 17.Kuraya M, Ming Z, Liu X, Matsushita M, Fujita T. Specific binding of L-ficolin and H-ficolin to apoptotic cells leads to complement activation. Immunobiology. 2005;209:689–697. doi: 10.1016/j.imbio.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Hartshorn KL, Crouch EC, White MR, Eggleton P, Tauber AI, Chang D, Sastry K. Evidence for a protective role of pulmonary surfactant protein D (SP-D) against influenza A viruses. J Clin Invest. 1994;94:311–319. doi: 10.1172/JCI117323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tecle T, White MR, Gantz D, Crouch EC, Hartshorn KL. Human neutrophil defensins increase neutrophil uptake of influenza A virus and bacteria and modify virus-induced respiratory burst responses. J Immunol. 2007;178:8046–8052. doi: 10.4049/jimmunol.178.12.8046. [DOI] [PubMed] [Google Scholar]
- 20.Hartshorn K, Sastry K, Chang D, White M, Crouch E. Enhanced antinfluenza activity of a recombinant pulmonary surfactant protein D and serum conglutinin fusion protein. Amer J Physiol. 2000;278:L90–98. doi: 10.1152/ajplung.2000.278.1.L90. [DOI] [PubMed] [Google Scholar]
- 21.Hartshorn KL, White MR, Shepherd V, Reid K, Jensenius JC, Crouch EC. Mechanisms of anti-influenza activity of surfactant proteins A and D: comparison with serum collectins. Am J Physiol. 1997;273:L1156–1166. doi: 10.1152/ajplung.1997.273.6.L1156. [DOI] [PubMed] [Google Scholar]
- 22.Qi L, Kash JC, Dugan VG, Jagger BW, Lau YF, Sheng ZM, Crouch EC, Hartshorn KL, Taubenberger JK. The ability of pandemic influenza virus hemagglutinins to induce lower respiratory pathology is associated with decreased surfactant protein D binding. Virology. 2011;412:426–434. doi: 10.1016/j.virol.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Job ER, Deng YM, Tate MD, Bottazzi B, Crouch EC, Dean MM, Mantovani A, Brooks AG, Reading PC. Pandemic H1N1 influenza A viruses are resistant to the antiviral activities of innate immune proteins of the collectin and pentraxin superfamilies. J Immunol. 185:4284–4291. doi: 10.4049/jimmunol.1001613. [DOI] [PubMed] [Google Scholar]
- 24.Hartshorn KL, Sastry K, White MR, Anders EM, Super M, Ezekowitz RA, Tauber AI. Human mannose-binding protein functions as an opsonin for influenza A viruses. J Clin Invest. 1993;91:1414–1420. doi: 10.1172/JCI116345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reading PC, Morey LS, Crouch EC, Anders EM. Collectin-mediated antiviral host defense of the lung: evidence from influenza virus infection of mice. J Virol. 1997;71:8204–8212. doi: 10.1128/jvi.71.11.8204-8212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reading PC, Bozza S, Gilbertson B, Tate M, Moretti S, Job ER, Crouch EC, Brooks AG, Brown LE, Bottazzi B, Romani L, Mantovani A. Antiviral Activity of the Long Chain Pentraxin PTX3 against Influenza Viruses. J Immunol. 2008;180:3391–3398. doi: 10.4049/jimmunol.180.5.3391. [DOI] [PubMed] [Google Scholar]
- 27.Hartshorn KL, Ligtenberg A, White MR, Van Eijk M, Hartshorn M, Pemberton L, Holmskov U, Crouch E. Salivary agglutinin and lung scavenger receptor cysteine-rich glycoprotein 340 have broad anti-influenza activities and interactions with surfactant protein D that vary according to donor source and sialylation. Biochem J. 2006;393:545–553. doi: 10.1042/BJ20050695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White MR, Helmerhorst EJ, Ligtenberg A, Karpel M, Tecle T, Siqueira WL, Oppenheim FG, Hartshorn KL. Multiple components contribute to ability of saliva to inhibit influenza viruses. Oral Microbiol Immunol. 2009;24:18–24. doi: 10.1111/j.1399-302X.2008.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White MR, Crouch E, van Eijk M, Hartshorn M, Pemberton L, Tornoe I, Holmskov U, Hartshorn KL. Cooperative anti-influenza activities of respiratory innate immune proteins and neuraminidase inhibitor. Am J Physiol Lung Cell Mol Physiol. 2005;288:L831–840. doi: 10.1152/ajplung.00365.2004. [DOI] [PubMed] [Google Scholar]
- 30.Garlatti V, Martin L, Lacroix M, Gout E, Arlaud GJ, Thielens NM, Gaboriaud C. Structural insights into the recognition properties of human ficolins. J Innate Immun. 2009;2:17–23. doi: 10.1159/000233475. [DOI] [PubMed] [Google Scholar]
- 31.Hartshorn KL, White MR, Mogues T, Ligtenberg T, Crouch E, Holmskov U. Lung and salivary scavenger receptor glycoprotein-340 contribute to the host defense against influenza A viruses. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1066–1076. doi: 10.1152/ajplung.00057.2003. [DOI] [PubMed] [Google Scholar]
- 32.Ma YJ, Doni A, Hummelshoj T, Honore C, Bastone A, Mantovani A, Thielens NM, Garred P. Synergy between ficolin-2 and pentraxin 3 boosts innate immune recognition and complement deposition. J Biol Chem. 2009;284:28263–28275. doi: 10.1074/jbc.M109.009225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benne CA, Kraaijeveld CA, van-Strijp JAG, Brouwer E, Harmsen M, Verhoef J, van-Golde LMG, van-Iwaarden JF. Interactions of surfactant protein A with influenza A viruses: Binding and neutralization. J Infect Dis. 1995;171:335–341. doi: 10.1093/infdis/171.2.335. [DOI] [PubMed] [Google Scholar]
- 34.van Eijk M, White MR, Crouch EC, Batenburg JJ, Vaandrager AB, Van Golde LM, Haagsman HP, Hartshorn KL. Porcine pulmonary collectins show distinct interactions with influenza A viruses: role of the N-linked oligosaccharides in the carbohydrate recognition domain. J Immunol. 2003;171:1431–1440. doi: 10.4049/jimmunol.171.3.1431. [DOI] [PubMed] [Google Scholar]
- 35.Hartshorn KL, Crouch EC, White MR, Eggleton P, Tauber AI, Chang D, Sastry K. Evidence for a protective role of pulmonary surfactant protein D (SP-D) against influenza A viruses. J Clin Invest. 1994;94:311–319. doi: 10.1172/JCI117323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gout E, Moriscot C, Doni A, Dumestre-Perard C, Lacroix M, Perard J, Schoehn G, Mantovani A, Arlaud GJ, Thielens NM. M-Ficolin Interacts with the Long Pentraxin PTX3: A Novel Case of Cross-Talk between Soluble Pattern-Recognition Molecules. J Immunol. 2011;186:5815–5822. doi: 10.4049/jimmunol.1100180. [DOI] [PubMed] [Google Scholar]
- 37.Mikerov AN, White M, Hartshorn K, Wang G, Floros J. Inhibition of hemagglutination activity of influenza A viruses by SP-A1 and SP-A2 variants expressed in CHO cells. Med Microbiol Immunol. 2008;197:9–12. doi: 10.1007/s00430-007-0051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hartshorn KL. Role of surfactant protein A and D (SP-A and SP-D) in human antiviral host defense. Front Biosci (Schol Ed) 2010;2:527–546. doi: 10.2741/s83. [DOI] [PubMed] [Google Scholar]
- 39.Hartshorn KL, White MR, Voelker DR, Coburn J, Zaner K, Crouch EC. Mechanism of binding of surfactant protein D to influenza A viruses: importance of binding to haemagglutinin to antiviral activity. Biochem J. 2000;351(Pt 2):449–458. [PMC free article] [PubMed] [Google Scholar]
- 40.Endo Y, Liu Y, Kanno K, Takahashi M, Matsushita M, Fujita T. Identification of the mouse H-ficolin gene as a pseudogene and orthology between mouse ficolins A/B and human L-/M-ficolins. Genomics. 2004;84:737–744. doi: 10.1016/j.ygeno.2004.07.006. [DOI] [PubMed] [Google Scholar]