Abstract
Objectives
In an entirely African-American cohort, we compared clinical characteristics, cardiac structure and function, and all cause mortality in heart failure (HF) with preserved ejection fraction (HFpEF) in relation to HF with reduced ejection fraction (HFrEF) and those without HF.
Background
African-Americans are at increased risk for HF. Nevertheless, there are limited phenotypic and prognostic data in African-Americans with HFpEF compared to those with HFrEF and those without HF.
Methods
Middle-aged African-Americans from the Jackson cohort of the Atherosclerosis Risk in Communities study (n=2,445) underwent echocardiography between 1993 and 1995. HF prevalence was available in 1,962 for whom left ventricular ejection fraction (LVEF) could be quantified. Participants with HF were categorized as having HFpEF (LVEF ≥ 50%) or HFrEF (LVEF < 50%), or no HF, with comparisons made between groups.
Results
HF was identified in 116 (5.9%) participants (n=85 [73%] HFpEF; n=31 [27%] HFrEF). Compared to those without HF, those with HFpEF were older, more likely to be female, had more frequent comorbidities, and concentric hypertrophy. In relation to HFrEF, those with HFpEF were more likely female, but less likely to have coronary heart disease, diabetes mellitus, chronic kidney disease, left atrial enlargement, and eccentric hypertrophy. Over a median 13.7 years of follow up, risk of death differed between groups, with age and sex adjusted hazard ratios of 1.51 (95%CI 1.01–2.25) for HFpEF vs. those without HF, and 2.50 (95%CI 1.37–4.58) for HFrEF vs. HFpEF.
Conclusions
In this cohort of middle-aged African-Americans, HFpEF was the most common form of HF, and was associated with a substantially better prognosis than HFrEF, but worse than those without HF.
Keywords: African-Americans, heart failure with preserved ejection fraction, heart failure with reduced ejection fraction, echocardiography, mortality
Introduction
African-Americans, as compared to other racial groups, are at increased risk for the development of heart failure (HF) (1–3). Additionally, HF in African-Americans occurs at a younger age and is associated with a higher prevalence of cardiovascular risk factors, particularly hypertension and diabetes mellitus, but lower frequency of coronary heart disease (CHD) (4–6). It has been suggested that the prevalence of HF and preserved ejection fraction (HFpEF) may be higher in African-Americans than current estimates of HFpEF in the general population (5,7,8). However, findings are mixed regarding survival in African-Americans with HF (3,4,6,9) and information is limited concerning prognosis in African-Americans with HFpEF (10,11). We therefore aimed to describe differences in clinical characteristics, cardiac structure and function, and prognosis in a community based sample of African-Americans with HFpEF as compared to those with HF and reduced ejection fraction (HFrEF) and those without HF.
Methods
Study population
The Atherosclerosis Risk in Communities (ARIC) study is an ongoing, prospective observational study of the natural history of atherosclerotic diseases and cardiovascular risk factors. Detailed study rationale, design, and procedures have been previously published (12). The original cohort was recruited between 1987–1989 using probability sampling of middle aged (45–64 years old) men and women from 4 communities in the United States (Forsyth County, NC; Jackson, MS; Minneapolis, MN; and Washington County, MD). The Jackson field center enrolled an entirely African American cohort. Subsequent follow up visits occurred at 3 year intervals up to 1998, with annual telephone interviews conducted between visits. Institutional review boards from each site approved the study and informed consent was obtained from all participants.
Transthoracic echocardiography was only performed in the Jackson cohort during visit 3 (1993–1995). Of 2,445 participants who underwent transthoracic echocardiography, 1,962 were included in this analysis after sequential exclusion of 318 in whom left ventricular ejection fraction (LVEF) could not be calculated, 32 with missing HF status, 3 in whom incident HF occurred after visit 3 but before the echocardiogram, and 130 with missing covariate data.
Echocardiography
Two-dimensional, M-mode, and Doppler images were acquired with an Acuson 128XP/10c cardiac ultrasound machine with 2.5, 3.5, and 5.0 MHz transducers (Acuson, Malvern, PA). Quality control measures have been previously described (13). Left ventricular (LV) end diastolic diameter (LVEDD), LV end systolic diameter, septal and posterior wall thickness were measured from 2-dimensional images according to American Society of Echocardiography criteria. LV mass was calculated using the simplified cubed equation and indexed (LVMI) to height (meters2.7) with left ventricular hypertrophy (LVH) defined as an LVMI ≥51g/m2.7 (14). Relative wall thickness was calculated as 2 × posterior wall thickness/LVEDD with ≤ 0.42 considered normal. LVEF was calculated utilizing 2-D Teichholz method, with a LVEF ≥ 50% and <50% defining preserved and reduced LVEF, respectively. Diastolic dysfunction was determined based upon transmitral Doppler E to A ratio, with ≤ 0.75 or > 1.5 considered abnormal (15).
Definition of Heart Failure
Prevalent HF at visit 3 was defined by a) stage 3 or manifest HF according to Gothenburg criteria or the use of medications for HF at visit 1 (n=82) or b) hospitalization with ICD-9 code for HF (428.x) listed at discharge between visit 1 and 3 (n=34) (3). The Gothenburg criterion is a validated scoring system composed of 3 components a) cardiac, b) pulmonary, and c) therapy, in which stage 3 or manifest HF requires 1 point from each component (16). Participants with quantifiable LVEF by echocardiography and prevalent HF were categorized as HFpEF (LVEF ≥ 50%) or HFrEF (LVEF < 50%).
Covariates
Established definitions for hypertension, obesity, diabetes mellitus, CHD, stroke, smoking status, and medication use as previously described in ARIC were utilized (17). Electrocardiographic LVH was determined by Cornell criteria. Pulmonary disease was defined as self reported history of physician diagnosed lung disease or asthma. Retinopathy, estimated glomerular filtration rate, ankle brachial index, hematologic parameters, lipids, and glucose were measured according to standardized protocols with chronic kidney disease (CKD) and peripheral arterial disease defined as previously described (12,18–20). Ankle brachial index was available in 1,239 participants. All covariates were ascertained at visit 3, except for hemoglobin, white blood cell count, and creatinine, for which the most recent prior measures were used.
Outcome
The primary outcome was death from any cause. The follow up period was defined as the time elapsed from the date of echocardiography to the date of death, date of last contact for those lost to follow-up, or December 31, 2008. Deaths were ascertained through annual phone calls to participants and ongoing surveillance of health department certificate files.
Statistical Methods
For each of the 3 groups (non HF, HFpEF, and HFrEF), summary statistics for covariates were calculated as counts and percentages, and medians and inter-quartile ranges for categorical and continuous data, respectively. Comparisons were then made between a) HFpEF and non-HF and b) HFpEF and HFrEF. Chi squared or Fisher’s exact test and Wilcoxon rank-sum test were used to compare baseline characteristics. All cause mortality rates were calculated (number of deaths divided by person-time at risk). Survival analysis was performed according to the Kaplan Meier method with the log-rank test used to assess for differences. Univariable and multivariable hazard ratios for death were estimated using Cox proportional hazards regression. Covariates included in multivariable models included age, gender, LVEF, and clinical characteristics. Propensity scores for HFpEF vs. non HF and HFrEF vs. HFpEF were calculated using clinical characteristics that significantly differed in univariate analyses between groups. The propensity score was then included in Cox proportional hazards models. Two-sided p values <0.05 were considered significant. Analyses were performed using Stata 11.2 (Stata Corp., College Station, Texas).
Results
Prevalent HF was identified in 116 (5.9%) participants and further classified as HFpEF (n=85, 73%) and HFrEF (n=31, 27%). Those with HFpEF were older than those without HF, but similar in age to those with HFrEF. Female sex was significantly more common in HFpEF as compared to those without HF and HFrEF. There were no differences with regard to heart rate or blood pressure. As expected, symptoms of HF, such as orthopnea, paroxysmal nocturnal dyspnea, and lower extremity edema were more common among those with HF as compared to those without HF (Table 1).
Table 1.
Characteristics of the Jackson participants of the ARIC cohort who underwent echocardiography stratified according to heart failure status.
| HFpEF N = 85 |
Non HF N = 1846 |
NonHF vs. HFpEF p-value |
HFrEF N = 31 |
HFrEF vs. HFpEF p-value |
|
|---|---|---|---|---|---|
| Age, years | 61.1 (57.1,66.5) | 58.5 (54.6,63.8) | 0.001 | 62.0 (54.9,65.7) | 0.89 |
| Gender (Female) | 72 (85) | 1156 (63) | <0.001 | 20 (65) | 0.036 |
| Orthopnea | 27 (32) | 175 (9) | <0.001 | 13 (42) | 0.38 |
| PND | 18 (21) | 116 (6) | <0.001 | 12 (39) | 0.09 |
| Self reported LE edema | 55 (65) | 588 (32) | <0.001 | 16 (52) | 0.28 |
| Hypertension | 72 (85) | 1088 (59) | <0.001 | 26 (84) | 1.00 |
| Obesity | 60 (71) | 831 (45) | <0.001 | 19 (61) | 0.37 |
| Diabetes mellitus | 36 (42) | 409 (22) | <0.001 | 21 (68) | 0.021 |
| Pulmonary Disease | 23 (27) | 129 (7) | <0.001 | 5 (16) | 0.33 |
| Peripheral Arterial Dis. | 8 (14) | 62 (5) | 0.013 | 2 (10) | 1.00 |
| Coronary Heart Dis. | 11 (13) | 69 (4) | 0.001 | 10 (32) | 0.027 |
| Chronic Kidney Dis. | 1 (1) | 55 (3) | 0.72 | 8 (27) | <0.001 |
| Stroke | 4 (5) | 42 (2) | 0.14 | 5 (16) | 0.056 |
| Current Tobacco Use | 10 (12) | 371 (20) | 0.07 | 4 (13) | 1.00 |
| Current Alcohol Use | 17 (20) | 579 (31) | 0.03 | 5 (16) | 0.79 |
| Aspirin | 50 (59) | 852 (46) | 0.026 | 13 (42) | 0.14 |
| Lipid Lowering Med | 4 (5) | 74 (4) | 0.77 | 4 (13) | 0.21 |
| Anti Hypertensive Med | 74 (87) | 910 (49) | <0.001 | 28 (90) | 0.76 |
| Beta blockers | 12 (14) | 163 (9) | 0.10 | 4 (13) | 0.87 |
| ACE Inhibitor | 17 (20) | 165 (9) | 0.002 | 14 (45) | 0.009 |
| Diuretic | 49 (58) | 412 (22) | <0.001 | 21 (68) | 0.39 |
| Digoxin | 8 (9) | 20 (1) | <0.001 | 11 (36) | 0.002 |
| Heart Rate, bpm | 68 (64,74) | 68 (64,72) | 0.32 | 72 (66,78) | 0.20 |
| Systolic BP, mmHg | 130 (120,141) | 128 (117,142) | 0.42 | 135 (122,154) | 0.14 |
| Diastolic BP, mmHg | 74 (68,81) | 76 (70,83) | 0.13 | 72 (64,92) | 0.76 |
| Pulse Pressure, mmHg | 52 (45,65) | 52 (43,63) | 0.15 | 60 (51,72) | 0.06 |
| Body mass index, kg/m2 | 32 (29,37) | 29 (26,33) | <0.001 | 34 (27,40) | 0.97 |
| Total cholesterol, mg/dL | 206 (182,227) | 204 (180,231) | 0.88 | 210 (184,245) | 0.24 |
| LDL cholesterol, mg/dL | 124 (105,154) | 126 (102,151) | 0.85 | 124 (104,156) | 0.88 |
| HDL cholesterol, mg/dL | 53 (41,64) | 54 (43,66) | 0.41 | 54 (42,66) | 0.55 |
| Triglycerides, mg/dL | 110 (85,150) | 98 (74,134) | 0.007 | 117 (84,157) | 0.85 |
| WBC | 5.3 (4.5,6.3) | 5.1 (4.2,6.3) | 0.25 | 6.4 (5.2,7.2) | 0.019 |
| Hemoglobin, g/dL | 12.6 (11.9,13.3) | 13.1 (12.2,13.9) | 0.003 | 12.5 (11.7,13.4) | 0.69 |
| Creatinine, mg/dL | 0.86 (0.76,0.96) | 0.86 (0.76,1.06) | 0.003 | 0.96 (0.86,1.26) | <0.001 |
| eGFR, ml/min/1.73m2 | 93 (79,114) | 90 (79,103) | 0.66 | 78 (59,100) | 0.006 |
| Glucose, mg/dL | 109 (96,156) | 102 (94,118) | 0.004 | 124 (97,203) | 0.30 |
| LVH on ECG (Cornell),% | 3 (4) | 125 (7) | 0.37 | 8 (26) | 0.001 |
| QRS duration, msec | 94 (87,103) | 93 (86,101) | 0.39 | 102 (92,109) | 0.042 |
| Advanced retinopathy,% | 17 (20) | 222 (12) | 0.037 | 12 (39) | 0.07 |
| Ankle Brachial Index | 1.05 (0.97,1.13) | 1.10 (1.03,.1.17) | 0.002 | 1.03 (0.99,1.14) | 0.99 |
Data presented as counts (percentages) and medians (interquartile ranges) for categorical and continuous variables, respectively. BMI = body mass index, BP = blood pressure, bpm = beats per minute, Dis = disease, ECG = electrocardiogram, eGFR = estimated glomerular filtration rate, HDL = high density lipoprotein, LDL = low density lipoprotein, LE = lower extremity, LVH = left ventricular hypertrophy, Med = medication, PND = paroxysmal nocturnal dyspnea, WBC = white blood cell. Obesity defined as BMI ≥ 30 kg/m2.
Comorbidities were common among those with HFpEF (Table 1). In particular, hypertension, obesity, diabetes mellitus, pulmonary disease, peripheral arterial disease, and CHD were more frequent among those with HFpEF as compared to those without HF. Of note, hypertension was highly prevalent even in those without HF, approaching 60%. Comorbidities were also common among those with HFrEF, with diabetes mellitus, CHD, and CKD present more frequently than in HFpEF.
Cardiac structure and function differed between groups (Table 2). Those with HFpEF had increased wall thickness and LVMI compared to those without HF, although prevalence of diastolic dysfunction and left atrial enlargement did not differ between these two groups. In contrast, those with HFrEF had the highest LV wall thickness and LVMI. Additionally, left atrial enlargement and mitral regurgitation were more frequent among those with HFrEF as compared to those with HFpEF. Concentric hypertrophy was the most common LV geometry; however, it was significantly more frequent in HFpEF as compared to those without HF. Hypertrophy was present in nearly all participants with HFrEF, with eccentric hypertrophy more frequent in HFrEF as compared to HFpEF.
Table 2.
Cardiac structure and function according to heart failure status and left ventricular ejection fraction among Jackson participants of the ARIC study.
| HFpEF N = 85 |
Non HF N = 1846 |
NonHF vs. HFpEF p-value |
HFrEF N = 31 |
HFrEF vs. HFpEF p-value |
||
|---|---|---|---|---|---|---|
| LVEF, % | 67 (59,75) | 64 (56,71) | 0.002 | 39 (28,44) | <0.001 | |
| FS 2D, % | 37 (31,44) | 34 (29,40) | 0.002 | 19 (13,22) | <0.001 | |
| Mitral E vel., cm/s | 76 (66,89) | 76 (65,87) | 0.50 | 79 (63,92) | 0.52 | |
| Mitral A vel., cm/s | 80 (67,91) | 75 (64,88) | 0.13 | 96 (72,106) | 0.001 | |
| Mitral E/A | 0.94 (0.79,1.12) | 1.00 (0.84,1.19) | 0.14 | 0.75 (0.66,1.10) | 0.054 | |
| Diastolic Dysfunction,% | 23 (27) | 460 (25) | 0.70 | 22 (71) | <0.001 | |
| SWT, cm | 1.22 (1.09,1.32) | 1.16 (1.04,1.29) | 0.028 | 1.27 (1.12,1.34) | 0.35 | |
| PWT, cm | 1.21 (1.09,1.36) | 1.17 (1.06,1.31) | 0.057 | 1.27 (1.20,1.41) | 0.07 | |
| LVEDD, cm | 4.35 (4.07,4.67) | 4.34 (3.98,4.72) | 0.76 | 5.43 (4.73,6.08) | <0.001 | |
| LVESD, cm | 2.60 (2.42,2.99) | 2.82 (2.45,3.20) | 0.014 | 4.32 (3.59,5.32) | <0.001 | |
| LV Mass, g | 239 (202,285) | 230 (188,276) | 0.10 | 366 (304,436) | <0.001 | |
| LVMI, g/m2.7 | 63 (51,74) | 56 (47,68) | 0.002 | 92 (75,101) | <0.001 | |
| LVH (LVMI ≥51g/m2.7) | 64 (75) | 1183 (64) | 0.037 | 29 (94) | 0.035 | |
| Relative Wall Thickness | 0.57 (0.51,0.62) | 0.54 (0.47,0.62) | 0.057 | 0.48 (0.42,0.56) | <0.001 | |
| RWT ≥ 0.42 | 79 (93) | 1668 (90) | 0.57 | 23 (74) | 0.01 | |
| LA Diameter, cm | 3.4 (3.1,3.8) | 3.4 (3.0,3.7) | 0.12 | 3.8 (3.3,4.3) | 0.008 | |
| LA enlargement (>4cm) | 16 (19) | 240 (13) | 0.14 | 12 (39) | 0.048 | |
| Aortic Root Diameter, cm | 3.1 (2.8,3.3) | 3.0 (2.8,3.2) | 0.08 | 3.0 (2.8,3.3) | 0.85 | |
| ≥ Mod Regurgitation | ||||||
| Mitral | 0 (0) | 13 (1) | 1.00 | 3 (10) | 0.02 | |
| Aortic | 1 (1) | 9 (1) | 0.35 | 0 (0) | 1.00 | |
| Tricuspid | 2 (2) | 29 (2) | 0.65 | 1 (3) | 1.00 | |
| LV Geometry | ||||||
| Normal | 4 (5) | 80 (4) | 0.79 | 1 (3) | 1.00 | |
| Concentric Remodeling | 17 (20) | 583 (32) | 0.023 | 1 (3) | 0.039 | |
| Concentric Hypertrophy | 62 (73) | 1085 (59) | 0.009 | 22 (71) | 0.82 | |
| Eccentric Hypertrophy | 2 (2) | 98 (5) | 0.32 | 7 (23) | 0.001 | |
Data presented as counts (percentages) and medians (interquartile ranges) for categorical and continuous variables, respectively. FS = fractional shortening, LA = left atrium, LV = left ventricular, LVEDD = left ventricular end diastolic diameter, LVEF = left ventricular ejection fraction, LVESD = left ventricular end systolic diameter, LVMI = left ventricular mass index, Mod = moderate PWT = posterior wall thickness, RWT = relative wall thickness, SWT = septal wall thickness.
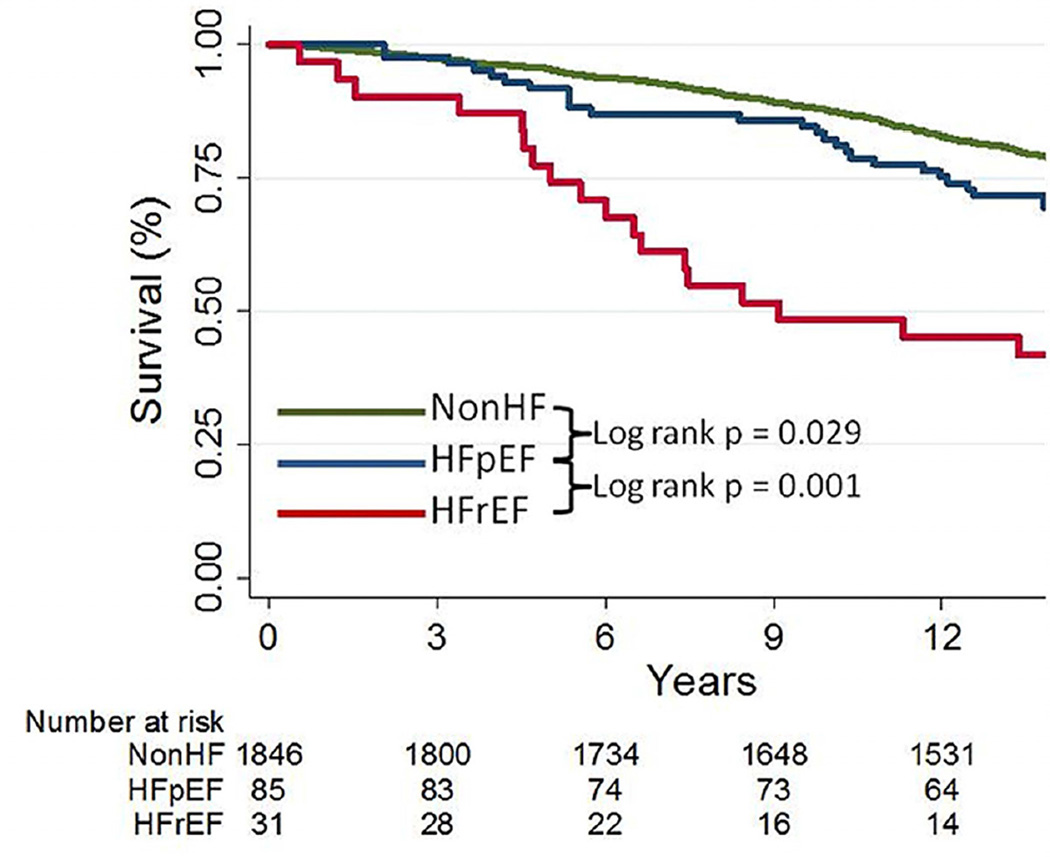
Over a median follow up of 13.7 years, deaths occurred in 21%, 31%, and 61% of those without HF, with HFpEF, and with HFrEF, respectively (Table 3). Death rates in HFpEF were increased compared to those without HF, but were lower than those with HFrEF, even when adjusted for age. In age and gender adjusted Cox Proportional Hazards Models, risk of death differed between groups. HFpEF was associated with a 51% increased risk of death as compared to those without HF (HR 1.51, 95%CI 1.01–2.25). HFrEF was associated with the worst survival, with 2.5 times the risk of death as compared to those with HFpEF (HR 2.50, 95%CI 1.37–4.58). When further adjusted for LVEF, HFpEF was associated with 61% increased risk of death as compared to those without HF (HR 1.61, 95%CI 1.08–2.41). Adjustment for differences in clinical characteristics attenuated the mortality risk associated with HFpEF vs. those without HF; however, those with HFrEF remained at significantly increased risk of death compared to those with HFpEF (Table 3, Figure 1).
Table 3.
Mortality rates and risk of death according to heart failure status and left ventricular ejection fraction in African-Americans.
| Non HF N=1846 |
HFpEF N=85 |
HFrEF N=31 |
|
|---|---|---|---|
| Deaths and mortality rates | |||
| Deaths, n (%) | 393 (21) | 26 (31) | 19 (61) |
| Person-Time (yrs) | 23,707 | 1,032 | 291 |
| Deaths / 100 person yrs* (95% CI) | 1.66 (1.50,1.83) | 2.52 (1.72,3.70) | 6.52 (4.16,10.22) |
| Hazard ratios (95% CI) |
HFpEF vs. Non HF |
HFrEF vs. HFpEF |
|
| Unadjusted | 1.55 (1.04,2.30) | 2.62 (1.45,4.75) | |
| Age, gender adjusted | 1.51 (1.01,2.25) | 2.50 (1.37,4.58) | |
| Age, gender, LVEF† adjusted | 1.61 (1.08–2.41) | n/a | |
| Age, gender, LVEF†, Propensity score‡ adjusted | 1.35 (0.87–2.09) | 2.29 (1.19–4.42) | |
Death rates standardized to median age (58.7 years) of cohort.
LVEF not included in comparison of HFrEF vs. HFpEF
Propensity score calculated utilizing baseline characteristics that significantly differed between groups.
LVEF = Left ventricular ejection fraction
Figure 1. Survival in African-Americans according to heart failure status.
Kaplan-Meier survival analysis in those without heart failure (nonHF), heart failure with preserved ejection fraction (HFpEF), and those with heart failure and reduced ejection fraction (HFrEF).
Discussion
In a community based sample of middle-aged African-Americans, we found that demographic and clinical characteristics as well as cardiac structure and function significantly differed between HFpEF, HFrEF, and those without HF. By comparing HFpEF to those without HF, we found that older age, female sex, hypertension, obesity, diabetes mellitus, and concentric hypertrophy were more common in HFpEF. Similarly, diabetes mellitus, CKD, CHD, and left atrial enlargement were more common in HFrEF than HFpEF. Survival differed between groups, with HFpEF portending a worse prognosis than those without HF, but not as severe as HFrEF. Together, these findings suggest that in African-Americans HFpEF and HFrEF may be distinct syndromes.
Representation of African-Americans in observational studies and clinical trials is typically low, thus HF in this population is not well understood (21,22). Moreover, few HF studies focus specifically on African-Americans (23), as most literature involving race in HF addresses differences between racial groups. This is in spite of a higher prevalence of cardiovascular risk factors and greater burden of HF among African-Americans. The existing literature suggests the development and progression of HF in African Americans may be characterized by predominantly non-ischemic etiologies, more severe natural history, and possibly a different response to pharmacotherapy when compared to predominantly Caucasian studies (9,21). By evaluating an entirely African-American cohort, our findings may advance understanding of HF in this high risk population.
Approximately three quarters of African-Americans with HF in our cohort had HFpEF. This is concordant with two population based studies of ambulatory HF patients, where 57–76% had preserved systolic function (7,24), but differs from an ambulatory Veterans Administration population where HFpEF was present in 25% (25). However, only 6% of those in the Veterans Administration study were female, and therefore may not be representative of the typical HFpEF population (25). In comparison, 65% of the Jackson cohort was female, which may be one explanation for the higher prevalence of HFpEF. Our findings are also consistent with population studies of ambulatory HF patients that included multiple ethnicities and demonstrated a higher prevalence of HFpEF than HFrEF (26–28). However, our results contrast with studies evaluating African-Americans hospitalized with acute decompensated HF where HFpEF was prevalent in 29–43% (5,6,29), suggesting the relative frequency of HFpEF and HFrEF may differ between hospitalized and ambulatory settings (30). Additionally, the finding that HFpEF was more common than HFrEF may be explained, in part, by the high prevalence of hypertension and relatively low frequency of CHD.
Regardless of preserved or reduced LVEF, comorbidities were common in HF. However, the pattern of clinical characteristics differed between HFpEF and HFrEF. In addition to diabetes mellitus and CKD, CHD was more frequent in HFrEF as compared to HFpEF, although it was only present in one third of those with HFrEF despite the high prevalence of atherosclerotic risk factors. Overall, CHD was not as common in HF as typically described in predominantly white populations, which may be partially explained by the relatively high proportion of women and middle age range in this study. Hypertension, however, was present in 85% of those with HF. Together, these findings suggest that hypertension, along with other comorbidities, such as obesity, diabetes mellitus, and CKD, rather than CHD, may be relatively more important factors in HF in African-Americans.
The most striking finding related to cardiac structure was the marked prevalence of LVH not only in those with HF, but also among those without HF. While hypertension may be the most common contributor to hypertrophy, other factors including obesity (31,32), diabetes mellitus (33,34), the metabolic syndrome (35), and CKD (36), have previously been demonstrated to be associated with LVH. Several studies have shown that these comorbidities are common in HF particularly among African-Americans and Hispanics (4,37). In our analysis, these comorbidities were frequent in HF, and a particularly worrisome finding was that concentric hypertrophy was present in 60% of those without HF. The high prevalence of hypertension and concentric LVH, a known marker of increased cardiovascular risk in African-Americans (38), portends a potential increase in HF among this group (39).
There is also uncertainty regarding the risk of death in chronic HF among African-Americans (5). Registries of hospitalized HF patients suggest similar or better survival in African-Americans vs. whites (4–6,40). This is congruent with previous reports from ARIC demonstrating similar mortality rates between races at 30 days and 1 year; however, the longer follow up time in ARIC revealed higher fatality rates in African-Americans at 5 years post HF hospitalization (3). Few studies have evaluated survival in HFpEF in African-Americans. In a predominantly male Veterans Administration population, survival over 5 years of follow up did not differ according to race among those with HFpEF, although among African-Americans HFpEF appeared to be associated with a similar or slightly better prognosis than HFrEF (10). Results from the Duke Databank of Cardiovascular Disease suggest a better 5 year survival in HFpEF (68%) (11), as compared to HFrEF (51%) (41), although a direct comparison of mortality between HFpEF and HFrEF in African-Americans was not made.
We found that HFpEF was associated with a more benign prognosis than HFrEF. This is consistent a meta-analysis of nearly 42,000 patients with HF that demonstrated a 32% lower risk of death in HFpEF as compared to HFrEF, although stratification according to race was not reported (42). It is also consistent with the Candesartan in Heart failure Assessment of Reduction in Mortality and morbidity (CHARM) trial (43), but differs from epidemiologic data from Olmsted County, Enhanced Feedback for Effective Cardiac Treatment, and the Framingham Heart Study where mortality rates were similar between HFpEF and HFrEF (44–46). This may, in part, be explained by differences in study populations, as those that capture patients during or immediately following an acute hospitalization find that HFpEF and HFrEF have similar mortality, particularly among the elderly (30). In contrast, ambulatory HF patients, such as those in the Cardiovascular Health and Strong Heart studies, which enrolled an older biracial population and Native Americans, respectively, demonstrate lower fatality rates in HFpEF vs. HFrEF (26,27). However, representation of African-Americans in these studies was generally low, limiting applicability to this race.
We also found the mortality rate in HFpEF to be greater than that of those without HF. The risk of death in HFpEF was 61% higher than age, gender, and LVEF matched participants without prevalent HF. However, adjustment for additional clinical characteristics attenuated this risk. It has been proposed that HFpEF is a collection of comorbidities and that the syndrome of HFpEF may not even exist (47). In contrast, pooled analyses of clinical trials of HFpEF and cardiovascular trials of patients without HF demonstrate higher mortality rates in HFpEF as compared to those without HF (48). Our data in African-Americans are consistent with these pooled analyses. Although adjustment for comorbidities attenuated this risk, the relatively small numbers of deaths in our HFpEF population limited our statistical power. Nevertheless, our findings highlight the importance of comorbidities in African Americans with HFpEF, but do not mitigate the broader literature demonstrating that HFpEF is associated with a worse prognosis than those without HF.
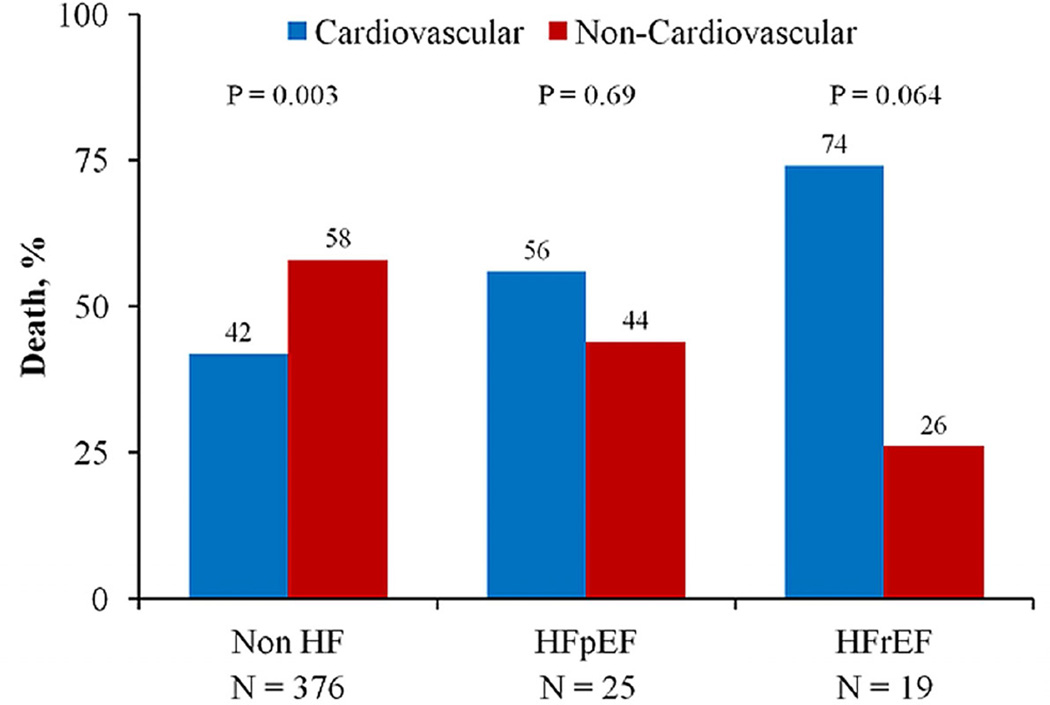
Recent literature also suggests that half of all deaths in HFpEF may not be related to cardiovascular causes (49–51), again emphasizing the impact of comorbidities (37,52). We extend these findings by showing that non-cardiovascular comorbidities are frequent among African-Americans with HF. Furthermore, using ICD codes (recognizing their limitation in identifying cause of death), we observed a similar trend to previous reports; namely that 58%, 44% and 26% of deaths in those without HF, with HFpEF, and HFrEF, respectively, were due to non-cardiovascular causes (Figure 2). Together these findings emphasize the importance of treating comorbidities, particularly among those with HFpEF, as a potential approach to improving outcomes (37,49,52,53).
Figure 2. Proportion of deaths due to cardiovascular and non-cardiovascular causes according to heart failure status in African-Americans.
Cause of death ascertained from ICD-9 codes. HFpEF = heart failure with preserved ejection fraction, HFrEF = heart failure with reduced ejection fraction, nonHF = no heart failure.
While we specifically evaluated an entirely African-American cohort over a long follow up period, limitations should be noted. The cross sectional design precludes assessment of causality between clinical characteristics, cardiac structure and function, and HF. However, differences between HFpEF, HFrEF, and those without HF may provide insight into targets for future investigation. The definition for prevalent HF was based upon Gothenburg criteria and unadjudicated hospitalization ICD-9 codes, although these methods have been validated in ARIC (3,54). Importantly, this approach captures participants with prior or current symptoms of HF, as recommended by ACC/AHA staging of HF (55). LVEF was not assessed at the time of incident HF and it is possible that LVEF may have recovered between the incident HF event and our assessment. However, LVEF has previously been demonstrated to be similar between acute and chronic HF (56). Moreover, our findings suggest LVEF measured after incident HF still imparts prognostic information. Teichholz’ method was used to calculate LVEF as volumetric measurements were not available. Diastolic function was assessed with transmitral Doppler E/A ratio as estimation of left atrial volumes, tissue Doppler imaging, transmitral E wave deceleration time, pulmonary venous flow, and isovolumic relaxation time were not obtained at echocardiography. While diastolic dysfunction is frequently reported in HFpEF, we found it to be present in 25% of participants with HFpEF, which did not differ between HFpEF and those without HF, likely reflecting limitations in diastolic assessment. There may have been selection bias with regard to participants who presented for echocardiography or had interpretable images, such that the sickest individuals, including more severe HF, may be underrepresented. Inherent to ARIC’s design, this study includes a selected age range and consisted entirely of African-Americans from Jackson. Therefore, our results may not be generalizable to younger, more elderly, or all African-Americans. However, the mean age of African-Americans in most HF registries was 63–64 years old, falling within the range of our cohort (4–6,25). The observed mortality rates may not be directly applicable to a more contemporary time period due to temporal changes in HF management. Finally, the relatively low numbers of participants with prevalent HF may limit statistical power.
In summary, we found in a community based sample of middle-aged African-Americans, that demographic and clinical characteristics, as well as cardiac structure and function, significantly differed between HFpEF, HFrEF, and those without HF. HFpEF was more common than HFrEF and portended a worse prognosis than those without HF, but not as severe as HFrEF. As this population bears a disproportionate burden of HF, focused investigation on African-Americans is an important step to understanding HF and developing strategies for prevention, detection, and treatment.
Acknowledgement
Financial Support:
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The authors thank the staff and participants of the ARIC study for their important contributions. Support was also provided by the National Heart, Lung, and Blood Institute training grant (T32 HL094301-02).
Abbreviations
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- ARIC
Atherosclerosis Risk in Communities
- LV
Left ventricular
- LVEF
Left ventricular ejection fraction
- LVEDD
Left ventricular end diastolic diameter
- LVMI
Left ventricular mass index
- CHD
Coronary heart disease
- CKD
Chronic kidney disease
- LVH
Left ventricular hypertrophy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bibbins-Domingo K, Pletcher MJ, Lin F, et al. Racial differences in incident heart failure among young adults. N Engl J Med. 2009;360:1179–1190. doi: 10.1056/NEJMoa0807265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bahrami H, Kronmal R, Bluemke DA, et al. Differences in the incidence of congestive heart failure by ethnicity: the Multi-Ethnic Study of Atherosclerosis. Arch Intern Med. 2008;168:2138–2145. doi: 10.1001/archinte.168.19.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study) Am J Cardiol. 2008;101:1016–1022. doi: 10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 4.Thomas KL, Hernandez AF, Dai D, et al. Association of race/ethnicity with clinical risk factors, quality of care, and acute outcomes in patients hospitalized with heart failure. Am Heart J. 2011;161:746–754. doi: 10.1016/j.ahj.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Kamath SA, Drazner MH, Wynne J, Fonarow GC, Yancy CW. Characteristics and outcomes in African American patients with decompensated heart failure. Arch Intern Med. 2008;168:1152–1158. doi: 10.1001/archinte.168.11.1152. [DOI] [PubMed] [Google Scholar]
- 6.Yancy CW, Abraham WT, Albert NM, et al. Quality of care of and outcomes for African Americans hospitalized with heart failure: findings from the OPTIMIZE-HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure) registry. J Am Coll Cardiol. 2008;51:1675–1684. doi: 10.1016/j.jacc.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 7.Martin TC. Comparison of Afro-Caribbean patients presenting in heart failure with normal versus poor left ventricular systolic function. Am J Cardiol. 2007;100:1271–1273. doi: 10.1016/j.amjcard.2007.05.047. [DOI] [PubMed] [Google Scholar]
- 8.Hogg K, Swedberg K, McMurray J. Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol. 2004;43:317–327. doi: 10.1016/j.jacc.2003.07.046. [DOI] [PubMed] [Google Scholar]
- 9.Dries DL, Exner DV, Gersh BJ, Cooper HA, Carson PE, Domanski MJ. Racial differences in the outcome of left ventricular dysfunction. N Engl J Med. 1999;340:609–616. doi: 10.1056/NEJM199902253400804. [DOI] [PubMed] [Google Scholar]
- 10.Agoston I, Cameron CS, Yao D, Dela Rosa A, Mann DL, Deswal A. Comparison of outcomes of white versus black patients hospitalized with heart failure and preserved ejection fraction. Am J Cardiol. 2004;94:1003–1007. doi: 10.1016/j.amjcard.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 11.East MA, Peterson ED, Shaw LK, Gattis WA, O'Connor CM. Racial differences in the outcomes of patients with diastolic heart failure. Am Heart J. 2004;148:151–156. doi: 10.1016/j.ahj.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 12.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 13.Skelton TN, Andrew ME, Arnett DK, et al. Echocardiographic left ventricular mass in African-Americans: the Jackson cohort of the Atherosclerosis Risk in Communities Study. Echocardiography. 2003;20:111–120. doi: 10.1046/j.1540-8175.2003.03000.x. [DOI] [PubMed] [Google Scholar]
- 14.Nunez E, Arnett DK, Benjamin EJ, et al. Optimal threshold value for left ventricular hypertrophy in blacks: the Atherosclerosis Risk in Communities study. Hypertension. 2005;45:58–63. doi: 10.1161/01.HYP.0000149951.70491.4c. [DOI] [PubMed] [Google Scholar]
- 15.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 16.Wilhelmsen L, Eriksson H, Svardsudd K, Caidahl K. Improving the detection and diagnosis of congestive heart failure. Eur Heart J. 1989;10(Suppl C):13–18. doi: 10.1093/eurheartj/10.suppl_c.13. [DOI] [PubMed] [Google Scholar]
- 17.Folsom AR, Yamagishi K, Hozawa A, Chambless LE. Absolute and attributable risks of heart failure incidence in relation to optimal risk factors. Circ Heart Fail. 2009;2:11–17. doi: 10.1161/CIRCHEARTFAILURE.108.794933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong TY, Rosamond W, Chang PP, et al. Retinopathy and risk of congestive heart failure. JAMA. 2005;293:63–69. doi: 10.1001/jama.293.1.63. [DOI] [PubMed] [Google Scholar]
- 19.Kottgen A, Russell SD, Loehr LR, et al. Reduced kidney function as a risk factor for incident heart failure: the atherosclerosis risk in communities (ARIC) study. J Am Soc Nephrol. 2007;18:1307–1315. doi: 10.1681/ASN.2006101159. [DOI] [PubMed] [Google Scholar]
- 20.Folsom AR, Peacock JM, Boerwinkle E. Variation in PCSK9, low LDL cholesterol, and risk of peripheral arterial disease. Atherosclerosis. 2009;202:211–215. doi: 10.1016/j.atherosclerosis.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yancy CW. Heart failure in African Americans. Am J Cardiol. 2005;96:3i–12i. doi: 10.1016/j.amjcard.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 22.Franciosa JA, Ferdinand KC, Yancy CW. Treatment of heart failure in African Americans: a consensus statement. Congest Heart Fail. 2010;16:27–38. doi: 10.1111/j.1751-7133.2009.00118.x. [DOI] [PubMed] [Google Scholar]
- 23.Taylor AL, Ziesche S, Yancy C, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049–2057. doi: 10.1056/NEJMoa042934. [DOI] [PubMed] [Google Scholar]
- 24.Magana-Serrano JA, Almahmeed W, Gomez E, et al. Prevalence of heart failure with preserved ejection fraction in Latin American, Middle Eastern, and North African Regions in the I PREFER study (Identification of Patients With Heart Failure and PREserved Systolic Function: an epidemiological regional study) Am J Cardiol. 2011;108:1289–1296. doi: 10.1016/j.amjcard.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 25.Deswal A, Petersen NJ, Urbauer DL, Wright SM, Beyth R. Racial variations in quality of care and outcomes in an ambulatory heart failure cohort. Am Heart J. 2006;152:348–354. doi: 10.1016/j.ahj.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Gottdiener JS, McClelland RL, Marshall R, et al. Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function. The Cardiovascular Health Study. Ann Intern Med. 2002;137:631–639. doi: 10.7326/0003-4819-137-8-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 27.Devereux RB, Roman MJ, Liu JE, et al. Congestive heart failure despite normal left ventricular systolic function in a population-based sample: the Strong Heart Study. Am J Cardiol. 2000;86:1090–1096. doi: 10.1016/s0002-9149(00)01165-6. [DOI] [PubMed] [Google Scholar]
- 28.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol. 1999;33:1948–1955. doi: 10.1016/s0735-1097(99)00118-7. [DOI] [PubMed] [Google Scholar]
- 29.Ilksoy N, Hoffman M, Moore RH, Easley K, Jacobson TA. Comparison of African- American patients with systolic heart failure versus preserved ejection fraction. Am J Cardiol. 2006;98:806–808. doi: 10.1016/j.amjcard.2006.03.066. [DOI] [PubMed] [Google Scholar]
- 30.Kitzman DW. Diastolic heart failure in the elderly. Heart Fail Rev. 2002;7:17–27. doi: 10.1023/a:1013745705318. [DOI] [PubMed] [Google Scholar]
- 31.Fox E, Taylor H, Andrew M, et al. Body mass index and blood pressure influences on left ventricular mass and geometry in African Americans: The Atherosclerotic Risk In Communities (ARIC) Study. Hypertension. 2004;44:55–60. doi: 10.1161/01.HYP.0000132373.26489.58. [DOI] [PubMed] [Google Scholar]
- 32.Lorber R, Gidding SS, Daviglus ML, Colangelo LA, Liu K, Gardin JM. Influence of systolic blood pressure and body mass index on left ventricular structure in healthy African-American and white young adults: the CARDIA study. J Am Coll Cardiol. 2003;41:955–960. doi: 10.1016/s0735-1097(03)00052-4. [DOI] [PubMed] [Google Scholar]
- 33.de Simone G, Palmieri V, Bella JN, et al. Association of left ventricular hypertrophy with metabolic risk factors: the HyperGEN study. J Hypertens. 2002;20:323–331. doi: 10.1097/00004872-200202000-00024. [DOI] [PubMed] [Google Scholar]
- 34.Lee M, Gardin JM, Lynch JC, et al. Diabetes mellitus and echocardiographic left ventricular function in free-living elderly men and women: The Cardiovascular Health Study. Am Heart J. 1997;133:36–43. doi: 10.1016/s0002-8703(97)70245-x. [DOI] [PubMed] [Google Scholar]
- 35.Burchfiel CM, Skelton TN, Andrew ME, et al. Metabolic syndrome and echocardiographic left ventricular mass in blacks: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2005;112:819–827. doi: 10.1161/CIRCULATIONAHA.104.518498. [DOI] [PubMed] [Google Scholar]
- 36.Astor BC, Arnett DK, Brown A, Coresh J. Association of kidney function and hemoglobin with left ventricular morphology among African Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am J Kidney Dis. 2004;43:836–845. doi: 10.1053/j.ajkd.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 37.Ather S, Chan W, Bozkurt B, et al. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol. 2012;59:998–1005. doi: 10.1016/j.jacc.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liao Y, Cooper RS, McGee DL, Mensah GA, Ghali JK. The relative effects of left ventricular hypertrophy, coronary artery disease, and ventricular dysfunction on survival among black adults. JAMA. 1995;273:1592–1597. [PubMed] [Google Scholar]
- 39.Kamath S, Markham D, Drazner MH. Increased prevalence of concentric left ventricular hypertrophy in African-Americans: will an epidemic of heart failure follow? Heart Fail Rev. 2006;11:271–277. doi: 10.1007/s10741-006-0228-8. [DOI] [PubMed] [Google Scholar]
- 40.Gordon HS, Nowlin PR, Maynard D, Berbaum ML, Deswal A. Mortality after hospitalization for heart failure in blacks compared to whites. Am J Cardiol. 2010;105:694–700. doi: 10.1016/j.amjcard.2009.10.051. [DOI] [PubMed] [Google Scholar]
- 41.Thomas KL, East MA, Velazquez EJ, et al. Outcomes by race and etiology of patients with left ventricular systolic dysfunction. Am J Cardiol. 2005;96:956–963. doi: 10.1016/j.amjcard.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 42.The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur Heart J. 2011 doi: 10.1093/eurheartj/ehr254. [DOI] [PubMed] [Google Scholar]
- 43.Solomon SD, Anavekar N, Skali H, et al. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation. 2005;112:3738–3744. doi: 10.1161/CIRCULATIONAHA.105.561423. [DOI] [PubMed] [Google Scholar]
- 44.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 45.Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 46.Lee DS, Gona P, Vasan RS, et al. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the Framingham Heart Study of the National Heart, Lung, and Blood Institute. Circulation. 2009;119:3070–3077. doi: 10.1161/CIRCULATIONAHA.108.815944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Packer M. Can brain natriuretic peptide be used to guide the management of patients with heart failure and a preserved ejection fraction? The wrong way to identify new treatments for a nonexistent disease. Circ Heart Fail. 2011;4:538–540. doi: 10.1161/CIRCHEARTFAILURE.111.963710. [DOI] [PubMed] [Google Scholar]
- 48.Campbell RT, Jhund PS, Castagno D, Hawkins NM, Petrie MC, McMurray JJ. What Have We Learned About Patients With Heart Failure and Preserved Ejection Fraction From DIG-PEF, CHARM-Preserved, and I-PRESERVE? J Am Coll Cardiol. 2012;60:2349–2356. doi: 10.1016/j.jacc.2012.04.064. [DOI] [PubMed] [Google Scholar]
- 49.Lee DS, Gona P, Albano I, et al. A systematic assessment of causes of death after heart failure onset in the community: impact of age at death, time period, and left ventricular systolic dysfunction. Circ Heart Fail. 2011;4:36–43. doi: 10.1161/CIRCHEARTFAILURE.110.957480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zile MR, Gaasch WH, Anand IS, et al. Mode of death in patients with heart failure and a preserved ejection fraction: results from the Irbesartan in Heart Failure With Preserved Ejection Fraction Study (I-Preserve) trial. Circulation. 2010;121:1393–1405. doi: 10.1161/CIRCULATIONAHA.109.909614. [DOI] [PubMed] [Google Scholar]
- 51.Henkel DM, Redfield MM, Weston SA, Gerber Y, Roger VL. Death in heart failure: a community perspective. Circ Heart Fail. 2008;1:91–97. doi: 10.1161/CIRCHEARTFAILURE.107.743146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kitzman DW. Outcomes in patients with heart failure with preserved ejection fraction: it is more than the heart. J Am Coll Cardiol. 2012;59:1006–1007. doi: 10.1016/j.jacc.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 53.Shah SJ, Gheorghiade M. Heart failure with preserved ejection fraction: treat now by treating comorbidities. JAMA. 2008;300:431–433. doi: 10.1001/jama.300.4.431. [DOI] [PubMed] [Google Scholar]
- 54.Avery CL, Mills KT, Chambless LE, et al. Long-term association between self-reported signs and symptoms and heart failure hospitalizations: the Atherosclerosis Risk In Communities (ARIC) Study. Eur J Heart Fail. 2010;12:232–238. doi: 10.1093/eurjhf/hfp203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–e479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 56.Gandhi SK, Powers JC, Nomeir AM, et al. The pathogenesis of acute pulmonary edema associated with hypertension. N Engl J Med. 2001;344:17–22. doi: 10.1056/NEJM200101043440103. [DOI] [PubMed] [Google Scholar]