Abstract
Objective
Deficits in cognitive functioning have been well-documented in persons with substance use disorders. In addition, some evidence suggests that poorer cognitive functioning predicts poorer engagement in substance abuse treatment and worse treatment outcomes.
TRIAL DESIGN
Non-blind, randomized clinical trial with parallel design.
Methods
Clients were recruited from a local methadone maintenance clinic within the first 30 days of treatment. All participants completed a comprehensive, computerized neuropsychological assessment (MicroCog) at the time they entered the clinical trial. Participants were randomized to receive 12 months of either standard methadone maintenance treatment, or methadone maintenance treatment with an integrated web-based intervention as part of treatment. The aims of the current study were to (1) characterize the cognitive functioning of clients entering methadone maintenance treatment; (2) evaluate the impact of cognitive functioning on the primary outcomes of treatment retention and opioid abstinence; and (3) determine whether cognitive functioning had a differential impact on these outcomes across treatment conditions. Randomization was non-blind and participants were stratified on past month cocaine use, prior history of methadone, LAAM or buprenorphine treatment, and counselor.
Results
Eighty participants were randomized to each condition (total n=160). Mean scores on MicroCog scales fell in the average and low average ranges and there were no differences in scores between treatment groups. Lower scores on General Cognitive Proficiency predicted longer study retention (χ2=5.03, p < .05), though this effect was quite small. Generalized linear modeling showed that scores on all MicroCog scales except for Spatial Processing significantly predicted opioid abstinence (defined as percent of total weeks and percent of tested weeks with continuous abstinence), with lower scores predicting smaller percentages of continuous weeks of abstinence. This pattern was not evident in regression analyses in which abstinence was defined as number of total weeks of abstinence. An interaction effect was observed, whereby lower cognitive scores predicted lower levels of abstinence for participants in standard methadone maintenance treatment, but not for those who received the web-based intervention as part of methadone maintenance treatment.
Conclusions
Technology-based interventions may hold promise for minimizing the impact of poorer cognitive functioning on treatment outcomes.
Keywords: cognitive, methadone maintenance treatment, technology, web-based intervention
Cognitive functioning may be comprised of multiple domains including executive functioning (impulsivity/cognitive control, abstract thinking, judgement, planning, problem solving, cognitive flexibility), verbal and nonverbal learning and memory, and processing speed (Powell, 1993; Lyvers, 2000). There is a preponderance of evidence documenting cognitive deficits in substance abusing populations (e.g., Erche et al., 2006; Rogers & Robbins 2001; Volkow, Fowler & Wang 2003). Deficits have been documented in a wide range of cognitive domains including executive functioning, memory, impulsivity, decision making, spatial and verbal abilities, and processing speed (e.g., Aharonovich 2003, 2006, 2008; Bates, LeBouvie, & Voebel, 2002; Bates et al., 2004, 2006; Carroll 2011; Ersche et al., 2006). Many of these deficits are thought to be associated with dysfunction in the prefrontal cortex, though there may be subtle, differential impact on particular neural pathways depending upon the drug of abuse (Volkow, Fowler, & Wang, 2003).
For example, the impact of chronic alcohol use on cognitive functioning has been widely studied, and indicates chronic drinkers demonstrate impaired memory and abstract reasoning (e.g., Allen, Goldstein, & Seaton, 1997; Bates & Convit, 1999; Bates, Labouvie, & Voelbel, 2002; Goldstein et al., 2004). Chronic stimulant abuse has been shown to cause impairments in visuo-motor performance, attention, and verbal memory (e.g., Roselli & Ardilla, 1993) with persistence of these deficits documented over time (O-Malley, Adamse, Heaton, & Gawin, 1992), possibly due to changes in the frontostriatal circuitry (Volkow et al., 1993). Opioid users have been much less studied than alcohol or stimulant users, though there is evidence that chronic opioid use may contribute to problems with decision making (Madden, Petry, Badger & Bickel, 1997). In studies of chronic drug users, amphetamine users demonstrated more difficulties with attention and spatial planning, whereas opioid users demonstrated more difficulties applying a learned strategy to new learning and with spatial memory (Ersche et al., 2006; Ornstein et al., 2000). It is still not known whether these cognitive deficits are caused by chronic drug use or are indicative of a pre-existing vulnerability that contributes to drug taking.
Complicating the picture, there is evidence that opioid pharmacological treatments, such as methadone maintenance, may serve to impair cognitive ability (Darke et al., 2000; Foreman et al., 2004; Mintzer & Stitzer 2002; Mintzer, Copersino, & Stitzer 2005; Zacny 1995). Methadone treatment has been shown to be safe and effective, especially when provided via long-term methadone maintenance treatment (Ball & Ross, 1991; Marsch, 1998; Strain et al., 1993). Unfortunately, cognitive impairments in attention, working memory, cognitive flexibility, problem solving and abstract reasoning have been demonstrated in persons maintained on methadone, compared to non-opioid using controls and abstinent opioid abusers (Darke, Sims, McDonald & Wickes, 2000; Mintzer & Stitzer, 2002; Pirastu et al., 2006; Rapeli et al., 2007; Verdejo et al., 2005). However, most of this prior work did not examine the length of time participants had been taking methadone. Newer research indicates that persons maintained on methadone for at least six months showed improved visuospatial functioning compared to those in their first 30 days of treatment (Soyka, Zingg, Koller, & Hennig-Fast, 2010), indicating that there may be some attenuation of possible methadone effects on cognitive functioning over time.
Non-pharmacological (i.e., behavioral) treatments have been shown to have strong efficacy in treating a variety of substance use disorders, particularly cognitive-behavioral approaches (Carroll, 1998; Dutra et al., 2008). In addition, numerous clinical trials have demonstrated that clients in methadone maintenance treatment who are provided with evidence-based psychosocial interventions are significantly more likely to remain in treatment and to experience markedly greater reductions in drug use than those receiving standard drug counseling in methadone maintenance treatment (e.g., Condelli & Dunteman, 1993; McLellan et al., 1993; O’Brien et al., 1995). For example, the Community Reinforcement Approach, a cognitive-behavioral therapy intervention, has been shown to decrease opioid and other drug use and improve psychosocial functioning (please see Abbott, 2009 for review). While cognitive-based treatments have shown good efficacy in the treatment of substance use disorders, some argue that cognitive deficits may interfere with problem solving, learning and applying new information/skills, and planning behavioral alternatives to substance use (Lyvers, 2000). This may make it difficult for treatment seekers to engage in or benefit from treatments that focus heavily on skills-building, coping, or other cognitive tasks.
The relationship between cognitive functioning and substance abuse treatment outcomes is complex. Lower levels of cognitive functioning appear to predict poorer treatment retention, but evidence of a direct relationship with substance abuse treatment outcomes is mixed (Aharonovich et al., 2003, 2006, 2008; Bates et al., 2006; Carroll et al. 2011; Passetti et al., 2008). One study found that impaired decision-making predicted lower levels of abstinence in a sample of opioid dependent persons receiving opioid substitution and behavioral therapy (Passetti et al., 2008). Other studies have shown that while cognitive functioning predicts treatment retention, it is indirectly associated with outcomes such as abstinence (Aharonovich et al., 2008; Bates et al., 2006). The impact of cognitive functioning is clinically meaningful, as retention in substance abuse treatment predicts improved outcomes (Grella et al., 1999; Smith & McCrady, 1991). Two studies of chronic alcohol users found that cognitive functioning predicted a variety of other factors (e.g., self-efficacy, treatment affiliation), which in turn were associated with abstinence, suggesting that mechanisms of change may differ in those with cognitive impairment (Bates et al. 2006; Morgenstern & Bates, 1999). For example, both studies showed a strong link between self-efficacy and abstinence in people without cognitive impairment, but a weak or non-significant impact in those with cognitive impairments.
Cognitive impairments may influence outcomes for certain types of substance abuse treatment, but not others. For example, patients with alcohol dependence who had lower levels of verbal learning, had poorer drinking outcomes in relapse prevention therapy but not supportive therapy. Conversely, higher levels of verbal learning were associated with better outcomes for relapse prevention therapy but not for supportive therapy (Jaffe et al., 1996). In addition, in a sample of patients with alcohol dependence, cognitive impairments led to poorer outcomes in cognitive-behavioral therapy, but not supportive therapy (Cooney, Kadden, Litt, & Getter, 1991). It may be that treatments that require more cognitive processing present specific challenges to those with cognitive impairments.
Some researchers are now starting to examine the impact of substance abuse treatment on cognitive deficits (Bates et al., 2004; Bates et al., 2005) and to explore treatments to improve cognitive functioning in substance abusing populations (e.g., Turner et al., 2009). One study examined a cognitive remediation approach consisting of exercises focused on increasing memory and attention through repetition. Patients in residential substance abuse treatment were examined and it was found that patients who received this intervention had significantly better retention and treatment outcomes than those who did not (Grohman & Fals-Stewart, 2003).
Technology-based interventions may present a unique opportunity to intervene with patients who present with a range of cognitive functioning, as they may be able to present material in multiple formats, tailor content and presentation to an individual’s needs, demonstrate and reinforce new skills at the individual’s own pace, etc. However, very little research exists on how technology-based interventions may impact treatment retention and outcomes in populations where cognitive functioning is associated with outcomes. A preliminary study examined the impact of cognitive functioning on retention and outcome for patients randomized to either standard counseling for alcohol or drug dependence or standard counseling plus a 6-session computer-delivered intervention based on cognitive-behavioral therapy (Carroll, et al. 2011). Results indicated that lower levels of overall cognitive functioning, executive functioning, visual memory and processing speed were not associated with retention or outcomes in either 8-week condition. Inattention (measured by the Continuous Performance Task II; Conners, 2004) and impulsivity (measured by the Balloon Analogue Risk Task; Lejuez et al., 2002) were negatively associated with treatment retention, homework completion and treatment outcomes in the patients receiving the computer-delivered treatment, but not with those receiving standard counseling. The authors suggest that substance users who are more impulsive may be less likely to learn or implement new skills or less likely to actively engage in treatment (e.g., completing homework assignments).
The aims of the current study are to comprehensively characterize multiple dimensions of cognitive functioning in methadone maintenance clients enrolled in a study designed to evaluate a web-based intervention; examine the impact of baseline cognitive functioning on retention and treatment outcome (i.e., abstinence from opioids) in the intervention study; and determine whether cognitive functioning had a differential impact on these outcomes across treatment conditions. We predict that: (1) participants will fall in the low average to average ranges of cognitive functioning, similar to drug abusing populations in other studies; and (2) participants with lower cognitive functioning at baseline will have poorer retention and lower levels of abstinence during the intervention study. We also will explore whether an interactive web-based intervention, which allows clients to control the pace of skills training and aims to train clients to become fluent in key skills and information despite their baseline knowledge / skills levels, may influence treatment outcomes among clients with varying levels of cognitive functioning.
METHODS
Participants
Participants were new adult clients (n=160; age > 18 years) within the first 30 days of initiating methadone maintenance treatment. All clients entering methadone maintenance treatment met DSM-IV criteria for opioid dependence and the criteria in the Federal Register outlining regulations regarding the use of opioid drugs in the treatment of opiate addiction.
Project Setting
The study was conducted in a large methadone maintenance treatment center (census of approximately 500) in an urban area of the northeastern US. As per the policy at the methadone maintenance treatment program, participants’ methadone doses generally were stabilized within the first two weeks of treatment, and they were provided with stable therapeutic maintenance doses of methadone thereafter (average of 80-120 mg daily), depending on treatment response.
Study Procedures
Clients were informed of the opportunity to participate in a research study at the time they entered methadone maintenance treatment. Interested clients were then able to speak with a research associate who had a complete discussion of the study, and subsequently conducted screening and written informed consent procedures with eligible and interested patients. The study was conducted in accordance with the Declaration of Helsinki and the National Development and Research Institutes, Inc. Institutional Review Board approved and monitored the study.
Random Assignment
Immediately following the baseline assessment, participants were randomly assigned to one of the two study conditions in an intent-to-treat design: (1) standard treatment or (2) reduced standard treatment plus a web-based behavior therapy program called the Therapeutic Education System. Participants were stratified on past month cocaine use and prior history of methadone, LAAM or buprenorphine treatment using permuted block randomization. Randomization was non-blind.
Treatment Conditions
Each study condition lasted for a period of 12 months. Participants who were randomly assigned to the standard treatment condition received the standard drug counseling offered at the methadone maintenance treatment program; participants had counseling once weekly for the first four weeks, then twice per month for an anticipated total of 26 hours over the 12 month study. Unfortunately, we were unable to collect specific data on what comprised standard counseling sessions at the clinic, as counselors were reluctant to participate if that information was collected. However, the program mandate, similar to many other methadone maintenance programs, focuses largely on compliance with program rules.
Participants who were randomly assigned to the reduced standard treatment condition plus the web-based intervention received the same standard drug counseling as those in the first condition, except that half of each scheduled counseling session was held with the therapist and the other half was replaced with the web-based intervention. In order to parallel the standard counseling schedule, participants were asked to complete one web-based intervention session per week during their first four weeks, then twice per month after that.
Web-based intervention
The content of the web-based intervention was based on the Community Reinforcement Approach (Budney & Higgins, 1998; Higgins et al., 1991). The Community Reinforcement Approach is a flexible, skills-based approach in which people are taught to identify patterns of drug or alcohol use, and to use specific strategies to avoid or cope with triggers for substance use. It also encompasses skills training areas such as problem solving, communication, relaxation or other skills that may be helpful in achieving and maintaining abstinence (Budney & Higgins, 1998; Higgins et al., 1991). The web-based intervention is comprised of 65 sections that address drug use and related issues (e.g., identifying and managing triggers for use, information about HIV and Hepatitis C), as well as more general skills such as financial management, communication skills, management of negative moods and depression, time management, etc. All text presented in the program is accompanied by audio to accommodate individuals with reading difficulties. The web-based intervention (Therapeutic Education System) employs a fluency-based Computer Assisted Instruction (e.g., Binder, 1993) approach, which requires the learner to develop a pre-determined level of accuracy and speed in responding during an active learning process (© copyright 1997, HealthSim, Inc.). It also includes interactive, video-based simulations (Issenberg et al., 2001) and a number of interactive exercises to enhance learning and personalize content for participants. The Therapeutic Education System has been used in a variety of drug abusing populations and has been shown to be effective in increasing retention and abstinence in these populations and to increase HIV prevention knowledge and intentions to reduce HIV risk behavior (Bickel et al., 2008; Marsch et al., 2011).
In the current study, participants accessed the Therapeutic Education System intervention using one of 4 computers set up in a room designated for this activity at the methadone maintenance treatment program. Participants used a unique password to log into the program and were provided with headphones to wear while using the intervention.
Urine Drug Testing
Participants provided weekly urine samples to research staff, approximately 25% of which were randomly observed by a research associate or clinic staff member of the same sex. Each sample was tested for the presence of: THC, cocaine, barbituates, benzodiazepines, methamphetamine, opiates, methadone, propoxyphene, and oxycontin using point-of-care qualitative urine test cups (Drug Check Drug Test Cup, Drug Test Systems, Dover, NH).
MicroCog Assessment of Cognitive Functioning (Powell, 1993)
As part of a larger baseline assessment, participants were administered the Short Form of the MicroCog Assessment of Cognitive Functioning. MicroCog was standardized in a US adults population sample (n=810, 50% male) stratified by age, gender, race/ethnicity, geographic region, and education. The test has demonstrated sound reliability, stability, and discriminant (i.e., can differentiate dementia versus depression) and convergent validity on a variety of assessments (Powell et al., 1993). This computerized battery is self-administered and normative scores are provided at three levels. Level 1 Index scores assess functioning in the domains of attention/mental control, memory (verbal and visual), spatial processing, abstract reasoning, and reaction time/processing speed (as measured by subscales contained within each domain). The Attention/Mental Control Index assesses attention span, vigilance, concentration, perseverance, and resistance to interference (Powell, 1993). The Memory Index assesses both short-term (immediate) and longer-term (delayed) memory abilities. The Reasoning/Calculation Index captures the ability to reason and perform abstract thinking tasks. Spatial Processing refers to visuospatial information processing and assesses a combination of visuospatial perception, visual recognition, and visual memory. Reaction Time measures the amount of time between the presentation of a stimulus and a designated response (e.g., key press; Powell, 1993).
Level 2 scores represent overall Information Processing Speed and Information Processing Accuracy. Information Processing Speed reflects average response time across MicroCog subscales, regardless of the correctness of the response. Processing speed has been shown to be sensitive to cognitive impairment, including brain injury. Information Processing Accuracy reflects average correct responses across MicroCog subscales, regardless of the speed of response. Finally, Level 3 scores (General Cognitive Functioning and General Cognitive Proficiency) are broadest and represent more global cognitive functioning. In the General Cognitive Functioning score, response accuracy and speed are weighed equally, while in the General Cognitive Proficiency score, accuracy is weighed more heavily than speed. The population mean for Index scores is 100 with a standard deviation of 15. For individual subtests, the mean score is 10 with a standard deviation of 1.5 (Powell, 1993).
Compensation
Participants received $50 for completing a baseline assessment consisting of several self-report measures and clinical interviews, and $10 for each urine sample provided.
Data Analyses
Descriptive statistics were run to characterize the sample. A survival analysis using Cox’s proportional hazards model was conducted to examine the impact of MicroCog scores on weeks retained in the intervention study (out of a possible 52 week window). Retention was calculated as the number of days each participant actively participated in the study (date of last contact subtracted from baseline). Opioid abstinence was based on urine analysis results for opiates, propoxyphene, and oxycodone. A missed test prior to study dropout was treated as positive for opioid use when summarizing abstinence. Opioid abstinence was calculated in three ways: percentage of total study weeks with continuous opioid abstinence, percentage of tested study weeks with continuous opioid abstinence, and total number of weeks of opioid abstinence. We chose to examine opioid abstinence in this manner as these are commonly used outcomes in substance abuse treatment research and may allow our findings to be more easily compared to other studies. Additionally, these varying ways of defining abstinence allow us to examine overall as well as continuous/maintenance patterns of abstinence. Ordinary least squares regressions were used to estimate the effect of MicroCog index scores on number of weeks of opioid abstinence (main effect model), and possible differential effects of MicroCog index scores across intervention groups (Micro-Cog index by intervention interaction model). Generalized linear modeling with binomial distribution was used to assess the effect of MicroCog index scores on percentage of total study weeks and percentage of tested study weeks with continuous opioid abstinence, and their possible differential effects across intervention groups.
RESULTS
Participants
Participants (n=160) ranged in age from 20 to 64 (M=40.66, SD=9.83) and most participants were male (75.0%). Participants reported their race to be White (44.3%), Black or African American (31.7%), or Other (24.1%), and 27.39% endorsed Hispanic ethnicity. Participants had an average of 12.2 years of education (SD=2.4), and there were no significant differences in years of education between treatment groups. The vast majority of participants reported that their primary opioid was heroin (96.3%). Approximately a third (33.8%) reported having Hepatitis C and 10.0% reported being positive for HIV.
Overall Cognitive Functioning
MicroCog Index scores fell in the average and low average ranges for our sample (See Table 1). No differences on any of the baseline MicroCog domains were observed between the standard group and the group who received reduced standard treatment group plus the web-based intervention.
TABLE 1.
Mean scores on MicroCog Indices
| Index | Standard Score* M(SD) |
Range |
Range of
Scores |
% Participants Below Average Range** |
|---|---|---|---|---|
| General Cognitive Functioning |
78.5(16.6) | Low Average | 50-115 | 33.1% |
| General Cognitive Proficiency |
77.7(13.9) | Low Average | 50-114 | 33.1% |
| Information Processing Speed | 85.0(19.5) | Average | 50-123 | 21.9% |
| Information Processing Accuracy |
80.8(16.2) | Low Average | 50-117 | 23.8% |
| Attention/Mental Control | 83.5(17.8) | Low Average | 50-116 | 23.8% |
| Memory | 82.8(17.5) | Low Average | 50-114 | 25.6% |
| Spatial Processing | 96.6(14.7) | Average | 50-130 | 5.6% |
| Reasoning/Calculation | 81.7(17.5) | Low Average | 50-119 | 24.4% |
| Reaction Time | 95.5(17.3) | Average | 50-120 | 10.0% |
normative mean = 100, SD = 15
Below Average range include scores of 69 or below and correspond to approximately 2.2% of the general population
Impact of Cognitive Functioning on Retention
Results from the survival analysis indicated that only General Cognitive Proficiency was a significant predictor of weeks retained in the intervention study; those with higher General Cognitive Proficiency were retained in the study for a briefer time than those with lower General Cognitive Proficiency scores (χ2=5.03, p < .05), though the magnitude of this effect was quite small (having higher General Cognitive Proficiency scores increased the chance of drop out by approximately 2%; Hazard ratio=1.016). There was no differential impact of any MicroCog Index on retention in the standard versus web-based treatment groups (all ps > .05).
Impact of Cognitive Functioning on Opioid Abstinence
Percentage of total weeks with continuous opioid abstinence
Regarding abstinence, all of the MicroCog indices were significant predictors of opioid abstinence expect for Spatial Processing (see Table 2). Higher cognitive scores predicted larger percentages of study weeks with continuous abstinence. In addition, the MicroCog Indices of Attention/Mental Control, Memory, Information Processing Accuracy and General Cognitive Functioning had significant differential effects (i.e., interaction effects) across treatment groups (see Table 3). Specifically, these MicroCog Indices were positively associated with abstinence for participants in standard treatment, but there was no relationship between these cognitive scores and abstinence for participants who received the Therapeutic Education System. Higher scores on Attention/Mental Control, Memory, Information Processing Accuracy and General Cognitive Functioning predicted larger percentages of study weeks with continuous abstinence, but only for participants in standard treatment.
TABLE 2.
MicroCog Indices predict Percentage of Study Weeks and Tested Study Weeks with Continuous Opioid Abstinence
| Percentage of Study Weeks with Continuous Opioid Abstinence |
Percentage of Tested Study Weeks with Continuous Opioid Abstinence |
|||||
|---|---|---|---|---|---|---|
| MicroCog Index | Estimate | SE | t | Estimate | SE | t |
| Attention/Mental Control | 0.008 | 0.002 | 4.13**** | 0.009 | 0.002 | 4.60**** |
| Reasoning | 0.007 | 0.002 | 3.72*** | 0.008 | 0.002 | 4.11**** |
| Memory | 0.006 | 0.002 | 2.86** | 0.006 | 0.002 | 3.05** |
| Spatial Processing | 0.004 | 0.002 | 1.76 | 0.005 | 0.002 | 1.91 |
| Reaction Time | 0.015 | 0.002 | 6.76**** | 0.016 | 0.002 | 6.92**** |
| Information Processing Speed | 0.006 | 0.002 | 3.62*** | 0.007 | 0.002 | 3.90*** |
| Information Processing Accuracy | 0.005 | 0.002 | 2.33* | 0.006 | 0.002 | 2.72* |
| General Cognitive Functioning | 0.008 | 0.002 | 3.89**** | 0.009 | 0.002 | 4.34**** |
| General Cognitive Processing | 0.008 | 0.003 | 3.02** | 0.010 | 0.003 | 4.00*** |
p < .0001,
p<0.001,
p <.01,
p <.05
TABLE 3.
Interaction of MicroCog Score by Treatment Condition on Percentage of Study Weeks and Percentage of Tested Study Weeks with Continuous Opioid Abstinence
| Percentage of Study Weeks with Continuous Opioid Abstinence |
Percentage of Tested Study Weeks with Continuous Opioid Abstinence |
|||||
|---|---|---|---|---|---|---|
| MicroCog Index | Estimate | SE | t | Estimate | SE | t |
| Attention/Mental Control | 0.023 | 0.003 | 7.05**** | 0.0217 | 0.003 | 6.66**** |
| Attention/Mental Control by Treatment Group | −0.025 | 0.004 | −6.01**** | −0.021 | 0.004 | −4.95**** |
| Reasoning | 0.011 | 0.003 | 3.60*** | 0.009 | 0.003 | 3.09*** |
| Reasoning by Treatment Group | −0.005 | 0.004 | −1.29 | 0.000 | 0.004 | 0.06 |
| Memory | 0.011 | 0.003 | 3.53*** | −0.010 | 0.003 | 3.26** |
| Memory by Treatment Group | −0.011 | 0.004 | −2.73** | −0.010 | 0.004 | −2.28* |
| Spatial Processing | −0.002 | 0.003 | −0.46 | −0.000 | 0.003 | −0.09 |
| Spatial Processing by Treatment Group | 0.008 | 0.005 | 1.64 | 0.005 | 0.005 | 1.16 |
| Reaction Time | 0.014 | 0.003 | 4.34**** | 0.014 | 0.003 | 4.33**** |
| Reaction Time by Treatment Group | 0.000 | 0.004 | 0.01 | 0.001 | 0.004 | 0.25 |
| Information Processing Speed | 0.005 | 0.003 | 2.02* | 0.005 | 0.003 | 1.86 |
| Information Processing Speed by Treatment Group | 0.001 | 0.003 | 0.41 | 0.003 | 0.003 | 0.95 |
| Information Processing Accuracy | 0.027 | 0.004 | 6.80**** | 0.026 | 0.004 | 6.49**** |
| Information Processing Accuracy by Treatment Group | −0.032 | 0.005 | −6.88**** | −0.030 | 0.005 | −6.23**** |
| General Cognitive Functioning | 0.016 | 0.003 | 5.10**** | 0.016 | 0.003 | 4.75**** |
| General Cognitive Functioning by Treatment Group | −0.016 | 0.004 | −3.71*** | −0.012 | 0.004 | −2.77*** |
| General Cognitive Processing | 0.012 | 0.004 | 2.96** | 0.0124 | 0.004 | 2.95** |
| General Cognitive Processing by Treatment Group | −0.008 | 0.005 | −1.62 | −0.004 | 0.005 | −0.78 |
p < .0001,
p<0.001,
p <.01,
p <.05
Percent of tested weeks with continuous abstinence
When abstinence was examined as percentage of tested weeks with continuous opioid abstinence, all MicroCog Indices again were significant predictors of abstinence except for Spatial Processing, which was marginally significant (see Table 2). Higher cognitive scores predicted higher percentages of tested weeks with continuous abstinence. Regarding differential effects across treatment groups, the MicroCog Indices of Attention/Mental Control, Memory, Information Processing Accuracy and General Cognitive Functioning had significant differential effects across treatment groups (see Table 3). Specifically, these MicroCog Indices were positively associated with abstinence for participants in standard treatment, but there was no relationship between these cognitive scores and abstinence for participants who received the Therapeutic Education System. Higher scores on Attention/Mental Control, Memory, Information Processing Accuracy and General Cognitive Functioning predicted higher percentages of study weeks with continuous abstinence, but only for participants in standard treatment.
Total number of weeks abstinent
Unlike findings for percent of total and tested weeks with continuous abstinence, none of the MicroCog Index scores were significant predictors of total number of weeks of opioid abstinence. In addition, there was no impact of study group (standard versus Therapeutic Education System) on the relationship between MicroCog scores and number of weeks of opioid abstinence.
DISCUSSION
The current study examined cognitive functioning and the impact of cognitive functioning on retention and treatment outcomes in a sample of 160 clients with opioid dependence entering methadone maintenance treatment. Mean levels of cognitive functioning fell in the average and low average ranges for our sample, which is consistent with other studies of substance abusing populations (e.g., Carroll et al., 2011; Aharanovich et al., 2006). Regarding the impact of cognitive functioning on retention and outcomes, our findings were mixed. In the current study, lower general cognitive functioning predicted longer study retention, which is in contrast to other research in similar populations (Aharonovich et al., 2003, 2006, 2008; Carroll et al., 2011). It is possible that the difference in treatment length in the current study compared with other studies that have examined this relationship may account in part for this discrepancy in the impact of cognitive functioning on retention. Specifically, the current study examined retention over a 52-week intervention period, whereas prior studies have examined retention over intervention periods of 8-12 weeks. Perhaps cognitive functioning has a differential impact on retention early in the treatment process versus later in the treatment process. For the current sample of clients in methadone maintenance treatment, the use of a longer intervention period may be more generalizable, as the mean length of methadone maintenance treatment ranges between 1.5 - 4.5 years (e.g., D’Aunno & Vaughn, 1992; Keen et al., 2000; Magura, Nwakeze, & Demsky, 1998). It is important to note that the impact of cognitive functioning on retention in our study was quite small, suggesting that we use caution when extrapolating from this finding.
Regarding the impact of cognitive functioning on opioid abstinence, we found that multiple domains of cognitive functioning significantly predicted abstinence. Significant cognitive predictors included executive functioning (i.e., attention/mental control), memory, reasoning, reaction time, information processing speed, information processing accuracy, and general cognitive functioning. The only cognitive domain that did not emerge as a predictor for abstinence was spatial processing. These findings contribute to the overall body of literature indicating that lower levels of cognitive functioning negatively impact substance abuse treatment outcomes (Cooney, Kadden, Litt, & Getter, 1991; Jaffe et al., 1996). Interestingly, the findings from the current study are the reverse of what has been observed in similar populations. Specifically, the current study demonstrated that lower levels of cognitive functioning did not lead to poorer retention, but did predict poorer treatment outcomes. Prior research in drug abusing populations has generally found that lower levels of cognitive functioning led to poorer treatment engagement/retention, though evidence for a direct impact on outcome is mixed (Aharonovich, Nunes, & Hasin, 2003; Aharonovich et al., 2006, 2008; Bates et al., 2006; Carroll et al., 2011; Passeti et al., 2008). Again, it is possible that the longer treatment period of our study compared to other studies (52 weeks versus 8-12 weeks) allowed the impact of cognitive functioning to emerge or strengthen over time.
Of particular importance, an interaction effect was seen in the current study, whereby lower cognitive scores predicted lower levels of abstinence for participants in standard methadone maintenance treatment, but not for those who received the web-based intervention as part of methadone maintenance treatment. This finding may indicate that technology-based interventions may be useful in minimizing the impact of poor cognitive functioning on treatment outcomes in populations with documented cognitive deficits. This finding is in contrast to prior reports in which lower cognitive functioning negatively impacted treatment outcomes for participants receiving cognitively-based interventions, and compared to more supportive or less cognitively-based approaches (Carroll et al., 2011; Cooney, Kadden, Litt, & Getter, 1991; Jaffe et al., 1996). Surprisingly, the current web-based intervention (i.e., Therapeutic Education System) also is based on a cognitive, skills-based approach. However, participants with lower levels of cognitive functioning who received the web-based intervention did not have worse outcomes than those involved in only standard care. In fact, participants with lower levels of cognitive functioning who received only standard methadone maintenance treatment had poorer treatment outcomes, suggesting a protective effect of the web-based intervention for those with lower levels of cognitive functioning. Perhaps the use of the web-based intervention served to “level the playing field” for these participants. It is possible that the self-paced nature of the intervention allowed participants to incorporate information at their own pace. Or perhaps the fluency learning approach of the web-based intervention (which required individuals to over-learn information presented in the intervention) is a good fit for a population with cognitive impairments. It also is possible that the amount of repeated exposure to the intervention may have allowed persons with lower levels of cognitive functioning to benefit. While it seems likely that exposure to our web-based intervention was able to attenuate the impact of cognitive functioning on treatment outcomes, additional research is needed to identify the potential mechanisms of action.
There are several limitations that should be noted. First, the current study examined a treatment length of 52 weeks, in contrast to the 8-12 weeks reported in most other published literature, which makes comparisons difficult. Therefore, our conclusions should be interpreted with caution. Second, because the only treatment outcome examined in the current study was opioid abstinence, it is difficult to know what the impact of cognitive functioning may have been on a broader array of substance-related, psychosocial, and psychiatric outcomes. Third, the use of a single, computer-administered cognitive assessment may limit the generalizability of our findings. We did not explore other cognitive domains, such as decision making, which have been shown to impact treatment retention and outcomes in substance-abusing populations. Fourth, the impact of withdrawal from illicit opioids and initiation of methadone treatment was not controlled for in the current trial. This may be problematic, as both withdrawal and initiation of methadone treatment may negatively impact cognitive functioning (Darke et al., 2000; Foreman et al., 2004; Mintzer & Stitzer 2002; Mintzer, Copersino, & Stitzer 2005; Zacny 1995). Participants were administered MicroCog early in their treatment episode before they may have been fully stabilized on methadone. One possibility is that cognitive functioning may have improved for participants after they were maintained on methadone for six months, as suggested by emerging research (i.e., Soyka, Zingg, Koller, & Hennig-Fast, 2010), which may have influenced retention and outcomes in the second half of our treatment window. However, preliminary findings from our parent trial do not support evidence of a delayed onset of effects. Finally, we were did not examine or control for psychiatric diagnoses that may have influenced cognitive functioning, such as schizophrenia and depression. Current evidence suggests a complex relationship between substance use and cognitive ability on functioning in persons with dual diagnoses (Potvin, Stavro, & Pelletier, 2012), indicating a need for more careful scrutiny of this issue.
Additional research is needed to clarify what appears to be a complex relationship between cognitive functioning and treatment retention and outcomes in substance-abusing populations; a replication study is needed in order to more fully understand these effects. However, the current study provides promising support that a web-based intervention that is cognitively/skills-based may be beneficial for individuals with varying levels of cognitive proficiency. Thus, it may be beneficial for web-based interventions to be made widely available for substance abusing and other populations.
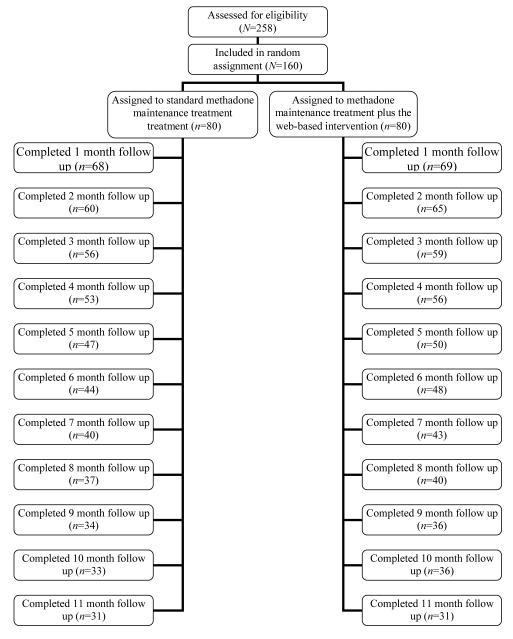
FIGURE 1.
CONSORT Diagram: Flow of participants through the study protocol
ACKNOWLEDGMENTS
The research study reported in this manuscript was supported by National Institute on Drug Abuse (NIH) R01 DA021818 (PI: L. Marsch). The preparation of this manuscript was partially supported by National Institute on Drug Abuse (NIH) P30DA029926 (PI: L. Marsch). Portions of this paper were presented at the Annual Meeting for the College on Problems of Drug Dependence in Palm Springs, CA in June 2012.
APPENDIX.
Description of Subscales Administered in MicroCog Short Form Assessment
Numbers Forward
The participant is shown a sequence of single digits on the computer screen. The participant must enter the digits in correct order using the computer number pad. The number of digits to be recalled varies from 2 to 9.
Wordlist 1
The participant is presented with a list of 16 words, containing five groups of category-related items, which is presented four times. Participants are asked to respond to words that are members of one of four categories and not to respond to words in the fifth category.
Wordlist 2
A list of 36 words (including the 16 from Wordlist 1) is presented and participants must identify which words were previously presented.
Math
Participants are presented with eight mathematical problems including addition, subtraction, multiplication, and division and are asked to type their answer using the keyboard.
Story
The participant is shown a brief story on the screen. After reading the story, the participant is asked 6 multiple choice questions about the story.
Address
The participant is presented with a name and address and asked to memorize this information for later recall.
Clocks
The participant is presented with a series of seven analog clocks and asked to choose which of five digital clocks is the correct match.
Analogies
The participant is shown 11 pairs of verbal analogies and asked to select the best answer from a multiple choice list for each pair.
Timers
The participant is asked to press the Enter key as quickly as possible in response to five auditory, five visual, and five combined auditory/visual signals.
Footnotes
DISCLOSURES In addition to her academic affiliation, Dr. Marsch is affiliated with HealthSim, LLC, the health-promotion software development organization that developed the web-based Therapeutic Education System referenced in this manuscript. Dr. Marsch has worked extensively with her institutions to manage any potential conflict of interest.
No other authors have any financial disclosures to make.
REFERENCES
- Abbott PJ. A review of the community reinforcement approach in the treatment of opioid dependence. Journal of Psychoactive Drugs. 2009;41(4):379–385. doi: 10.1080/02791072.2009.10399776. [DOI] [PubMed] [Google Scholar]
- Aharonovich E, Nunes E, Hasin D. Cognitive impairment, retention and abstinence among cocaine abusers in cognitive-behavioral treatment. Drug and Alcohol Dependence. 2003;71(2):207–211. doi: 10.1016/s0376-8716(03)00092-9. doi:10.1016/S0376-8716(03)00092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug and Alcohol Dependence. 2006;81(3):313–322. doi: 10.1016/j.drugalcdep.2005.08.003. doi:10.1016/j.drugalcdep.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Aharonovich E, Amrhein PC, Bisaga A, Nunes EV, Hasin DS. Cognition, commitment language, and behavioral change among cocaine-dependent patients. Psychology of Addictive Behaviors. 2008;22(4):557–562. doi: 10.1037/a0012971. doi:10.1037/a0012971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DN, Goldstein G, Seaton BE. Cognitive rehabilitation of chronic alcohol abusers. Neuropsychology Review. 1997;7:21–39. doi: 10.1007/BF02876971. doi:10.1007/BF02876971. [DOI] [PubMed] [Google Scholar]
- Ball JC, Ross A. The effectiveness of methadone maintenance treatment: Patients, programs, services and outcomes. Springer-Verlag; New York, NY: 1991. [Google Scholar]
- Bates M, Convit A. Neuropsychology and neuroimaging of alcohol and illicit drug abuse. In: Calev A, editor. The assessment of neuropsychological functions in psychiatric disorders. American Psychiatric Press; Washington, DC: 1999. pp. 373–445. [Google Scholar]
- Bates ME, Labouvie EW, Voelbel G. Individual differences in latent neuropsychological abilities at addictions treatment entry. Psychology of Addictive Behaviors. 2002;16:35–46. doi: 10.1037//0893-164x.16.1.35. doi:10.1037/0893-164X.16.1.35. [DOI] [PubMed] [Google Scholar]
- Bates ME, Barry D, Labouvie EW, Buckman JF, Fals-Stewart W, Voelbel G. Risk factors and neuropsychological recovery in alcohol use disordered clients exposed to different treatments. Journal of Consulting and Clinical Psychology. 2004;72:1073–1080. doi: 10.1037/0022-006X.72.6.1073. doi:10.1037/0022-006X.72.6.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates ME, Voelbel GT, Buckman JF, Labouvie EW, Barry D. Short term neuropsychological recovery clients with substance use disorders. Alcoholism: Clinical and Experimental Research. 2005;29:367–377. doi: 10.1097/01.alc.0000156131.88125.2a. doi: 10.1097/01.ALC.0000156131.88125.2A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates ME, Pawlak AP, Tonigan JS, Buckman JF. Cognitive impairment influences drinking outcome by altering therapeutic mechanisms of change. Psychology of Addictive Behaviors. 2006;20:241–253. doi: 10.1037/0893-164X.20.3.241. doi:10.1037/0893-164X.20.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Marsch LA, Buchhalter A, Badger G. Computerized behavior therapy for opioid dependent outpatients: A randomized, controlled trial. Experimental and Clinical Psychopharmacology. 2008;16:132–143. doi: 10.1037/1064-1297.16.2.132. doi:10.1037/1064-1297.16.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder C. Behavioral fluency: A new paradigm. Educational Technology. 1993 Oct;8:14. [Google Scholar]
- Budney AJ, Higgins ST. Therapy manuals for drug addiction, a community reinforcement plus vouchers approach: Treating cocaine addiction. National Institute on Drug Abuse; Rockville, MD: 1998. [Google Scholar]
- Carroll KM, Kiluk BD, Nich C, Babuscio TA, Brewer JA, Potenza MN, Lejuez CW. Cognitive function and treatment response in a randomized clinical trial of computer-based training for cognitive behavioral therapy (CBT4CBT) Substance Use and Misuse. 2011;46:23–34. doi: 10.3109/10826084.2011.521069. doi:10.3109/10826084.2011.521069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM. Therapy Manuals for Drug Addiction. National Institute of Drug Abuse; Rockville, MD: 1998. A cognitive-behavioral approach: Treating cocaine addiction. [Google Scholar]
- Cooney NL, Kadden RM, Litt MD, Getter H. Matching alcoholics to coping skills or interactional therapies: Two-year follow-up results. Journal of Consulting and Clinical Psychology. 1991;59:598–601. doi: 10.1037//0022-006x.59.4.598. doi:10.1037//0022-006X.59.4.598. [DOI] [PubMed] [Google Scholar]
- Condelli WS, Dunteman GH. Exposure to methadone programs and heroin use. American Journal of Drug and Alcohol Abuse. 1993;19(1):65–78. doi: 10.3109/00952999309002666. doi:10.3109/00952999309002666. [DOI] [PubMed] [Google Scholar]
- D’Aunno T, Vaughn TE. Variations in methadone treatment practices. Results from a national study. JAMA: The Journal of the American Medical Association. 1992;267(2):253–258. [PubMed] [Google Scholar]
- Darke S, Sims J, McDonald S, Wickes W. Cognitive impairment among methadone maintenance patients. Addiction. 2000;95:687–695. doi: 10.1046/j.1360-0443.2000.9556874.x. doi:10.1046/j.1360-0443.2000.9556874.x. [DOI] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden S, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. American Journal of Psychiatry. 2008;165:179–187. doi: 10.1176/appi.ajp.2007.06111851. doi:10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Clark L, London M, Robbins TW, Sahakian BJ. Profile of executive and memory function associated with amphetamine and opiate dependence. Neuropsychopharmacology. 2006;31:1036–1047. doi: 10.1038/sj.npp.1300889. doi:10.1038/sj.npp.1300889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Dougherty GG, Casey BJ, Siegle GJ, Braver TS, Barch DM, Lorensen E. Opiate addicts lack error-dependent activation of rostral anterior cingulate. Biological Psychiatry. 2004;55:531–537. doi: 10.1016/j.biopsych.2003.09.011. doi:10.1016/j.biopsych.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Leskovjan AC, Hoff AL, Hitzemann R, Bashan F, Khalsa SS, Volkow ND. Severity of neuropsychological impairment in drug addiction: Association with metabolism in the brain reward circuit. Neuropsychologia. 2004;42:1447–1458. doi: 10.1016/j.neuropsychologia.2004.04.002. doi:10.1016/j.neuropsychologia.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Grella CE, Hser Y-I, Joshi V, Anglin MD. Patient histories, retention and outcome modules for younger and older adults in DATOS. Drug and Alcohol Dependence. 1999;57:151–166. doi: 10.1016/s0376-8716(99)00082-4. doi:10.1016/S0376-8716(99)00082-4. [DOI] [PubMed] [Google Scholar]
- Grohman K, Fals-Stewart W. Computer-assisted cognitive rehabilitation with substance-abusing patients: Effects on treatment response. The Journal of Cognitive Rehabilitation. 2003;21:2–9. [Google Scholar]
- Higgins ST, Delaney DD, Budney AJ, Bickel WK, Hughes JR, Foerg F, Fenwick JW. A behavioral approach to achieving initial cocaine abstinence. American Journal of Psychiatry. 1991;148:1218–1224. doi: 10.1176/ajp.148.9.1218. [DOI] [PubMed] [Google Scholar]
- Issenberg SB, Gordon MS, Gordon DL, Safford RE, Hart IR. Simulation and new learning technologies. Medical Teacher. 2001;23(1):16–23. doi: 10.1080/01421590020007324. [DOI] [PubMed] [Google Scholar]
- Jaffe AJ, Rounsaville B, Chang G, Schottenfeld RS, Meyer RE, O’Malley SS. Naltrexone, relapse prevention, and supportive therapy with alcoholics: An analysis of patient treatment matching. Journal of Consulting and Clinical Psychology. 1996;64(5):1044–1053. doi: 10.1037//0022-006x.64.5.1044. doi:10.1037/0022-006X.64.5.1044. [DOI] [PubMed] [Google Scholar]
- Keen J, Rowe G, Mathers N, Campbell M, Seivewright N. Can methadone maintenance for heroin-dependent patients retained in general practice reduce criminal conviction rates and time spent in prison? British Journal of General Practice. 2000;50:48–49. [PMC free article] [PubMed] [Google Scholar]
- Lyvers M. “Loss of control” in alcoholism and drug addiction: A neuroscientific interpretation. Experimental and Clinical Psychopharmacology. 2000;8:225–249. doi: 10.1037//1064-1297.8.2.225. doi:10.1037//1064-1297.8.2.225. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: Drug and monetary rewards. Experimental and Clinical Psychopharmacology. 1997;5:256–262. doi: 10.1037//1064-1297.5.3.256. doi:10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- Magura S, Nwakeze PC, Demsky S. Pre- and in-treatment predictors of retention in methadone treatment using survival analysis. Addiction. 1998;93(1):51–60. doi: 10.1046/j.1360-0443.1998.931516.x. doi:10.1046/j.1360-0443.1998.931516.x. [DOI] [PubMed] [Google Scholar]
- Marsch LA. The efficacy of methadone maintenance interventions in reducing illicit opiate use, HIV risk behavior and criminality: A meta-analysis. Addiction. 1998;93(4):515–532. doi: 10.1046/j.1360-0443.1998.9345157.x. doi:10.1046/j.1360-0443.1998.9345157.x. [DOI] [PubMed] [Google Scholar]
- Marsch LA, Grabinski MJ, Bickel WK, Desrosiers A, Guarino H, Muehlbach B, Acosta M. Computer-assisted HIV prevention for youth with substance use disorders. In: Marsch LA, editor. Special Issue on Technology and Substance Use Disorders. Substance Use and Misuse. Vol. 46. 2011. pp. 46–56. doi:10.3109/10826084.2011.521088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Arndt IO, Metzger DS, Woody GE, O’Brien CP. The effects of psychosocial services in substance abuse treatment. JAMA: The Journal of the American Medical Association. 1993;269(15):1953–1959. doi:10.1001/jama.1993.03500150065028. [PubMed] [Google Scholar]
- Mintzer MZ, Copersino ML, Stitzer ML. Opioid abuse and cognitive performance. Drug and Alcohol Dependence. 2005;78:225–230. doi: 10.1016/j.drugalcdep.2004.10.008. doi:10.1016/j.drugalcdep.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Stitzer ML. Cognitive impairment in methadone maintenance patients. Drug and Alcohol Dependence. 2002;67:41–51. doi: 10.1016/s0376-8716(02)00013-3. doi:10.1016/S0376-8716(02)00013-3. [DOI] [PubMed] [Google Scholar]
- Morgenstern J, Bates ME. Effects of executive function impairment on change processes and substance use outcomes in 12-step treatment. Journal of Studies on Alcohol and Drugs. 1999;60(6):846–855. doi: 10.15288/jsa.1999.60.846. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Woody GE, McLellan AT. Enhancing the effectiveness of methadone using psychotherapeutic interventions. NIDA Research Monograph. 1995;150:5–18. [PubMed] [Google Scholar]
- O’Malley S, Adamse M, Heaton RK, Garwin FH. Neuropsychological impairment in chronic cocaine abusers. American Journal of Drug and Alcohol Abuse. 1992;18(2):131–44. doi: 10.3109/00952999208992826. doi:10.3109/00952999208992826. [DOI] [PubMed] [Google Scholar]
- Ornstein TJ, Iddon JL, Baldacchino AM, Sahakian BJ, London M, Everitt BJ, Robbins TW. Profiles of cognitive dysfunction in chronic amphetamine and heroin abusers. Neuropsychopharmacology. 2000;23:113–126. doi: 10.1016/S0893-133X(00)00097-X. doi:10.1016/S0893-133X(00)00097-X. [DOI] [PubMed] [Google Scholar]
- Passetti F, Clark L, Mehta MA, King JM. Neuropsychological predictors of clinical outcome in opiate addiction. Drug and Alcohol Dependence. 2008;94:82–91. doi: 10.1016/j.drugalcdep.2007.10.008. doi:10.1016/j.drugalcdep.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Pirastu R, False R, Messina M, Binio V, Spiga S, Falconieri D, Diana M. Impaired decision-making in opiate-dependent subjects: Effect of pharmacological therapies. Drug and Alcohol Dependence. 2006;83:163–168. doi: 10.1016/j.drugalcdep.2005.11.008. doi:10.1016/j.drugalcdep.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Powell DH, Kaplan EF, Whitla D, Caitlin R, Funkenstein HH. MicroCog: Assessment of cognitive functioning. The Psychological Corporation; San Antonio, TX: 1993. [Google Scholar]
- Rapeli P, Fabritius C, Alho H, Salaspuro M, Wahlbeck K, Kalska H. Methadone vs. buprenorphine/naloxone during early opioid substitution treatment: A naturalistic comparison of cognitive performance relative to healthy controls. BMC Clinical Pharmacology. 2007;7(5) doi: 10.1186/1472-6904-7-5. doi:10.1186/1472-6904-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Robbins TW. Investigating the neurocognitive deficits associated with chronic drug misuse. Current Opinion in Neurobiology. 2001;11:250–257. doi: 10.1016/s0959-4388(00)00204-x. doi:10.1016/S0959-4388(00)00204-X. [DOI] [PubMed] [Google Scholar]
- Roselli M, Ardilla A. Cognitive effects of cocaine and polydrug abuse. Journal of Clinical and Experimental Neuropsychology. 1993;18:122–135. doi: 10.1080/01688639608408268. doi:10.1080/01688639608408268. [DOI] [PubMed] [Google Scholar]
- Smith DE, McCrady BS. Cognitive impairment among alcoholics: Impact of drink refusal skill acquisition and treatment outcome. Addictive Behaviors. 1991;16:265–274. doi: 10.1016/0306-4603(91)90019-e. doi:10.1016/0306-4603(91)90019-E. [DOI] [PubMed] [Google Scholar]
- Soyka M, Zingg C, Koller G, Hennig-Fast K. Cognitive function in short- and long-term substitution treatment: Are there differences? The World Journal of Biological Psychiatry. 2010;11(2):400–408. doi: 10.1080/15622970902995604. doi:10.3109/15622970902995604. [DOI] [PubMed] [Google Scholar]
- Strain EC, Stitzer ML, Liebson IA, Bigelow GE. Dose-response effects of methadone in the treatment of opioid dependence. Annals of Internal Medicine. 1993;119(1):23–27. doi: 10.7326/0003-4819-119-1-199307010-00004. [DOI] [PubMed] [Google Scholar]
- Turner TH, LaRowe S, Horner MD, Herron J, Malcolm R. Measures of cognitive functioning as predictors of treatment outcome for cocaine dependence. Journal of Substance Abuse Treatment. 2009;37:328–334. doi: 10.1016/j.jsat.2009.03.009. doi:10.1016/j.jsat.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Verdejo A, Toribio I, Orozco C, Puente KL, Perez-Garcia M. Neuropsychological functioning in methadone maintenance patients versus abstinent heroin abusers. Drug and Alcohol Dependence. 2005;78:283–288. doi: 10.1016/j.drugalcdep.2004.11.006. doi:10.1016/j.drugalcdep.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain: Insights from imaging studies. The Journal of Clinical Investigation. 2003;111(10):1444–1451. doi: 10.1172/JCI18533. doi:10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacny JP. A review of the effects of opioids on psychomotor and cognitive functioning in humans. Experimental and Clinical Psychopharmacology. 1995;3:432–466. doi:10.1037//1064-1297.3.4.432. [Google Scholar]