Abstract
This paper investigates the relationship between the concept of activity (including both professional and nonprofessional) and cognitive functioning among older European individuals. In this research, we used data collected during the first wave of SHARE (Survey on Health, Ageing and Retirement in Europe), and a measurement approach known as stochastic frontier analysis, derived from the economic literature. SHARE includes a large population (n > 25,000) geographically distributed across Europe, and analyzes several dimensions simultaneously, including physical and mental health activity. The main advantages of stochastic frontier analysis are that it allows estimation of parametric function relating cognitive scores and driving factors at the boundary and disentangles frontier noise and distance to frontier components, as well as testing the effect of potential factors on these distances simultaneously. The analysis reveals that all activities are positively related to cognitive functioning in elderly people. Our results are discussed in terms of prevention of cognitive aging and Alzheimer’s disease, and regarding the potential impact that some retirement programs might have on cognitive functioning in individuals across Europe.
Keywords: cognitive aging, cognitive reserve, retirement, Alzheimer’s disease
Introduction
Over the last two decades, a steadily growing body of evidence indicates that aging is accompanied by a systematic decline in performance of a wide variety of cognitive tasks, observed both in the laboratory setting and in everyday life.1 For instance, it is widely accepted that age influences several general factors, such as processing speed, inhibition, and working memory, which in turn affect other cognitive functions, such as episodic memory and language.2
Moreover, this age-related cognitive decline is associated with structural changes in the brain.3 Therefore, even early in the aging process, global changes, such as cerebral atrophy, ventricular enlargement, and hippocampal atrophy, can be evident in some but not all individuals.4 Further, the underlying pathologic basis of cognitive decline is the loss of synapses, neurons, neurochemical inputs, and neuronal networks.5 However, although this age-related cognitive decline has been largely defined (both on a functional and neurological level) and may impair quality of life, it is not inevitable. In this regard, nature provides clear examples of elderly people who maintain cognitive vitality, even in extreme old age.6 The belief that cognitive decline is inevitable has also been dispelled by the observation of centenarians who retain a good intellectual level7 and avoid dementia.8 Based on these findings, Fillit et al9 suggested that individuals have varying degrees of “functional reserve” in their brains. Thus, despite age-related changes, those with high functional reserve could continue to learn and to adapt.
This point of view was also supported and developed by Stern10,11 as the concept of “cognitive reserve”. According to Stern,10 cognitive reserve relates back to the fact that innate intelligence or aspects of life experience, such as educational or occupational attainment, provide a reserve represented by a set of skills that would protect individuals from the cognitive decline associated with normal aging or Alzheimer’s disease. However, the processes leading to the formation of this reserve remain unclear. Two hypotheses have been put forward to explain the neurophysiologic substrate of cognitive reserve,10 ie, the passive and active hypotheses. The passive hypothesis suggests that differences in brain reserve capacity, ie, the brain’s ability to cope with damage, depend on anatomical features, eg, the number of neurons and synaptic density. Therefore, individuals with higher brain reserve, (ie, larger brain, more neurons and synapses) could sustain more brain damage before clinical impairment arose. Indeed, because individuals with high reserve have more neurons and more synaptic density, they would have more remaining neurons available when a pathological process affects a certain amount of them. On the other hand, the active hypothesis is characterized by differences in how individuals process tasks rather than their physiologic differences. In this regard, cognitive reserve could take the form of using brain networks or cognitive paradigms that are more efficient or flexible. Thus, once normal aging or a pathological process begins to occur, individuals with high cognitive reserve could tolerate more brain lesions because they would use alternative brain networks in order to perform a cognitive task successfully.11
However, the direct influence of environment and activities on the brain remains subject to discussion, and in particular the thorny problem of the “causal relationship”, ie, are activities predictive of cognitive functioning, or is it the reverse? The most important argument in favor of a direct effect of environment and activity on the brain and cognitive functioning can be found in the animal literature. Several experimental studies in rats suggest that animals reared in enriched environments have greater dendritic density in the hippocampus and an increased number of glial cells in comparison with animals bred in standard conditions.12 In addition, Winocur showed that these brain modifications affect the cognitive abilities of older rats (ie, rats bred in an enriched environment performed better on a memory test compared with those bred in a standard environment).13 A second argument has been found in studies showing the presence of brain plasticity in adult primates and that an enriched environment can modulate brain plasticity.14
From a more functional point of view, recent studies have attempted to identify parameters contributing to the development of cognitive reserve. For example, education is widely recognized as having a significant impact on cognitive function, and is thought to support cognitive reserve capacity.15 Some studies have suggested that educational attainment completed in early adulthood or socioeconomic environment throughout the course of life can influence cognitive functioning in midlife, and even in later life, with a lower risk of cognitive impairment in old age and of developing dementia.16,17
Factors other than education may also build up cognitive reserve and influence cognitive functioning of elderly people. In this regard, several studies have suggested that differential susceptibility to age-related cognitive decline or to Alzheimer’s disease is related to variables such as occupation,18 professional or leisure activities,19 and lifestyle.9
For example, studies have reported that there is a positive association between participation in intellectual, social, and physical activity and performance on a wide range of cognitive tasks. In a 6-year longitudinal study, Newson and Kemps obtained results suggesting that engaging in general lifestyle activities may help to promote successful cognitive aging.20 Conversely, low-complexity occupations have been identified as risk factors for age-related cognitive decline,21 and social isolation seems to accelerate this decline.22 Other studies have focused on the effect of profession. Indeed, work can be seen as a rich activity contributing to the development of cognitive reserve. Schooler et al showed that complex intellectual work increases the cognitive functioning of older workers.18 Professional activity may also increase social interaction and a sense of self-efficacy, both of which are considered to be key factors contributing to the maintenance of cognitive reserve.23
Moreover, it is worth noting that most of the variables mentioned earlier have also been associated with parameters such as well-being and mental health in older people; see, for example, Greenfield and Marks24 for the positive effect of nonprofessional activity on well-being, Hao25 for the effect of professional activity on psychological well-being, and Berkman et al22 for the impact of social networks on health.
Taking into account this theoretical background, the aims of our study are to further explore the relationship between cognitive performance and occupational activity, defined in a broad sense (ie, including professional, leisure, physical, and other activities), while also taking into account the influence of age and educational attainment, as well as factors related to social and economic status. For this purpose, we used data collected in the first wave of SHARE (Survey on Health, Ageing and Retirement in Europe),26 which simultaneously analyzes several dimensions, including physical and mental health and occupational activity, in the European population aged 50 years and over, including several cognitive tests (for a complete description of SHARE, see http://www.share-project.org). The key strength of our study is that it is based on a very large population (n > 25,000) and provides an international framework, whereas most studies include at most 2000–3000 participants limited to one region or country with specific policies in terms of employment. In addition, SHARE allows to take into account a large number of dimensions simultaneously. At the time this study was conducted, only the first wave of SHARE was available, and the study presented here is purely cross-sectional.
For estimation purposes, we opted in favor of the frontier analysis techniques used in economic modeling. The main advantage of frontier analysis is that it offers a methodology specifically conceived to measure performance in a benchmark setting built upon all available observations. More precisely, among the alternative frontier measurement approaches, we were inclined to use a statistical approach known as stochastic frontier analysis.27 This approach, originally developed to measure the performance of firms, has been used to measure individual performance in other fields of human behavior eg, poverty and educational attainment, the measurable outcomes of which are driven by observable factors. This is also the case in this paper, because we were interested in estimation of individual cognitive function which by definition is a performance measure.
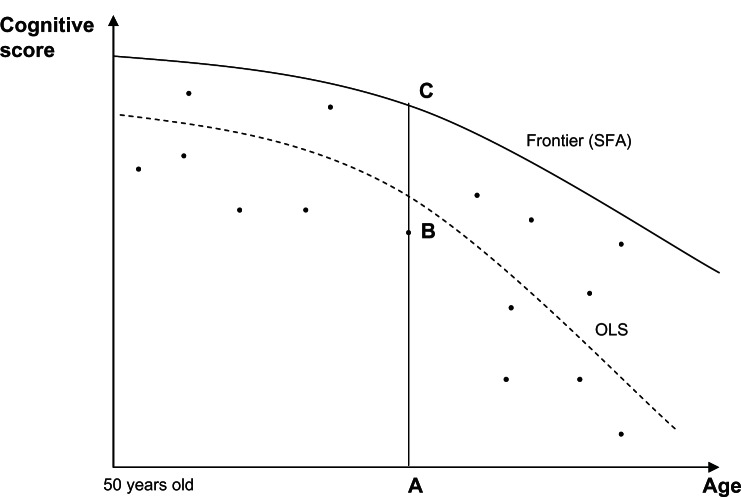
In practice, a stochastic frontier is estimated assuming that an individual’s cognitive functioning, represented by cognitive test scores, is determined by age and years of education. In other words, age and education are the main variables entered in the analysis as factors to explain an individual’s cognitive functioning (for a complete review of this subject see Coelli et al).28 Figure 1 illustrates these concepts in a simple two-dimensional setting, with age as the driving factor (horizontal axis) and cognitive test score as the outcome (vertical axis). Contrary to traditional regression analysis that estimates the average parametric relationship between cognitive scores and age, stochastic frontier analysis allows estimation of the parametric function that describes all individual scores.
Figure 1.
The distance concept.
Abbreviations: SFA, stochastic frontier analysis; OLS, ordinary least squares.
In Figure 1, the ordinary least squares function passes through the scatter points for individual observations, while the stochastic frontier corresponds to the boundary function. For each age, thereby for each individual, stochastic frontier analysis estimates an optimum cognitive score on the basis of the available information. In this way, segment measures the distance the cognitive score of individual B is from the boundary, and the ratio is a measure of performance . Assuming that individual B is aged 70 years, given her/his age, she/he would be quite able to reach a score like C on the “best practice” frontier. Why is this not the case? Stochastic frontier analysis allows us to test simultaneously the effect of other factors on individual distances from the frontier. Here, we are particularly interested in testing the assumption that lack of activity, specifically retirement from a professional activity, may be one of the factors associated with lower cognitive scores at the individual level.
The advantages of the frontier analysis approach are that: it allows estimation of a parametric function relating cognitive scores and driving factors at the boundary (best practice), in contrast with the average relationships studied in the literature up to now, which are mainly through regression analysis; it adopts rather simple assumptions about error distribution, allowing frontier noise and distance to the frontier components to be disentangled; and the model can be extended in order to test simultaneously the association between potential explanatory factors and the distance that separates an individual’s cognitive functioning from the stochastic frontier.
Materials and methods
Sample
SHARE is a pan-European interdisciplinary panel dataset including more than 25,000 individuals aged 50 years and over, coming from 11 European countries ranging from Scandinavia to the Mediterranean.26 The survey brings together many disciplines (demography, economics, epidemiology, psychology, sociology, statistics). Data were collected for this survey using a computer-assisted personal interviewing program, supplemented by a self-completed paper-and-pencil questionnaire. Note that one feature of SHARE is its high level of coordination across countries in terms of random sampling procedures, questionnaire contents, and fieldwork methodology.
For the analysis, we first excluded observations from Israel because of missing values for the variables of interest, and individuals of less than 50 years of age, leaving 27,320 observations (the average response rate for the first wave of SHARE was estimated to be 61.8%). Among those observations, there were 879 proxy interviews for which the cognitive functioning module was not asked and hence these observations were excluded from the analysis (“proxy” interviews are conducted when physical and cognitive limitations make it too difficult for a respondent to complete the interview unaided). We also excluded observations with missing values for cognitive scores (296 observations) or for one of the explanatory variables (387 observations) and outliers (ie, observations for which the residual from a simple ordinary least squares model of memory or fluency score on all explanatory variables used in our stochastic frontier model is higher than three times the standard deviation of these residuals) for either the memory or fluency score (606 observations). Given that our sample contains a significant proportion of missing values regarding work status and retirement period (944 observations), we computed an additional modality that controls these missing data in the model, softening the potential selectivity bias that may arise in such cases. Finally, 25,152 participants were selected for our analysis. As expected, missing information for cognitive tests generally corresponded to older and less well educated people. The results presented in this paper were obtained without any specific treatment to correct for potential selection bias. However, we tested for potential bias arising from lost observations (ie, information missing for variables such as education) using an imputation procedure and dummy variables. Nevertheless, our results remained unchanged.
Cognitive tests
Cognitive functioning was measured using short and simple tests of orientation, episodic memory, executive functioning, and numeracy. However, for the subsequent analysis, we decided to compute a global measure of cognitive functioning by focusing on two key measures, ie, the word list recall task known as an episodic memory task and a semantic fluency task, which is a multidetermined task considered to be a measure of executive functioning, but also includes other processes, such as semantic memory and processing speed. The rationale for this choice was two-fold: first, from a psychometric point of view, the distribution and variability of the raw scores for all measures allows selection of sensitive cognitive scores that are not affected by ceiling/floor effects (ie, with mean raw scores too close to the maximum or the minimum score), or that have limited variability, eg, the ordinal variable result of numeracy tests; and second, from a more theoretical point of view, it is widely recognized that free recall tasks and the fluency task are sensitive to cognitive aging.29
In our study, the episodic memory task was a test of verbal learning and recall tests whereby the participants were required to learn a list of ten common words. At encoding, the words were presented automatically on a computer screen, and respondents were asked to read each word aloud. Immediate recall followed the encoding phase, with a short waiting period (about five minutes during which verbal fluency and numeracy questions were asked) inserted before the delayed recall. During immediate and delayed recall, participants were asked to recall the ten words in any order. The score was calculated by adding the number of target words recalled at the immediate and delayed recall phases (score ranging from 0 to 20).
In the semantic fluency task, subjects had to provide as many different animal names as possible in one minute. Performance was defined as the total number of different animal names given by the participant.
For all the analyses, we used the raw scores of fluency and memory tasks. Moreover, we created a general cognitive score by averaging the standardized memory and fluency scores. In this way, we obtained a single value representing a more global and sensitive assessment of cognitive functioning. Of note, a complementary analysis was run with the sum of correct answers to all numeracy tests as the dependent variable. These results largely confirmed those presented here.
Statistical analysis
In order to identify the main factors driving cognitive functioning in individuals, we propose the stochastic frontier model: (1) where ri is the cognitive score of individual i; Xi is a vector containing the two main determinants of cognitive functioning (ie, age and education), along with a vector of control variables, Di, and εi is an error term of the form (2) where the vi term is assumed to be a two-sided random (stochastic) disturbance designed to account for statistical noise and distributed iid , and ui is a random term assumed to be independently distributed as truncations at zero of the distribution.
Both terms, vi and ui, have an immediate interpretation in the frontier analysis literature. The vi term, which gives a stochastic nature to the frontier, is expected to capture the effect of a large number of unobserved factors, among them innate abilities (intelligence quotient) and life events, which might affect an individual’s cognitive functioning in a random way. The ui term corresponds to the distance, seen as the segment in Figure 1, from the best practice boundary, represented by the stochastic frontier . In the case analyzed here, best practice would correspond to the maximum cognitive functioning each individual would be expected to achieve given his/her age and education (and Di controls). In other words, the estimated frontier must be interpreted as an extended benchmark set, corresponding in this case to all the individuals who participated in the first wave of SHARE.
We chose a logarithmic and quadratic (translogarithmic) specification for the relationship between cognitive functioning and age and education as explanatory factors in equation (1). The proposed function corresponds to a second-order approximation of these two variables, as well as the dm,i (m = 1,2, …, M) control variables. The estimated function is as follows:
where and are parameters to be estimated. Therefore, ui corresponds to the performance ratio (0 ≤ ui ≤ 1). The main advantage of the translogarithmic specification is its great flexibility. Other than the logarithmic transformation of variables, second-order terms allow for nonlinear relationships and interactions between age and education.
Moreover, we introduce here the stochastic frontier analysis model specification proposed by Battese and Coelli30 which allows simultaneous testing of the influence of other individual characteristics, denoted by zj,i variables (j = 1, …, J), on cognitive performance ui, through the truncation parameter φi,, as follows:
| (4) |
where δ0 and δj are parameters to be estimated jointly, with the βk and λ0 parameters in equation (3) using a maximum likelihood optimization algorithm (for estimations, we used Frontier version 4.1 [Armidale, NSW, Australia]).31 In addition, two other parameters are simultaneously estimated: first, the variance of the total error term , where indicates the variance of the two-sided disturbance term and indicates the variance of the distance to the frontier term; and second , corresponding to the share of the inefficiency term (distance to the frontier) variance in the total error variance.
Survey variables included in the analysis
As mentioned earlier, the two main variables assumed to determine cognitive performance are age (x1) and years of education (x2). Years of education (a continuous variable corresponding to the number of years of school successfully completed) was constructed for the different countries according to the 1997 International Standard Classification of Education.32
Next, we selected several indicators among the SHARE variables that could potentially explain poor individual cognitive performance (these indicators correspond to zj variables). Different categories of these variables can be distinguished.
First, professional activity status is represented by several different categories, being active (more specifically that the individual is employed or self-employed), inactive for 0 to 4 years, …, inactive for 15 or more years, or never worked professionally. Being professionally inactive refers to complete cessation of paid employment. Somebody who retires and continues to work part-time was considered to be professionally active, whereas somebody who is not working and is receiving an unemployment benefit was considered to be professionally inactive. Second, a vector of dummy variables was related to nonprofessional activities in which a person was engaged during the previous month, including “voluntary or charity work”, “taking care of a sick or disabled adult”, “providing help to family, friends or neighbors”, “attending an educational or training course”, “going to a sports, social or other kind of club”, “taking part in a religious organization”, or “taking part in a political or community-related organization”. Third, physical activity was summarized by a set of dummy variables indicating, respectively, the frequency (never or hardly ever, 1–3 times a month, once a week, more than once a week) of vigorous (mainly playing sports) and moderate (gardening, cleaning the car, or going for a walk) activities.
Two other variables are related to the potentiality of activities. First, mobility limitation is a variable that refers to the number of mobility limitations encountered while doing everyday activities, such as walking 100 meters or sitting for about two hours. Second, a single-person household is expected to be associated with lower cognitive performance, potentially as a result of social isolation.
Several dummy variables, dm, were integrated into the model as controls. Country dummies are expected to capture differences across countries that may be the consequence of language and cultural differences; female gender and being born outside the country of residence are two dummy variables expected to control for differences in cognitive test scores due to gender and origin. Thus, these variables cannot be considered as reflecting differences in cognitive functioning but rather should be viewed as reflecting the consequence of particular life circumstances: a variable corresponding to the respondent’s willingness to answer, as assessed by the interviewer, according to four categories (very high, high, average, and low); and a variable corresponding to the household’s quartile position within distribution of wealth in the country as a control for individual socioeconomic characteristics.
Regarding the gender variable, some authors suggest possible gender differences in the consideration of work and retirement, which could have consequences for cognitive status. Sharabi and Harpaz34 have reported that the transition between professional activity and retirement seems to be steeper for men compared with women, perhaps as a result of men being more work-centered than women and family centrality being greater for women than for men. Considering this, the consequences of retirement would not be the same for women and men. Further research should be conducted to address this question.
Finally, there is a set of variables used to control for health status. These include number of chronic diseases (eg, heart problems, high blood pressure, diabetes, chronic lung disease, asthma), number of symptoms (eg, pain in the back, knees, hips or any other joint, heart trouble, or angina), number of activities of daily living (the respondent was asked about his/her ability to perform some activities, eg, dressing, walking across the room, bathing or showering, eating, getting in and out of bed, and using the toilet, including getting up or down), and two mental health indicators, ie, “past stay in psychiatric institution” and a dummy variable for depression symptoms based on the EURO-D scale, which takes into account symptoms of depression, such as pessimism, suicidal tendency, and guilt.33
It is important to underscore that these variables may also represent potential confounding factors, both for cognitive decline and for diminishing participation in professional and/or nonprofessional activity. It was not possible to reconstruct the sequence of events and direction of causality, mainly because of the cross-sectional nature of the data. This is why we preferred to include these variables directly as controls in the model.
Results
Table 1 shows the mean fluency, memory, and global assessment scores by age and years of education. As expected, fluency and memory scores diminished with age and increased with educational level. Moreover, variations were greater in older and less educated categories in the population. Table 2 presents the results of three stochastic frontier estimations corresponding to alternative cognitive test indicators. In the first and second columns, the dependent variables are the fluency and memory scores while in the third column it is global cognitive assessment.
Table 1.
Cognitive performance by country, age group and education (Mean scores)
| Observations | Fluency | Memory | Global assessment | |
|---|---|---|---|---|
| Age group | ||||
| 50–54 | 4,760 | 21.1 | 9.8 | 0.11 |
| 55–59 | 4,843 | 20.6 | 9.3 | 0.08 |
| 60–64 | 4,360 | 19.8 | 8.8 | 0.04 |
| 65–69 | 3,845 | 18.7 | 8.1 | −0.01 |
| 70–74 | 3,114 | 17.3 | 7.3 | −0.09 |
| 75–79 | 2,234 | 16.3 | 6.7 | −0.14 |
| 80–84 | 1,330 | 15.2 | 5.8 | −0.22 |
| 85–99 | 666 | 13.6 | 4.8 | −0.32 |
| Years of education | ||||
| 0–2 | 1,491 | 13.0 | 5.2 | −0.31 |
| 3–5 | 2,200 | 13.8 | 6.1 | −0.23 |
| 6–9 | 7,309 | 17.4 | 7.5 | −0.07 |
| 10–12 | 6,079 | 20.2 | 9.0 | 0.06 |
| 13–15 | 5,296 | 21.9 | 9.6 | 0.12 |
| 16+ | 2,777 | 22.2 | 10.2 | 0.15 |
| Total | 25,152 | 19.0 | 8.4 | 0.00 |
Note: Mean raw scores for memory and fluency tasks and mean index of global cognitive assessment are presented.
Table 2.
Stochastic frontier model of cognitive performance
| Fluency |
Memory |
Global assessment |
||||
|---|---|---|---|---|---|---|
| Parameter | t-ratio | Parameter | t-ratio | Parameter | t-ratio | |
| Frontier parameters | ||||||
| Intercept | 0.262* | 19.9 | 0.251* | 27.4 | 0.205* | 23.0 |
| Age (ln x1) | −0.282* | −20.3 | −0.386* | −29.6 | −0.348* | −31.9 |
| (ln x1)2 | −0.617* | −8.5 | −0.735* | −10.3 | −0.682* | −11.7 |
| Years of education (ln x2) | 0.170* | 33.7 | 0.163* | 33.3 | 0.171* | 42.7 |
| (ln x2)2 | 0.051* | 19.0 | 0.049* | 18.6 | 0.051* | 24.0 |
| (ln x1)(ln x2) | −0.022 | −1.3 | 0.116* | 6.5 | 0.047* | 3.3 |
| Country | ||||||
| Austria | Ref | Ref | Ref | |||
| Belgium | −0.060* | −8.3 | −0.042* | −5.9 | −0.055* | −9.5 |
| Denmark | −0.033* | −3.8 | 0.013 | 1.5 | −0.010 | −1.5 |
| France | −0.005 | −0.6 | −0.037* | −4.9 | −0.022* | −3.5 |
| Germany | −0.062* | −8.2 | 0.001 | 0.1 | −0.032* | −5.3 |
| Greece | −0.269* | −34.4 | −0.017 | −2.3 | −0.139* | −22.3 |
| Italy | −0.234* | −28.6 | −0.084* | −10.6 | −0.161* | −24.7 |
| The Netherlands | −0.078* | −10.3 | 0.006 | 0.9 | −0.038* | −6.4 |
| Spain | −0.164* | −19.2 | −0.122* | −14.8 | −0.148* | −21.9 |
| Sweden | 0.065* | 8.6 | 0.028* | 3.9 | 0.047* | 8.0 |
| Switzerland | −0.068* | −6.9 | 0.015 | 1.6 | −0.029* | −3.7 |
| Female | 0.004 | 1.3 | 0.077* | 24.5 | 0.042* | 16.4 |
| Born outside the country | −0.085* | −14.5 | −0.032* | −5.7 | −0.059* | −12.7 |
| Wealth quartile | ||||||
| 1st | Ref | Ref | Ref | |||
| 2nd | 0.016* | 3.7 | 0.015* | 3.4 | 0.015* | 4.2 |
| 3rd | 0.033* | 7.4 | 0.026* | 5.9 | 0.030* | 8.4 |
| 4th | 0.043* | 9.4 | 0.025* | 5.7 | 0.034* | 9.4 |
| Number of chronic diseases | 0.002 | 1.3 | 0.001 | 0.9 | 0.001 | 1.3 |
| Number of symptoms | 0.004* | 2.9 | 0.000 | 0.2 | 0.002 | 2.0 |
| Number of activities of daily living | −0.015* | −4.8 | −0.005 | −1.6 | −0.009* | −3.5 |
| Past stay in psychiatric institution | −0.020 | −1.9 | −0.019 | −1.8 | −0.021 | −2.5 |
| Depression scale (EURO-D) | −0.015* | −3.7 | −0.028* | −6.8 | −0.023* | −6.7 |
| Willingness to answer | ||||||
| Very high | Ref | Ref | Ref | |||
| High | −0.045* | −12.6 | −0.036* | −10.5 | −0.040* | −14.2 |
| Average | −0.089* | −16.0 | −0.079* | −14.6 | −0.081* | −18.4 |
| Low | −0.202* | −11.3 | −0.113* | −6.5 | −0.154* | −10.7 |
| Explanatory factors for distance to frontier | ||||||
| Intercept | 0.128* | 3.8 | −0.059 | −1.5 | 0.020 | 0.6 |
| Single-person household | 0.018 | 1.8 | 0.067* | 5.7 | 0.045* | 5.0 |
| Number of mobility limitations | 0.008* | 3.3 | 0.017* | 6.1 | 0.014* | 6.6 |
| Nonprofessional activity | ||||||
| Charity/voluntary work | −0.096* | −4.7 | −0.063* | −3.6 | −0.082* | −5.1 |
| Caring for sick/disabled individuals | −0.072* | −3.0 | −0.003 | −0.2 | −0.037 | −2.1 |
| Helping family/friends/neighbors | −0.120* | −6.5 | −0.087* | −6.2 | −0.099* | −7.3 |
| Educational or training course | −0.139* | −4.1 | −0.147* | −5.2 | −0.154* | −5.2 |
| Going to a social/sport club | −0.107* | −5.9 | −0.097* | −6.4 | −0.100* | −7.1 |
| Participating in a religious organization | −0.030 | −2.0 | −0.004 | −0.3 | −0.012 | −1.0 |
| Participating in a political or community-related organization | −0.079* | −2.6 | −0.106* | −3.5 | −0.100* | −3.6 |
| Vigorous physical activity | ||||||
| Never or hardly ever | Ref | Ref | Ref | |||
| 1–3 times a month | −0.065* | −4.0 | −0.081* | −4.4 | −0.072* | −4.9 |
| Once a week | −0.037* | −2.7 | −0.067* | −4.1 | −0.056* | −4.4 |
| More than once a week | −0.067* | −5.2 | −0.040* | −3.2 | −0.057* | −5.4 |
| Moderate physical activity | ||||||
| Never or hardly ever | Ref | Ref | Ref | |||
| 1–3 times a month | −0.060* | −3.2 | −0.059 | −2.5 | −0.052* | −3.1 |
| Once a week | −0.044* | −3.0 | −0.067* | −3.5 | −0.040* | −3.0 |
| More than once a week | −0.125* | −7.7 | −0.075* | −4.7 | −0.082* | −7.0 |
| Employment and retirement | ||||||
| Working | Ref | Ref | Ref | |||
| Inactive for 0–4 years | 0.050* | 3.0 | 0.079* | 4.3 | 0.064* | 4.3 |
| Inactive for 5–9 years | 0.054* | 3.2 | 0.119* | 6.2 | 0.077* | 5.0 |
| Inactive for 10–14 years | 0.069* | 4.0 | 0.140* | 7.0 | 0.095* | 5.9 |
| Inactive for ≥15 years | 0.068* | 4.2 | 0.179* | 9.3 | 0.109* | 7.1 |
| Never worked professionally | 0.124* | 6.5 | 0.207* | 9.4 | 0.154* | 8.7 |
| Employment status missing | 0.115* | 5.0 | 0.275* | 10.0 | 0.180* | 8.4 |
| Other parameters: | 0.092 | 0.145 | 0.072 | |||
| 0.580 | 0.844 | 0.715 | ||||
| n | 25,152 | 25,152 | 25,152 | |||
Notes: For each model fluency, memory, and global assessment the parameters reported in Tables 2 were estimated simultaneously using frontier analysis; *Significant at the 1% level. The t-ratio is the coefficient estimate divided by its estimated standard error.
Not surprisingly, the age and education coefficients have the expected signs, ie, negative for increasing age and positive for increasing years of education. In all cases, these coefficients were highly significant, with the exception of age and education cross-effect under the fluency model. The results are slightly different between the models. In particular, memory test scores seem to be more negatively affected by aging and less sensitive to increasing level of education.
Figure 2 illustrates these results in a simulated three-dimensional space. The cognitive global assessment frontier, corresponding to combinations of all age groups and years of education, is drawn using the estimated parameters. The boundary hyperplane has a negative slope on the age axis and a positive slope on the education axis. The derivative (tangent) to this hyperplane at each point allows estimation of the effect of education on cognitive aging. Figure 2 indicates that, all else being equal, for an average individual aged 60 years with ten years of education, one additional year of school “compensates” for four years of cognitive aging.
Figure 2.
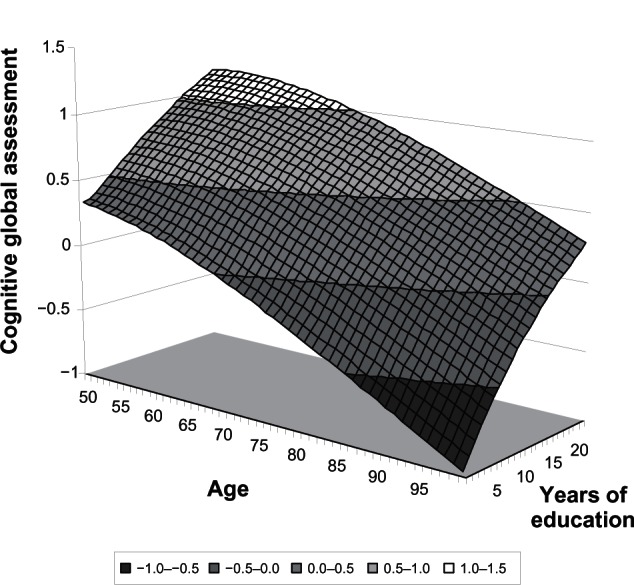
Stochastic frontier: cognitive global assessment as a function of age and years of education.
In most cases, the control variables represent significant parameters indicating that they are important, as expected. Unexpectedly, most physical health indicators, with the exception of activities of daily living, had no significant effect on cognitive outcomes. However, depression had a significant negative effect on memory and fluency scores. Note also the significant positive effect of wealth, which we interpret as an indicator of socioeconomic status at the individual level. Overall, we consider that the cognitive efficiency frontier estimated here is statistically well defined, mainly as a function of age and education. Therefore, most of our attention is focused on the parameters presented at the bottom of Table 2. These parameters correspond to the zj variables considered to be potential factors affecting poor performance of the individual, or in other words, distances from the estimated frontier (note that negative values indicate less distance to the frontier, or better cognitive performance, while positive signs indicate worse performance). Almost all the parameters are statistically significant and their signs are in line with our expectations. Specifically, most types of occupational activity, professional or otherwise, are positively related to cognitive performance.
As mentioned earlier, we were particularly interested in the potential effect of professional activity or, more precisely, in the consequences of inactivity after retirement. As shown in Table 2, the distance to the cognitive frontier increases after leaving the labor force. That is, for a given age and educational level, the cognitive functioning of two individuals, one still professionally active and the other retired (all other things being equal), will differ significantly in favor of the former. The distance to the frontier is even higher for individuals who have never worked professionally.
This negative association between professional inactivity and cognitive performance can be well compensated by engaging in nonprofessional activity, particularly attending educational and training courses. However, there are exceptions, eg, “taking care of sick/disabled individuals” and “taking part in religious organizations”. Indeed, for these variables, we did not observe a significant association with global cognitive functioning. As expected, mobility limitations are significantly and positively associated with cognitive inefficiency. Moreover, the results indicate that living in a single-person household is negatively associated with cognitive functioning.
In order to illustrate the estimated relationship of the z variables and cognitive function, we present in Table 3 the results of a simulation performed using individuals aged 60 years. The outcome of this simulation is estimated in terms of cognitive aging, ie, in years of cognitive decline. These estimates were calculated in two steps using the parameters shown in Table 2. First, we calculated cognitive performance change for each individual, corresponding to a change in a specific z characteristic, all other characteristics being equal. Next, we computed the equivalent change in cognitive aging due to the z factors. For this purpose, we assumed that the slope of the cognitive frontier is at 60 years of age, and for a given education level, remains invariate (see Figure 1).
Table 3.
Impact of z variables on cognitive performance (equivalent years of cognitive aging for 60-year-old individuals)
| Fluency | Memory | Global assessment | |
|---|---|---|---|
| Professional activity status | |||
| Active | Reference | Reference | Reference |
| Inactive for 0–4 years | 1.59 | 1.08 | 1.38 |
| Inactive for 5–9 years | 1.71 | 1.59 | 1.64 |
| Inactive for 10–14 years | 2.17 | 1.86 | 2.03 |
| Inactive for ≥15 years | 2.11 | 2.35 | 2.32 |
| Never worked professionally | 3.76 | 2.70 | 3.23 |
| Nonprofessional activity | |||
| Charity/voluntary work | −2.90 | −0.86 | −1.75 |
| Caring for sick/disabled individuals | −2.21 | −0.05 | −0.81 |
| Helping family/friends/neighbors | −3.55 | −1.18 | −2.09 |
| Educational or training course | −3.96 | −1.93 | −3.08 |
| Going to a social/sport club | −3.19 | −1.30 | −2.10 |
| Participating in a religious organization | −0.97 | −0.06 | −0.26 |
| Participating in a political or community-related organization | −2.40 | −1.41 | −2.08 |
| Physical activities | |||
| Vigorous: never or hardly ever | Ref | Ref | Ref |
| 1–3 times a month | −2.05 | −1.11 | −1.57 |
| Once a week | −1.20 | −0.92 | −1.23 |
| More than once a week | −2.12 | −0.56 | −1.26 |
| Moderate: never or hardly ever | Ref | Ref | Ref |
| 1–3 times a month | −1.97 | −0.82 | −1.16 |
| Once a week | −1.49 | −0.93 | −0.91 |
| More than once a week | −3.80 | −1.03 | −1.78 |
| Other factors | |||
| Number of mobility limitations | 0.27 | 0.25 | 0.32 |
| Single-person household | 0.61 | 0.93 | 1.00 |
Table 3 quantifies the positive relationship between cognitive functioning and variables directly associated with the notion of activity. For example, based on our global assessment model results, a 60-year-old individual delays cognitive aging by 1.38 years by continuing to work, and by 1.75 years by undertaking regular charity or voluntary work. Our analysis also shows that the impact of physical activity differs according to its frequency. Thus, the estimated potential benefit in terms of years of cognitive aging for a 60-year-old individual changes by 1.26 years when physical activity is performed more than once a week instead of never or hardly ever and, even more strikingly, by 1.78 years in the case of moderate physical activity. However, the involvement of the last two variables (ie, mobility limitations or being in a single-person household) can cause a potential cognitive decline of less than one year.
Discussion
In this research, we used a parametric stochastic frontier approach27 to study the association between potential factors (more specifically occupational activity) and cognitive function in the European population aged 50 years and over. For this purpose, we used individual data collected during the first wave of SHARE. In contrast with the majority of relevant studies, this survey includes a large population distributed geographically across Europe. In addition, the multidisciplinary nature of SHARE allowed us to analyze several dimensions of participants’ lives simultaneously.
As expected, our results show that cognitive performance has a negative association with advancing age and a positive association with years of education. The latter result is in accordance with other research suggesting that education is one of the major factors contributing to the development of cognitive reserve.35 Taking into account these potential relationships with age and education, we used stochastic frontier analysis36 to create a “frontier” corresponding to the optimum cognitive functioning that each individual is expected to achieve given age and level of education. This model then allows us to test simultaneously the relationship of various factors (associated directly or indirectly with the notion of activity) and cognitive scores driven by cognitive reserve. Our results show that, after controlling for the effects of factors not associated with the notion of activity (such as gender, being born inside or outside the country, and suffering from physical or mental disease), most types of occupational activity are associated with cognitive reserve. More specifically, all other things being equal, individuals who continue to work or engage in a nonprofessional activity have better cognitive performance.
These results confirm the observations of others, such as the six-year longitudinal study reported by Menec showing a relationship between everyday activity and successful aging.37 It is not possible here to distinguish between cognitively stimulating and nonstimulating professional activity. However, in light of research showing that the level of work complexity positively influences the level of intellectual functioning,18 it can be assumed that the relationship between professional activity and cognitive aging should be more pronounced for activities that mobilize more cognitive resources.
With regard to nonprofessional activity, the strength of the association between cognition and activity differs according to the subtype of nonprofessional activity. The strongest positive association is observed for the variable of attending an educational or training course. This result can be interpreted along the same lines as a study by Hultsch et al who showed a significant relationship between cognitive performance and engagement in new stimulating intellectual activities.38 Indeed, attending an educational or training course could be considered to be more intellectually stimulating than other nonprofessional activity. Our results also show a moderate association between global cognitive functioning and some nonprofessional activities, such as “going to a social/sports club”, “taking part in a political or community-related organization”, “helping family/friends/neighbors”, and “doing charity/voluntary work”. It seems that the common feature of these activities is the notion of social interaction or affiliation, which has been associated in several studies with a decreased risk of cognitive impairment in elderly people.39 Finally, we did not observe a significant association between two nonprofessional activities, ie, “taking care of sick/disabled individuals” and “taking part in religious organizations”, and global cognitive functioning. This result is not surprising for the former because elderly people caring for impaired individuals experience physical, psychological, social, and financial problems, all of which are known to have a negative influence on cognitive function. The absence of an association with membership of a religious organization is discordant with other research showing that religious attendance is associated with a reduction in cognitive aging.40 This conflicting result could be explained by the multicultural nature of our sample compared with the regional nature of the samples used by other researchers (eg, Mexican Americans in the southwestern United States for the study reported by Reyes-Ortiz et al)40. We can hypothesize that religious practice in Europe is very heterogeneous from one country or region to another. For instance, engaging in religious practice might be highly participative or socially rich in some places or religions, but be more passive in others, and such heterogeneity may have a differential impact on the association with cognitive functioning. Further research would be necessary to address this question more directly.
In addition to the estimated association between professional and nonprofessional activity and cognitive function, our study highlights a significant positive relationship with physical activity, both vigorous and moderate, which is consistent with the literature.41 Further, living alone (a variable indirectly associated with the notion of activity) was negatively associated with cognitive functioning, and is consistent with other research showing that social isolation or social disengagement is a risk factor for cognitive impairment in older individuals.42
Globally, and as discussed in the introduction to this paper, most of the variables associated with cognitive functioning have also been related to the notion of psychological well-being and mental health. For example, a recent study showed that higher levels of psychological well-being were associated with better cognitive functioning in a large population of community-living adults.43 However, the question remains as to how these variables interact. In this regard, Gerstorf et al examined cross-domain associations between one dimension of cognitive functioning (ie, perceptual speed) and well-being,44 and showed that well-being has an effect on subsequent decline in perceptual speed, while no evidence was found in the opposite direction. This finding indicates that well-being is not only a consequence but also a source of successful cognitive aging.
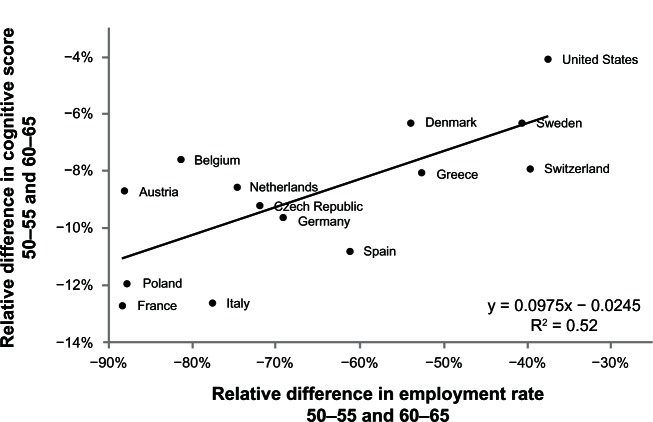
Finally, although our results confirm the relationship between activity and cognitive functioning, we are aware that, mainly because of the cross-sectional nature of the data, our approach does not take into account the causality question of whether the decrease in cognitive functioning is the consequence of reduction in activity or vice versa, with the same question applying to the relationship between activity and well-being. As suggested by Schooler and Mulatu,45 there is probably a reciprocal relationship between cognitive functioning and activity. However, while the impact of cognitive deficits on activities of daily living has been clearly demonstrated at both the clinical and empirical levels, the reverse relationship appears less evident. In that sense, we have recently addressed the causal impact of activity on cognitive functioning in older people by focusing on the relationship between cognitive functioning and retirement.46,47 Indeed, retirement implies major changes in the individual’s lifestyle, and is likely to affect involvement in activities that may contribute to maintaining cognitive function in older age. If individuals have on average more cognitively stimulating activities during their professional life compared with post-retirement, than we would expect a decline in cognitive functioning during retirement as a result of the decrease in stimulating activities. Our studies confirmed this hypothesis. Indeed, we identified the causal effect of retirement on cognitive functioning by using the data from two surveys (SHARE and the Health and Retirement Study, a longitudinal survey among individuals aged 50 years and over living in the United States) and cross-country differences in the age pattern of retirement.46Figure 3 highlights a strong relationship between the relative decrease in cognitive score (as measured by a ten-word recall test when we compared the groups aged 50–55 years and 60–65 years in the different countries, see vertical axis) and the relative decrease in employment rate (using the same age groups, see horizontal axis). In other words, cognitive scores in the elderly are better for countries in which the age of eligibility for retirement benefits is higher (eg, 65 years in Sweden) as compared with countries in which the eligibility age is lower (eg, 60 years in France). The coefficient of the regression line fitting the relationship between the relative drop in employment rate and the relative drop in cognitive functioning suggests that retirement decreases cognitive functioning by about 10%.
Figure 3.
Employment rate and memory score.
Notes: Relative difference between men aged 60–65 years and 50–55 years. Survey of health, Ageing and Retirement in Europe 2004–2006. Health Retirement Study 2004 for the US. The relative difference in employment rate/cognitive score is defined as (Y60–65–Y50–55)/Y50–55 for Yi = the average employment rate/cognitive score for the age category i.
Reproduced from Bonsang E, Adam S, Perelman S. Does retirement affect cognitive functioning? Netspar discussion paper 11/2010-069;2010.46
Finally, we investigated longitudinal data on older Americans from 1998 to 200847 (Health and Retirement Study, six waves). Our analyses confirm a significant negative causal impact of retirement on cognitive functioning. This negative effect remains even after controlling for individual heterogeneity and the endogeneity of the retirement decision. Furthermore, using eligibility for social security as an instrument for retirement, we demonstrated that this relationship is unlikely to be due to reverse causality. Most importantly, our analysis suggests that the effect of retirement on cognitive functioning is not immediate, but occurs with a delay of about one year post retirement.1 For example, in the United States, we observed that the rate of retirement is higher at 62, 65, and 66 years, while in parallel, there is a significant cognitive decline at the age of 63, 66, and 67 years.
Taking into account these elements, the hypothesis that activities in old age are associated with formation and preservation of cognitive reserve has several important implications for the prevention of cognitive aging and Alzheimer’s disease, and also for socioeconomic decisions, particularly with regard to the structure of retirement. Indeed, over the last few decades, decisions to retire in most industrialized countries have been driven mainly by institutions, such as social security systems with regulations that encourage early retirement by financial incentives and tightly restrict professional activity after retirement. These issues have been well documented in the economic literature.48 One of the main reasons invoked to justify these early retirement programs is that shortage in labor demand and its consequences for unemployment would be better absorbed by reducing the number of older workers in the labor market. Therefore, particularly in European countries, an increasing number of workers retire from professional activity before reaching the age of 60 years, and even before 55 years. Nowadays, these policies seem counterproductive from an economic point of view. Given our results, the same applies for the public health point of view.
Nevertheless, our observations should be interpreted with caution. One should not consider that increasing the age of retirement would be beneficial to the health of all. Indeed, further research would be necessary to clarify the effect of professional activity on cognition (and more particularly on memory functioning). Indeed, the first question to be investigated is whether the impact of retirement on cognitive function depends on the type of professional activity undertaken while employed, ie, physical versus intellectual work, a light versus heavy workload, and whether work is stressful or not. For example, some studies have shown that intellectually demanding jobs during adulthood are associated with better cognitive functioning in later life, whereas manual labor is associated with worse cognitive functioning.49 A second important question to be answered is whether the relationship between retirement and cognition is direct and/or whether there are some intermediate variables between retirement and cognition. Indeed, work is known to increase social interaction and the sense of self-efficacy, with both these variables being considered to be important factors contributing to the maintenance of cognitive reserve.23
Conclusion
This study highlights that, after controlling for extraneous variables not associated with the notion of “activity” (such as gender or being born inside or outside the country), all types of occupational activity (professional and nonprofessional) clearly have a positive effect on cognitive functioning. In fact, our results underscore the importance of activity in a general sense, and not just professional activity. For professional activity, further research is necessary to clarify the effect of retirement on cognitive functioning by taking into account several associated parameters, such as the type of professional activity undertaken while employed (eg, manual versus intellectual work) and the modality of retirement (eg, voluntary versus forced retirement). With regard to the concept of activity in a more global sense, it appears that being active is important for elderly people. However, some issues remain concerning what constitutes a constructive activity for an elderly person and which activities should be implemented. For instance, whether it is more beneficial to work in a group or independently or to engage in new activities versus old ones, and whether all activities have the same impact on cognitive functioning. When we look at the results presented in Table 3, it seems that some activities are more related to cognitive functioning compared to others, eg, taking part in a religious organization has the least impact on cognitive performance whereas educational training or training courses have the most impact.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Dixon RA, Bäckman L, Nilsson L-Gr. New Frontiers in Cognitive Aging. Oxford, UK: Oxford University Press; 2004. [Google Scholar]
- 2.See ST, Ryan EB. Cognitive mediation of adult age differences in language performance. Psychol Aging. 1995;10:458–468. doi: 10.1037//0882-7974.10.3.458. [DOI] [PubMed] [Google Scholar]
- 3.Raz N. The aging brain: structural changes and their implications for cognitive aging. In: Dixon RA, Bäckman L, Nilsson L-Gr, editors. New Frontiers in Cognitive Aging. Oxford, UK: Oxford University Press; 2004. [Google Scholar]
- 4.Meyer JS, Rauch GM, Crawford K, et al. Risk factors accelerating cerebral degenerative changes, cognitive decline and dementia. Int J Geriatr Psychiatry. 1999;14:1050–1061. doi: 10.1002/(sici)1099-1166(199912)14:12<1050::aid-gps56>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 5.Honig LS, Rosenberg RN. Apoptosis and neurologic disease. Am J Med. 2000;108:317–330. doi: 10.1016/s0002-9343(00)00291-6. [DOI] [PubMed] [Google Scholar]
- 6.Berkman LF, Seeman TE, Albert M, et al. High, usual and impaired functioning in community-dwelling older men and women: findings from the MacArthur Foundation Research Network on Successful Aging. J Clin Epidemiol. 1993;46:1129–1140. doi: 10.1016/0895-4356(93)90112-e. [DOI] [PubMed] [Google Scholar]
- 7.Silver M, Newell K, Hyman B, Growdon J, Hedley-Whyte ET, Perls T. Unraveling the mystery of cognitive changes in old age: correlation of neuropsychological evaluation with neuropathological findings in the extreme old. Int Psychogeriatr. 1998;10:25–41. doi: 10.1017/s1041610298005122. [DOI] [PubMed] [Google Scholar]
- 8.Perls T. Centenarians who avoid dementia. Trends Neurosci. 2004;27:633–636. doi: 10.1016/j.tins.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Fillit HM, Butler RN, O’Connell AW, et al. Achieving and maintaining cognitive vitality with aging. Mayo Clin Proc. 2002;77:681–696. doi: 10.4065/77.7.681. [DOI] [PubMed] [Google Scholar]
- 10.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–460. [PubMed] [Google Scholar]
- 11.Stern Y. The concept of cognitive reserve: a catalyst for research. J Clin Exp Neuropsychol. 2003;25:589–593. doi: 10.1076/jcen.25.5.589.14571. [DOI] [PubMed] [Google Scholar]
- 12.Rosenzweig MR, Bennett EL. Cerebral changes in rats exposed individually to an enriched environment. J Comp Physiol Psychol. 1972;80:304–313. doi: 10.1037/h0032978. [DOI] [PubMed] [Google Scholar]
- 13.Winocur G. Environmental influences on cognitive decline in aged rats. Neurobiol Aging. 1998;19:589–597. doi: 10.1016/s0197-4580(98)00107-9. [DOI] [PubMed] [Google Scholar]
- 14.Dobrossy MD, Dunnett SB. The influence of environment and experience on neural grafts. Nat Rev Neurosci. 2001;2:871–879. doi: 10.1038/35104055. [DOI] [PubMed] [Google Scholar]
- 15.Le Carret N, Lafont S, Letenneur L, Dartigues F, Mayo W, Fabrigoule C. The effect of education on cognitive performances and its implication for the constitution of the cognitive reserve. Dev Neuropsychol. 2003;23:317–337. doi: 10.1207/S15326942DN2303_1. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z, Gu D, Hayward MD. Early life influences on cognitive impairment among oldest old Chinese. J Gerontol B Psychol Sci Soc Sci. 2008;63:S25–S33. doi: 10.1093/geronb/63.1.s25. [DOI] [PubMed] [Google Scholar]
- 17.Letenneur L, Gilleron V, Commenges D, Helmer C, Orgogozo JM, Dartigues JF. Are sex and educational level independent predictors of dementia and Alzheimer’s disease? Incidence data from the PAQUID project. J Neurol Neurosurg Psychiatry. 1999;66:177–183. doi: 10.1136/jnnp.66.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schooler C, Mulatu MS, Oates G. The continuing effects of substantively complex work on the intellectual functioning of older workers. Psychol Aging. 1999;14:483–506. doi: 10.1037//0882-7974.14.3.483. [DOI] [PubMed] [Google Scholar]
- 19.Wilson RS, Mendes De Leon CF, Barnes LL, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA. 2002;287:742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- 20.Newson RS, Kemps EB. General lifestyle activities as a predictor of current cognition and cognitive change in older adults: a cross-sectional and longitudinal examination. J Gerontol B Psychol Sci Soc Sci. 2005;60:P113–P120. doi: 10.1093/geronb/60.3.p113. [DOI] [PubMed] [Google Scholar]
- 21.Capurso A, Panza F, Solfrizzi V, et al. All’età declino cognitivo correlato: valutazione e strategia di prevenzione [Age-related cognitive decline: evaluation and prevention strategy.] Recenti Prog Med. 2000;91:127–134. Italian. [PubMed] [Google Scholar]
- 22.Berkman LF, Glass T, Brissette I, Seeman TE. From social integration to health: Durkheim in the new millennium. Soc Sci Med. 2000;51:843–857. doi: 10.1016/s0277-9536(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 23.Rowe JW, Kahn RL. Successful Aging. New York, NY: Pantheon Books; 1998. [Google Scholar]
- 24.Greenfield EA, Marks NF. Continuous participation in voluntary groups as a protective factor for the psychological well-being of adults who develop functional limitations: evidence from the national survey of families and households. J Gerontol B Psychol Sci Soc Sci. 2007;62:S60–S68. doi: 10.1093/geronb/62.1.s60. [DOI] [PubMed] [Google Scholar]
- 25.Hao Y. Productive activities and psychological well-being among older adults. J Gerontol B Psychol Sci Soc Sci. 2008;63:S64–S72. doi: 10.1093/geronb/63.2.s64. [DOI] [PubMed] [Google Scholar]
- 26.Börsch-Supan A, Jürges H. The Survey of Health, Ageing and Retirement in Europe – Methodology. Mannheim, Germany: Mannheim Research Institute for the Economics of Aging; 2005. [Google Scholar]
- 27.Aigner DJ, Lovell CAK, Schmidt P. Formulation and estimation of stochastic production function models. J Econom. 1977;6:21–37. [Google Scholar]
- 28.Coelli T. An Introduction to Efficiency and Productivity Analysis. 2nd ed. New York, NY: Springer; 2005. [Google Scholar]
- 29.Souchay C, Isingrini M, Espagnet L. Aging, episodic memory feeling-of-knowing, and frontal functioning. Neuropsychology. 2000;14:299–309. doi: 10.1037//0894-4105.14.2.299. [DOI] [PubMed] [Google Scholar]
- 30.Battese GE, Coelli T. A model for technical inefficiency effects in a stochastic frontier production function for panel data. Empir Econ. 1995;20:325–332. [Google Scholar]
- 31.Coelli T. A Guide to Frontier Version 4.1: A Computer Program for Stochastic Production and Cost Function Estimation. Armidale, NSW, Australia: Department of Econometrics, University of New England; 1994. [Google Scholar]
- 32.OECD . Classifying Educational Programmes Manual for ISCED-97 Implementation in OECD Countries 1999 Edition. Paris, France: OECD Publishing; 1999. [Google Scholar]
- 33.Prince MJ, Reischies F, Beekman AT, et al. Development of the EURO-D scale – a European Union initiative to compare symptoms of depression in 14 European centres. Br J Psychiatry. 1999;174:330–338. doi: 10.1192/bjp.174.4.330. [DOI] [PubMed] [Google Scholar]
- 34.Sharabi M, Harpaz I. Gender and the relative centrality of major life domains: changes over the course of time. Community Work and Family. 2011;14:57–62. [Google Scholar]
- 35.Scarmeas N, Albert SM, Manly JJ, Stern Y. Education and rates of cognitive decline in incident Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2006;77:308–316. doi: 10.1136/jnnp.2005.072306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumbhakar SC, Knox Lovell CA. Stochastic Frontier Analysis. Cambridge, UK: Cambridge University Press; 2002. [Google Scholar]
- 37.Menec VH. The relation between everyday activities and successful aging: a 6-year longitudinal study. J Gerontol B Psychol Sci Soc Sci. 2003;58:S74–S82. doi: 10.1093/geronb/58.2.s74. [DOI] [PubMed] [Google Scholar]
- 38.Hultsch DF, Hertzog C, Small BJ, Dixon RA. Use it or lose it: engaged lifestyle as a buffer of cognitive decline in aging? Psychol Aging. 1999;14:245–263. doi: 10.1037//0882-7974.14.2.245. [DOI] [PubMed] [Google Scholar]
- 39.Bassuk SS, Glass TA, Berkman LF. Social disengagement and incident cognitive decline in community-dwelling elderly persons. Ann Intern Med. 1999;131:165–173. doi: 10.7326/0003-4819-131-3-199908030-00002. [DOI] [PubMed] [Google Scholar]
- 40.Reyes-Ortiz CA, Berges IM, Raji MA, Koenig HG, Kuo YF, Markides KS. Church attendance mediates the association between depressive symptoms and cognitive functioning among older Mexican Americans. J Gerontol A Biol Sci Med Sci. 2008;63:480–486. doi: 10.1093/gerona/63.5.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Albert MS, Jones K, Savage CR, et al. Predictors of cognitive change in older persons: MacArthur studies of successful aging. Psychol Aging. 1995;10:578–589. doi: 10.1037//0882-7974.10.4.578. [DOI] [PubMed] [Google Scholar]
- 42.Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3:343–353. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- 43.Llewellyn DJ, Lang IA, Langa KM, Huppert FA. Cognitive function and psychological well-being: findings from a population-based cohort. Age Ageing. 2008;37:685–689. doi: 10.1093/ageing/afn194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gerstorf D, Lovden M, Rocke C, Smith J, Lindenberger U. Well-being affects changes in perceptual speed in advanced old age: longitudinal evidence for a dynamic link. Dev Psychol. 2007;43:705–718. doi: 10.1037/0012-1649.43.3.705. [DOI] [PubMed] [Google Scholar]
- 45.Schooler C, Mulatu MS. The reciprocal effects of leisure time activities and intellectual functioning in older people: a longitudinal analysis. Psychol Aging. 2001;16:466–482. doi: 10.1037//0882-7974.16.3.466. [DOI] [PubMed] [Google Scholar]
- 46.Bonsang E, Adam S, Perelman S. Does retirement affect cognitive functioning? Netspar discussion paper 11/2010-069. 2010. [DOI] [PubMed] [Google Scholar]
- 47.Bonsang E, Adam S, Perelman S. Does retirement affect cognitive functioning? J Health Econ. 2012;31:490–501. doi: 10.1016/j.jhealeco.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 48.Gruber J, Wise DA. Social Security Programs and Retirement Around the World: Micro-Estimation. Chicago, IL: University of Chicago Press; 2004. [Google Scholar]
- 49.Potter GG, Helms MJ, Plassman BL. Associations of job demands and intelligence with cognitive performance among men in late life. Neurology. 2008;70(19 Pt 2):1803–1808. doi: 10.1212/01.wnl.0000295506.58497.7e. [DOI] [PMC free article] [PubMed] [Google Scholar]