Abstract
Many stroke patients are subject to limited hand functions in the paretic arm due to a significant loss of Corticospinal Tract (CST) fibers. A possible solution for this problem is to classify surface Electromyography (EMG) signals generated by hand movements and uses that to implement Functional Electrical Stimulation (FES). However, EMG usually presents an abnormal muscle coactivation pattern shown as increased coupling between muscles within and/or across joints after stroke. The resulting Abnormal Muscle Synergies (AMS) could make the classification more difficult in individuals with stroke, especially when attempting to use the hand together with other joints in the paretic arm. Therefore, this study is aimed at identifying the impact of AMS following stroke on EMG pattern recognition between two hand movements. In an effort to achieve this goal, 7 chronic hemiparetic chronic stroke subjects were recruited and asked to perform hand opening and closing movements at their paretic arm while being either fully supported by a virtual table or loaded with 25% of subject’s maximum shoulder abduction force. During the execution of motor tasks EMG signals from the wrist flexors and extensors were simultaneously acquired. Our results showed that increased synergy-induced activity at elbow flexors, induced by increasing shoulder abduction loading, deteriorated the performance of EMG pattern recognition for hand opening for those with a weak grasp strength and EMG activity. However, no such impact on hand closing has yet been observed possibly because finger/wrist flexion is facilitated by the shoulder abduction-induced flexion synergy.
I. Introduction
Abnormal muscle synergies have been described as stereotypical coactivation pattern between certain groups that result in a loss of independent joint control following stroke induced brain injury. One example is the pronounced coupling between shoulder abduction and elbow flexion [1]–[2]. Because of the expression of this so-called flexion synergy, when the loads on shoulder progressively increase in individuals with stroke, an increased obligatory activity from hand/wrist flexors starts to emerge. As a result of both paralysis and the flexion synergy, a more severely impaired individual with stroke can totally lose his/her voluntary control for hand opening and closing. The expression of these impairments following stroke may result in great difficulty in performing EMG-based detection of hand movements even when employing the latest signal pattern recognition techniques [3]–[6]. Therefore, the objective of this study will be trying to find out how Abnormal Muscle Synergy (AMS) affects pattern recognition for two different hand movements based on surface EMG in individuals with chronic stroke. The current study is anticipated to shed lights on three questions: 1) whether the EMG signal is a reliable source for the detection of hand movement intention after stroke; 2) How does AMS influence intention detection across stroke patients? 3) Depending on the outcome, are other signals such as EEG required to complement EMG-driven intention detection. These are essential issues that need to be addressed before development of any EMG-driven neural prosthetics aimed at regaining hand function in individuals with stroke.
II. Methods
A. Participants
A total of 7 chronic hemiparetic stroke subjects (age: 55.29±8.04) with moderate to severe impairment were recruited for this study. The level of motor impairment was evaluated using the Fugl-Meyer motor assessment index [7] of all the stroke participants. This assessment includes the evaluation of tendon reflexes and performance of proximal and distal voluntary movements of the impaired arm. The test was administered by a licensed physical therapist. Subjects with a score around 60/66 were classified as mildly impaired, while those with a score less than 20/66 as severely impaired. Relative Grasp Strength (RGS) was also measured during each individual’s first visit. Every participant was asked to grasp a hand dynamometer as hard as possible while sitting in a chair and maintaining his/her shoulder at 90° shoulder abduction and 90° elbow flexion. RGS is defined as the ratio of grasp strength of paretic arm to non-paretic arm. Clinical information regarding the stroke subjects is listed in Table I. All participants provided written consent prior to participation in the study that was approved by the Institutional Review Board of Northwestern University and in compliance with the principles of the Declaration of Helsinki.
Table I.
CLINICAL INFORMATION OF PARTICIPANTS
| Gender | Age | Affected Hand |
Years | FM Score |
RGS | |
|---|---|---|---|---|---|---|
| 1 | M | 61 | L | 3 | 20 | 0.242 |
| 2 | M | 40 | L | 5 | 36 | 0.260 |
| 3 | M | 62 | L | 7 | 34 | 0.251 |
| 4 | M | 59 | R | 9 | 14 | 0.246 |
| 5 | M | 54 | L | 3 | 25 | 0.199 |
| 6 | F | 61 | R | 6 | 16 | 0.197 |
| 7 | M | 50 | L | 11 | 12 | 0.128 |
Relative Grasp Strength (RGS) is defined as the ratio of grasp strength of paretic arm to non-paretic arm.
B. Experiment Setup
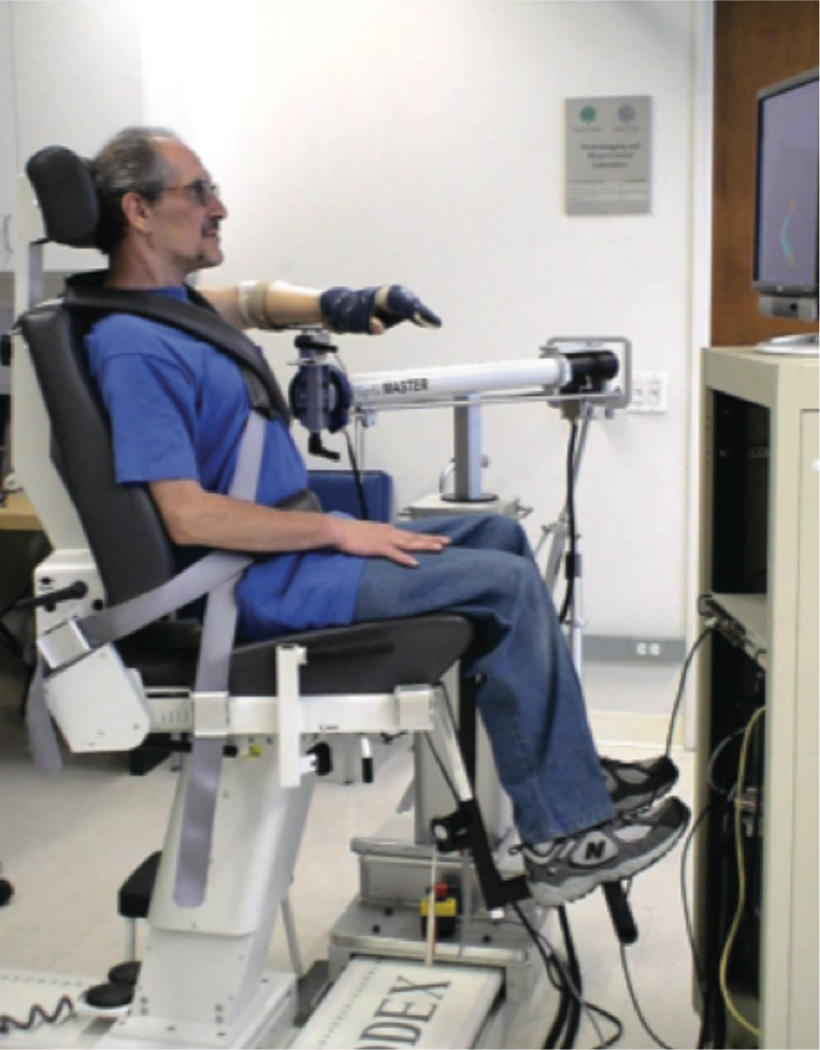
Participants were asked to sit in a Biodex chair (Biodex Medical Systems, Shirley, NY) and restrained by a pair of belts crossing their chest and abdomen to limit any unnecessary movements of trunk and pelvis while the arm was strapped to a forearm-hand orthosis attached to the ACT3D. The ACT3D can generate different levels of shoulder loads to induce AMS (Fig. 1). Four pairs of EMG active differential surface electrodes each with a 1 cm interelectrode distance (16-channel Bagnoli EMG System; Delsys, Boston, MA) were placed on Extensor Digitorum Communis (EDC), Extensor Carpi Radialis (ECR), Flexor Digitorum Profundus (FDP), Flexor Carpi Radialis (FCR) respectively before the arm was strapped to the orthosis. The paretic arm was manually positioned at 75° shoulder abduction, 40° shoulder flexion and 90° elbow flexion. An avatar of the arm, which mimics the arm position and movement, was shown on a flat screen display placed in front of the participants so they were able to position their hand right on the target following the display of a cue.
Figure 1.
Experiment Setup
C. Data Acquisition, Algorithm and Processing
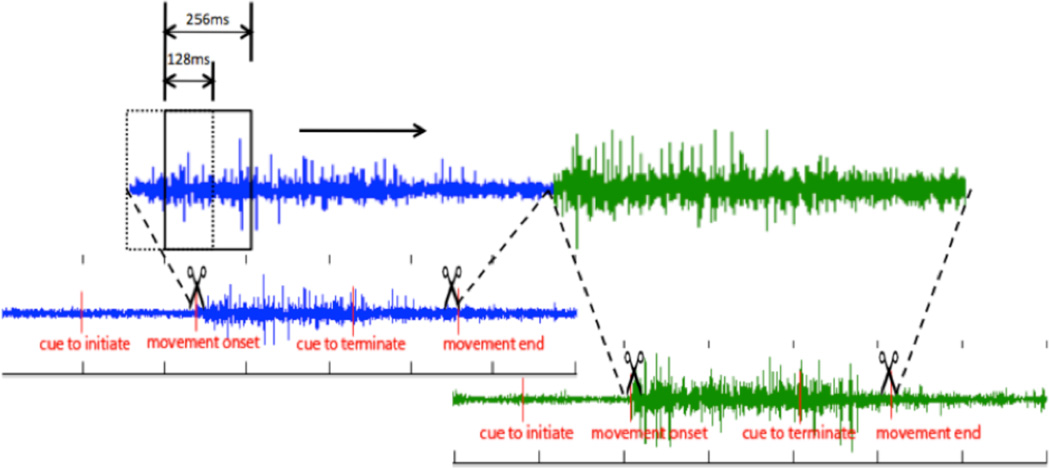
After subject’s arm was placed on the orthosis, a specific load level was calculated according to the subject’s maximum shoulder abduction force, which was measured using a six-freedom cell. In this study, subjects were asked to open and close their affected hand under two conditions, either with their arm being completely supported on a haptic table generated by the robot or at 25% of maximum shoulder abduction (Table II) requiring the participant to lift their arm of the haptic table prior to their two hand movements. Both arm lifting and hand movements were cued by a spherical target shown on the display with three colors indicating rest, lift or movement, respectively. The experiment consists of 6 sessions, each of which includes 4 sets of 11 randomized trials, covering 66 trials of hand opening and 66 trials of hand closing for each supporting condition. In order to avoid fatigue, a 10-minute break was offered between sessions. All EMG signals were manually segmented and concatenated according to the onset and termination of the movement (Fig. 2). A 256-ms long sliding window that was implemented from the beginning to the end of the segmented EMGs with a 128-ms increment and a 128-ms overlap between adjacent windows. Within each moving window, signal features in time domain were extracted based on the method proposed by Hudgins [8]. This method calculated four parameters in the time domain: mean absolute value, zero crossings, slope sign changes and waveform length [9]. Therefore, in each moving window, 16 feature parameters (4 channel EMGs times 4 parameters) were used to represent myoelectric firing pattern and then send to a linear discriminant classifier (LDC) to be recognized as either a hand opening or hand closing tasks. LDC is aimed at finding a linear combination of features which characterize or separate two or more classes of objects or events. LDC approaches a classification problem based on two assumptions (x⃗ is features in training set, y is classification set): 1) the conditional probability density functions p(x⃗|y = 0) and p(x⃗|y = 1) are both normally distributed with mean and covariance parameters and , respectively; 2) The class covariances are identical, so Σy=0 = Σy=1 = Σ. Therefore, the decision criterion for LDA is w⃗ · x⃗ < c, for some threshold c, where . So classification of an input x⃗ as in y is a function of the linear combination of the known observations. For each of the single hand movements, if the outcome of the judgment from the moving windows for hand opening outnumbered those for hand closing, this piece of signal was counted as a hand-opening task, and vise versa.
Table II.
TWO LOADING LEVELS OF SHOULDER ABDUCTION
| Loading level | Description | Illustration |
|---|---|---|
| 0% | Supported by the force that equals to the weight of the subject’s arm, so the subject will experience a weightless arm. However, the subject cannot push down to obtain extra supporting force to facilitate hand extension. | 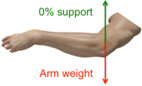 |
| 25% | Loaded by the force that amounts to 25% of the weight of the subject’s arm. Subjects have to lift a weight that equals to 125% of their arm. |  |
Figure 2.
EMG signal segmentation and concatenation. A 256ms-long sliding window slides which moves over a signal sequence with at a 128ms increment each time. Four feature parameters are extracted within the window and then go transfered to classifier.
III. Results
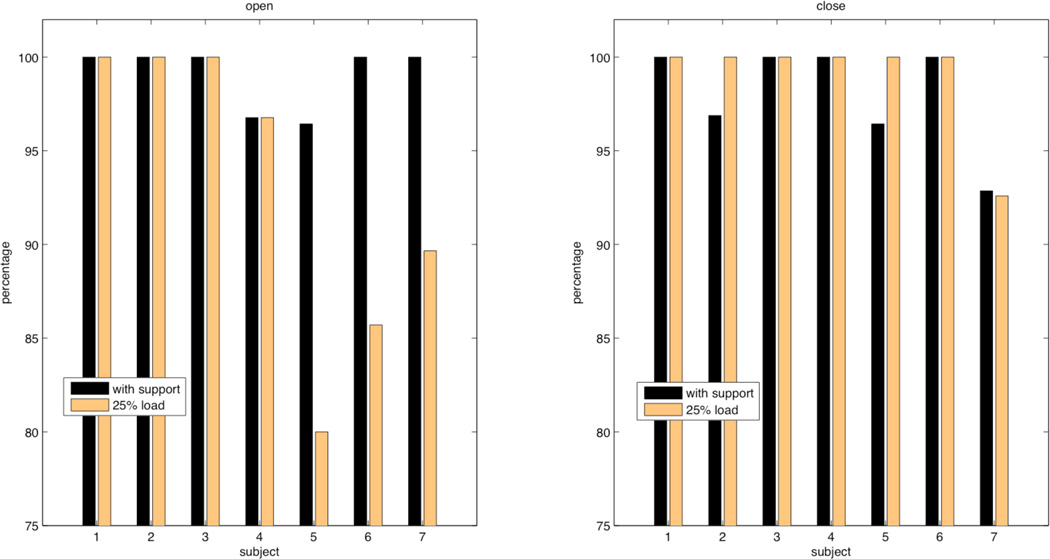
Hand movement classification rates for the 2 different shoulder loads in each of subjects were listed in Table III in the format of ‘the number of trials that were correctly classified’/’ the number of total available trials for a specific condition.’ Classification rates across all seven individuals with stroke for both abduction levels were further plotted in Fig. 3. Statistical t-test analysis investigating the effect of shoulder abduction loading on overall classification rates for hand closing did not reach significance across subjects and was 98% when supported and 99% during shoulder loading. Conversely, for hand opening results do reach significance and the overall mean classification rate is 99% when supported on the haptic table and 93% during shoulder abduction loading.
TABLE III.
CLASSIFICATION RESULTS
| Subject | Group | Level | Open | Close |
|---|---|---|---|---|
| 1 | I | 0% | 30/30 | 33/33 |
| 25% | 30/30 | 32/32 | ||
| 2 | I | 0% | 32/32 | 31/32 |
| 25% | 32/32 | 32/32 | ||
| 3 | I | 0% | 29/29 | 30/30 |
| 25% | 30/30 | 30/30 | ||
| 4 | I | 0% | 30/31 | 30/30 |
| 25% | 30/31 | 30/30 | ||
| 5 | II | 0% | 27/28 | 27/28 |
| 25% | 24/30 | 30/30 | ||
| 6 | II | 0% | 26/26 | 28/28 |
| 25% | 24/28 | 27/27 | ||
| 7 | II | 0% | 29/29 | 26/28 |
| 25% | 26/29 | 25/27 |
Group I: subjects with RGS > 0.23; Group II: subjects with RGS ≤ 0.23
Figure 3.
Comparison between hand opening and closing pattern recognition with either shoulder being fully supported or loaded with 25% arm weight. Group I (subject 1, 2, 3, 4) shows a consistently high rates for both opening and closing; while in Group II (subject 5, 6, 7) with a smaller RGS, lower recognition rates with a loaded arm were observed for hand opening only, but not for closing.
When we divide the subjects in two groups based on grip strength (above and below 0.23), we did observe differences in the recognition rate for hand opening, as shown in figure 3. Subjects in Group I (> 0.23) showed consistently high (> 95%) recognition rate for both hand opening and closing regardless of shoulder loading. In contrast, recognition rates for hand opening in participants in Group II are more sensitive to changes in shoulder loading as recognition rates dropped from 10% to 16%.
IV. Discussion
A. EMG Signal and Hand Movement Intention Detection
This study provides high recognition rates for hand opening and closing from moderately to severely impaired stroke subjects. This may be due to the fact that hand opening and closing represent relatively simple movements at the level of the hand. The original signals from all stroke subjects show consistently weaker EMG signals from extensors as compared to flexors therefore classification of hand opening and closing may rely mainly on the signals from two flexors. Weak muscle innervations in extensors are a common problem in individuals with stroke [2], [10]. Such a problem is likely to become critical when additional movements are to be classified.
One solution to this dilemma may be the introduction of EEG to compensate the loss of extensors EMG signals. Signal acquisition directly from Central Nervous System (CNS) is likely to be less affected by AMS and thus may supply supplementary information to classify additional wrist/finger movements [11].
Another explanation of our results is probably related to the inclusion of stroke subjects with less muscle weakness. Even though these individuals are equally incapable performing the hand opening and closing tasks, they are able to generate stronger EMG signals that are likely to benefit pattern our classification analysis.
B. Possible Neural Mechanism
Our study demonstrates that shoulder loading has a distinctive impact on subjects from the second group. The sensitivity to load changes increases for the subjects with a smaller RGS and less EMG signal strength. These preliminary results indicate that the success of intention detections may be linked to overall motor impairment as measured with FMA and RGS; however, a great participant number is required to conclusively answer this question.
Although the current pattern recognition method has shown great successes in healthy and amputee subjects who can still activate their muscles, the same is not necessarily expected in individuals with stroke. Yet our results have shown that even in the case of a compromised CNS the algorithm still preforms well albeit for a simple set of motor tasks. This could be seen from Group I in Fig. 3, both hand opening and closing intention of these individuals can still be easily detected presumably because the loss of their neural substrates like CST fibers is not as significant as in individuals from group II. In the Group II individuals where hand strength is more impaired, possibly because of an additional loss of CST tract fibers, the detection of intention is less successful. As mentioned earlier these individuals, may benefit from a combination of both central and periphery signals for intention detection.
V. Conclusion
This study demonstrates that shoulder abduction loading can have an impact on the detection of hand opening in stroke survivors with a weak RGS and associated EMG activity. At the same time, hand closing will not be affected to the same extent as hand opening and therefore still results high recognition rates even in more paralyzed individuals. Finally, in individuals with extreme weak peripheral signals, such as during the hand opening task, EEG-driven methods could be considered to augment detection rates as obtained with an EMG based pattern recognition approach.
Acknowledgment
This work was supported in part by a NIH/NCRR Grant, UL1 RR025741, Yao(PI) (sponsor and financial support acknowledgment goes here).
The author thanks to Carolina Carmona and Justin Drogos for helping collect the data.
Contributor Information
Yiyun Lan, Graduate student in Interdepartmental Neuroscience Program and Department of Physical Therapy and Human Movement Sciences at Northwestern University, Feinberg School of Medicine, Chicago, IL 60611, USA (phone: 312-503-4431; fax: 312-908-0741; yiyunlan2009@u.northwestern.edu)..
Jun Yao, Assistant Professor at Department of Physical Therapy and Human Movement Sciences at Northwestern University..
Julius P.A. Dewald, Professor and Chairman at Department of Physical Therapy and Human Movement Sciences and Biomedical Engineering at Northwestern University..
References
- 1.Dewald JP, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain. 1995 Apr;vol. 118(Pt 2):495–510. doi: 10.1093/brain/118.2.495. [DOI] [PubMed] [Google Scholar]
- 2.Dewald JP, Beer RF. Evidence for abnormal joint torque patterns in the paretic upper limb of subjects with hemiparesis. Muscle & Nerve. 2001;vol. 24(2):273–283. doi: 10.1002/1097-4598(200102)24:2<273::aid-mus130>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 3.Englehart K, Hudgins B, Parker PA, Stevenson M. Classification of the myoelectric signal using time-frequency based representations. Medical Engineering & Physics. 1999 Jul;vol. 21:431–438. doi: 10.1016/s1350-4533(99)00066-1. [DOI] [PubMed] [Google Scholar]
- 4.Englehart K, Hudgin B, Parker PA. A wavelet-based continuous classification scheme for multifunction myoelectric control. IEEE Trans. Biomedical Engineering. 2001 Mar;vol. 48:302–311. doi: 10.1109/10.914793. [DOI] [PubMed] [Google Scholar]
- 5.Englehart K, Hudgins B. A robust, real-time control scheme for multifunction myoelectric control. IEEE Trans. Biomedical Engineering. 2003 Jul;vol. 50:848–854. doi: 10.1109/TBME.2003.813539. [DOI] [PubMed] [Google Scholar]
- 6.Reaz MBI, Hussain MS, Mohd-Yasin F. Techniques of EMG signal analysis: detection, processing, classification and applications. Biological Procedures Online. 2006;vol. 8:11–35. doi: 10.1251/bpo115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fugle-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975;vol. 7:13–31. [PubMed] [Google Scholar]
- 8.Hudgins B, Parker P, Scott RN. A new strategy for multifunction myoelectric control. IEEE Transl. Biomedical Engineering. 1993 Jan;vol. 40 doi: 10.1109/10.204774. 82–49. [DOI] [PubMed] [Google Scholar]
- 9.Chan ADC, Green GC. Myoelectric control development toolbox. 30th conference of the Canadian Medical & Biological Engineering Society; Toronto, Canada, M0100: 2007. [Google Scholar]
- 10.Sukal TM, Ellis MD. Shoulder abduction-induced reductions in reaching work area following hemiparetic stroke: neuroscientific implications. Exp. Brain Res. 2007 Nov;vol. 183:215–223. doi: 10.1007/s00221-007-1029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buch E, et al. Think to move, a neuromagnetic brain-computer interface (BCI) system for chronic stroke. Stroke. 2008 Feb;vol. 39:910–917. doi: 10.1161/STROKEAHA.107.505313. [DOI] [PMC free article] [PubMed] [Google Scholar]