Abstract
Reactive oxygen species (ROS) such as superoxide (O2−) and hydrogen peroxide (H2O2) have long been implicated as pro-inflammatory, yet the sources of ROS and the molecular mechanisms by which they enhance inflammation have been less clear. Recent advances in the understanding of the molecular basis of inflammation mediated by the innate immune system have allowed these issues to be revisited. Although the Nox2 NADPH oxidases generate the bulk of ROS for antimicrobial host defense, recent studies have found that NADPH oxidase-dependent ROS production can actually dampen macrophage inflammatory responses to sterile pro-inflammatory stimuli. Instead, production of mitochondrial ROS has emerged as an important factor in both host defense and sterile inflammation. Excess mitochondrial ROS can be generated by either damage to the respiratory chain or by alterations of mitochondrial function such as those that increase membrane potential and reduce respiratory electron carriers. In autoinflammatory diseases, where key components of innate immune responses are activated by genetic mutations or environmental stimuli, inflammation has been found to be particularly sensitive to inhibition of mitochondrial ROS production. These findings have highlighted mitochondrial ROS as a novel generator of pro-inflammatory ROS and a potential therapeutic target in inflammatory diseases.
Keywords: reactive oxygen species, mitochondria, inflammation, metabolism, signaling
Introduction: The dual role of ROS in inflammation
Our understanding of the role of ROS in biology has undergone a transformation over the past decade in parallel with new insights into the roles of ROS in inflammation [1]. Initially, ROS were seen as an unwanted byproduct of metabolism that increased during pathological conditions and led to non-specific oxidative damage to biological molecules. In inflammation, the production of superoxide by NADPH oxidase in the phagosome leads to the formation of further damaging ROS, such as hydrogen peroxide and hypochlorite, which contribute to bacterial killing. This led to a straightforward view of ROS production as essential to bacterial killing, with the potential to damage surrounding cells and tissues if excessive, misdirected or inappropriate activation of inflammatory pathways occurred. However, over the past decade, a more nuanced view of the role of ROS in biology has emerged and it is now clear that ROS, e.g. hydrogen peroxide, can also act as signaling molecules [2, 3]. This redox signaling can occur when a ROS is produced by a metabolic process and then diffuses to a target, such as a transcription factor or enzyme, to alter its activity [4, 5]. A typical scenario would be hydrogen peroxide altering the activity of a target protein through the reversible oxidation of a particular thiol [3]. On ce the redox signal ends, the thiol modification would be reversed by the action of the thioredoxin or glutathione systems. A good example is tyrosine phosphatases that have redox sensitive thiols at their active sites, thus rendering many tyrosine kinase cascades redox sensitive [6]. The level of hydrogen peroxide can be further modulated by the activity of enzymes that degrade hydrogen peroxide, notably peroxiredoxins. With this new view of ROS, it is clear that these molecules could have a dual role in inflammation, by mediating bacterial killing while also acting as a redox signal that can alter the activation and duration of the inflammatory processes. There is now considerable evidence to support such a dual role for ROS in inflammation, particularly in the innate immune response, with a compelling body of data pointing to a specific role for mitochondrial hydrogen peroxide. However, significant questions remain about how the ROS coming from mitochondria are generated and regulated, and how ROS signaling would fit in with the many other pathways that regulate inflammation. To address the dual role of ROS in inflammation, and in particular to unravel the role of mitochondria, here we survey possible sources of ROS that may drive inflammatory immune responses, and discuss recent insights into the role for mitochondrial ROS in these processes (Table 1).
Table 1.
Sources of ROS important for inflammatory responses and host defense
| ROS generating enzyme system | Cellular signaling | Sterile Inflammation | Host Defense |
|---|---|---|---|
| Cytoplasmic | |||
| NADPH oxidases (NOX1-5), DUOX enzymes | Non-apoptotic cell death, NOX3 in STAT1 signaling, NOX5 in ERK signaling, Duox2 in TLR5 signaling | Dampens sterile inflammation | Important for host defense against bacterial pathogens |
| Xanthine Oxidase | Upregulates EGR-1 | Promotes chemokine production | Triggered by rhinovirus infection in epithelial cells |
| Polyamine catabolism (sperimine oxidase) | Induced by TNF and IL-6 | Polyamine-dependent ROS may protect against H. Pylori infection through triggering cell death | |
| Mitochondrial | |||
| Respiratory Chain complexes I, III, others | Enhances MAPK activation, promotes gene expression of components of the NLRP3 inflammasome | Enhances sterile inflammation in setting of TNFR1 mutations and autoinflammatory disease | Antiviral host defense |
NADPH oxidases: Critical for host defense, but also anti-inflammatory
NADPH oxidases are multicomponent enzyme systems that transfer electrons across biological membranes and generate superoxide. Downstream reactions of superoxide produce other ROS, the most significant of which is hydrogen peroxide that arises from the dismutation of superoxide. The enzyme family has seven members: NADPH oxidase (Nox)1, 2, 3, 4 and 5, and dual oxidase (Duox)1 and 2. These proteins share common structural similarities, with NADPH- and FAD-binding sites, and each member is expressed in, although not restricted to, specific tissues. For their activation, the Nox proteins usually require the assembly of additional components, such as p22phox, Noxo2/p47phox and Noxa2/p67phox, the small GTPase Rac, and the modulatory p40phox. The function of the Nox proteins go well beyond their role in host defense, and have been linked to cell proliferation, differentiation and death, regulation of gene expression, posttranslational processing of proteins, and cellular signaling [7, 8].
Through its role in the generation of the respiratory burst in phagocytes, Nox2 has a major role in host defense, although its role extends beyond this function, and Nox2 may even inhibit inflammatory responses to sterile inflammatory stimuli. The heightened susceptibility to a wide variety of infections observed in chronic granulomatous disease (CGD) patients harboring deleterious mutations in the Nox2 gene and other components of the Nox2 enzyme complex confirm its importance in host defense [9]. In addition, Nox2 is involved in the full activation of phagocytes through promoting the cleavage of the extracellular domain of the Mer tyrosine kinase, an integral membrane protein that inhibits inflammatory signaling [10]. A role for Nox2 in promoting TNF-induced inflammatory responses has also been found, particularly in lung epithelial cells [11, 12]. This may be mediated through induction of riboflavin kinase activity by TNF, which would provide more substrate for Nox2 and other flavin-containing enzymes [13].
Paradoxically, anti-inflammatory functions have also been found for NADPH oxidases, particularly Nox2. Mice lacking multiple components of the Nox2 enzymatic complex have exaggerated neutrophil responses in sterile peritonitis [14], and spontaneously develop inflammatory arthritis and demonstrate preferred development of CD11b+Gr -1+ myeloid cells with increased production of pro-inflammatory cytokines [15–17]. Macrophages from patients with CGD can also produce increased amounts pro-inflammatory cytokines [18]. Altered differentiation of myeloid cells and reduced numbers of regulatory T cells have been suggested to account for these effects.
Nox1, 3, 4 and 5 are restricted to particular cell lineages and play roles in tissue development and inflammatory responses. Nox1 is primarily expressed in the colonic epithelium where it regulates enterocyte differentiation [19]. Nox1 is also expressed in mast cells where it has been shown to play a role in promoting the secretion of the chemokine CXCL8/IL-8 in response to the pro-inflammatory cytokine IL-1β [20]. ROS derived from Nox1 also enhance inflammatory pain, as thermal and mechanical hyperalgesia was attenuated in mice lacking Nox1 [21]. Nox1 may also promote inflammation through its involvement in TNF-induced necrosis [22]. Nox4 was originally thought to be expressed exclusively in the kidney, but has since been found in numerous other cell types including fibroblasts, hematopoetic stem cells and macrophages. In macrophages, Nox4 has recently been found to mediate inflammatory signaling by oxidized phospholipids [23] and LPS [24]. Nox4 is also unique in that it does not require activation by Rac1 or other cytosolic Nox subunits besides p22 [25], and it localizes to distinct intracellular membranes, mainly the ER, nucleus and/or mitochondria [26, 27]. There is only limited evidence supporting a role for Nox3 or Nox5 in inflammatory responses. Nox3 has been linked to inflammatory processes in the cochlea where it is primarily expressed, as it was observed that Nox3 couples TRPV1 (transient receptor vanilloid 1) to Stat1-mediated inflammation and hearing loss [23]. Nox5 has been implicated in mediating ERK1/2 activation and growth and inflammatory responses signaling by angiotensin II in endothelial cells [28]. Although primarily thought to be expressed in the thyroid gland, Duox2 has been found to play a pivotal role in inflammatory responses of nasal airway epithelium to the Toll-like receptor 5 (TLR5) agonist flagellin [29].
Oxidases and ROS generation in non-mitochondrial metabolism
In addition to NADPH oxidases, a number of catabolic enzymes with oxidase activity produce ROS as a byproduct of their enzymatic activity, and ROS produced by these enzymes have been linked to inflammatory responses in certain situations. Xanthine oxidase plays an important role in purine degradation, catalyzing the oxidation of hypoxanthine to xanthine, and further oxidation of xanthine to uric acid. Under certain conditions, such as tissue hypoxia [30] or the conversion of retinol to retinoic acid [31], xanthine oxidase can produce significant quantities of superoxide and hydrogen peroxide. Rhinovirus infection of epithelial cells induces activation of xanthine oxidase, leading to superoxide production and NF-κB activation while reduction of xanthine oxidase levels in infected neutrophils decreased production of the pro-inflammatory cytokines CXCL8/IL-8 and CXCL1/Gro-α [32]. ROS derived from xanthine oxidase can also upregulate Egr-1 (early growth response-1), a transcription factor involved in fibrosis and inflammation, via the activation of ERK1/2 in pulmonary artery smooth muscle cells [33]. Oxidative catabolism by spermine oxidase and N1-acetylpolyamine oxidase of spermine and spermidine, two polyamines important for cellular proliferation, generates hydrogen peroxide which can cause DNA damage [34]. Production of ROS by these enzymes is induced by the pro-inflammatory cytokines TNF and IL-6 [35]. How much the ROS produced by these metabolic oxidases contribute to sterile inflammation is not yet clear. Other metabolic oxidases such as the microsomal monoxygenases [36] and monoamine oxidases [37] can produce ROS as a byproduct of their activity, but the role of ROS produced by these sources in acute inflammation is not known.
Mitochondrial production of ROS
Superoxide is generated as a consequence of oxidative phosphorylation due to the ‘leak’ of electrons being transferred by enzyme complexes in the respiratory chain from electron carriers such as NADH to oxygen [38]. The one electron reduction of oxygen by the respiratory chain produces superoxide. This molecule, itself, is not particularly reactive, but it does react with iron sulfur center-containing proteins such as aconitase, as well as nitric oxide to produce the reactive nitrogen species peroxynitrite. Most superoxide produced is efficiently converted to hydrogen peroxide by SOD2 (MnSOD) in the mitochondrial matrix, and by SOD1 (Cu/ZnSOD) in the intermembrane space. Unlike superoxide, H2O2 can diffuse through membranes into the cytoplasm and the extracellular milieu, and thereby can potentially act as a redox signal from mitochondria to the cytosol [39]. In the presence of ferrous or cuprous ions, hydrogen peroxide is converted to the extremely reactive hydroxyl radical, which reacts indiscriminately with protein, lipid and nucleic acid and is thought to be the major source of oxidative damage within mitochondria. A number of systems regulate levels of hydrogen peroxide within mitochondria, the most significant of which is peroxiredoxin 3, which is linked to the thioredoxin system [40]. In addition, mitochondrial glutathione peroxidase-1 also regulates the level of hydrogen peroxide. Similar systems exist in the cytosol to control the level of hydrogen peroxide, and catalase also acts to degrade excess hydrogen peroxide [41]. Thus, the generation and spread of ROS are tightly regulated and controlled, and these systems are important in preserving cell integrity and inhibiting ROS-dependent signaling and cellular damage [42].
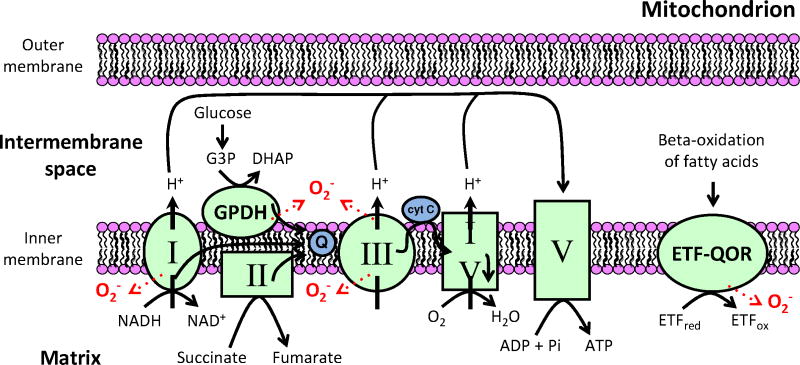
A number of mitochondrial enzyme complexes can produce superoxide [43] (Figure 1). In the respiratory chain, ROS are primarily thought to be byproducts of complex I (NADH dehydrogenase) and complex III (coenzyme Q: cytochrome c – oxidoreductase) activity. When this occurs, ROS produced by complex I are released in the mitochondrial matrix while those of complex III are released in both the matrix and the intermembrane space. However, it is important to bear in mind that there are many other redox active mitochondrial enzymes that can also produce superoxide under various conditions. The mitochondrial inner membrane enzyme glycerol-3-phosphate dehydrogenase (GPDH) is capable of superoxide production during the conversion of glycerol-3-phosphate (G3P) to form dihydroxyacetone phosphate (DHAP), and the electron transferring flavoprotein ubiquinone oxidoreductase (ETF-QOR) can release ROS in the matrix during β-oxidation of fatty acids. Their contribution to the generation of mitochondrial ROS in vivo is uncertain but may be significant [38].
Figure 1. Mitochondrial sources of reactive oxygen species.
In the respiratory chain, complex I (NADH dehydrogenase) and complex III (coenzyme Q: cytochrome c – oxidoreductase) can both produce ROS. ROS produced by complex I are released in the mitochondrial matrix while those of complex III are generated in both the matrix and the intermembrane space. The mitochondrial inner membrane enzyme glycerol-3-phosphate dehydrogenase (GPDH) is also capable of superoxide production during the conversion of glycerol-3-phosphate (G3P) to form dihydroxyacetone phosphate (DHAP). During β-oxidation of fatty acids, the electron transferring flavoprotein ubiquinone oxidoreductase (ETF-QOR) catalyzes the oxidation of electron transferring protein (ETF). During this process, ROS can be released in the matrix.
As the level of mitochondrial ROS production is a potential redox signal in inflammatory conditions, it is important to consider how this might be modified. The rate of superoxide production by the mitochondrial respiratory chain can be altered dramatically by a number of factors [38]. Superoxide production by complexes I and III is increased by conditions that lead to a reduced state of electron carriers such as NADH and CoQ and by a large proton motive force. In particular, complex I can produce large amounts of superoxide by reverse electron transport, which depends critically on a high proton motive force. Consequently, mitochondrial superoxide production is influenced by the metabolic and redox conditions inside the cell. For example, fatty acid oxidation leads to the induction of a more negative reduction potential in a number of mitochondrial redox couples and leads to superoxide production in both isolated mitochondria [44] and intact cells [45]. Superoxide may control its own production through activating uncoupling proteins (UCPs) that allow protons to leak back through the inner mitochondrial membrane and reduce the production of superoxide by the respiratory chain [46]. Thus, metabolic alterations in substrate supply to mitochondria, and potentially other post-translational modification of mitochondrial proteins, could act to modulate mitochondrial superoxide production. Once formed, the superoxide is rapidly converted to hydrogen peroxide by the action of MnSOD. The hydrogen peroxide itself is likely to be a significant redox signal as it can easily diffuse from mitochondria into the cytosol. Altering the activity of matrix peroxiredoxins can, in principle, modulate the level of hydrogen peroxide within mitochondria, and levels within the cytosol can be further altered by the activity of cytosolic peroxidases. Thus, there are several levels of modulation for the production and diffusion of hydrogen peroxide from mitochondria. Once produced, hydrogen peroxide can act to modulate the activity of target proteins by the reversible modification of redox-active cysteine residues that can then be reversed by the action of the thioredoxin and glutathione systems [4, 5].
A newly appreciated role for mitochondrial ROS in immunity and inflammation
Recent evidence has implicated mitochondria and mitochondrial ROS in innate immunity to viruses and bacteria as well as sterile inflammation [47, 48] (Table 2). Antiviral host defense depends on cellular sensors of RNA replication intermediates, such RIG-I, MDA5 and related RNA helicases (RLR proteins). MAVS (also termed IPS-1, CARDIFF, and VISA) is a key mediator of RLR signaling required for activation of type I interferon and NF-κB-mediated antiviral responses. MAVS resides on the mitochondrial outer membrane, and this localization is necessary for its signaling function. Interestingly, depletion of the mitochondrial membrane potential (Δψm) or inhibition of mitochondrial fusion, which depends on intact Δψm, reduces the ability of MAVS to transduce antiviral signals [49]. One of the proteins which may regulate MAVS signaling is NLRX1 (also known as NOD5), a member of the NOD-like receptor family that localizes to the mitochondrial matrix where it interacts with the mitochondrial complex III subunit UQCRC2 [50–52]. Initial reports suggested that NLRX1 was a negative regulator of MAVS signaling, although cells from NLRX1-deficient mice generate normal responses to MDA5 ligands and respond normally to Sendai virus infection [51, 53]. The exact role for NLRX1 and mitochondrial ROS in this pathway of antiviral immunity is still being defined, but mitochondria remain an important integration point for antiviral responses.
Table 2.
Regulators of Mitochondrial Metabolism and ROS production rrelevant to inflammation and host defense
| Regulator | Mechanism | Sterile Inflammation | Host Defense |
|---|---|---|---|
| Mitophagy | Disposes of damaged mitochondria, limits mitochondrial ROS production | Reduces sterile inflammation | Limits antiviral host defense (VSV) |
| Uncoupling mediated by UCP2 | Mechanism unclear, but decreases proton motive force | Limits monocyte cytokine production | Limits host defense (Toxoplasmosis) |
| ECSIT | Ubiquitinated by TRAF6, promotes assembly of complex I and enhances complex I function and ROS production | Host defense against salmonella | |
| TXNIP | Released from Thioredoxin upon oxidation and binds NLRP3 | Promotes NLRP3 inflammasome and IL-1β production |
Another line of evidence supporting a role for mitochondrial ROS in antiviral responses are the consequences of reducing autophagic disposal of mitochondria, a process known as mitophagy. Autophagy-related gene 5 (Atg5) and Atg7 are required for cellular autophagy and mitophagy, and it has been shown that cells lacking Atg5 or Atg7 accumulate dysfunctional mitochondria and have increased mitochondrial ROS production [54]. Treatment with the N-acetyl-L-cysteine (NAC) or the propyl gallate (PG) antioxidants or the complex I respiratory chain inhibitor rotenone reduced type I IFN production by Atg5-deficient cells back to wild type levels, supporting the idea that mitochondrial ROS support the pro-inflammatory effects of reduced autophagy [55]. Interestingly, cells from Atg5-deficient mice display enhanced RIG-I and MAVS dependent type I IFN production and resistance to infection with vesicular stomatitis virus (VSV) [55, 56].
Although mitochondrial UCP2 can mitigate oxidant damage by decreasing mitochondrial proton motive force and superoxide production, mitochondrial superoxide may be important for host defense in some cases, revealing a positive role for mitochondrial ROS. Mice lacking UCP2 have little overt phenotype, but have remarkable resistance to Toxoplasma gondii infection, clearing the chronic brain infection that usually follows acute infection. Cells from UCP2-deficient mice display increased toxoplasmacidal activity and intracellular killing of Salmonella enterica and Listeria monocytogenes. These enhanced antibacterial responses may result from increased mitochondrial ROS production, as cells from UCP2-deficient mice have higher baseline mitochondrial ROS levels [57], heightened activation of ROS-sensitive NF-κB and MAPK signaling pathways, and increased production of pro-inflammatory cytokines in response to TLR stimuli [58, 59]. Thus, the potential therapeutic benefits of blocking UCP2 function to enhance immunity may be balanced by the increased oxidative damage seen in UCP2-deficient mice.
Some aspects of signaling by TLRs and host defense previously ascribed to NADPH oxidases may actually be mediated through mitochondrial ROS. Stimulation of TLR1, TLR2, or TLR4 promotes TRAF6 translocation to the mitochondria. Once in the mitochondria, TRAF6 catalyzes ubiquitinylation of ECSIT (Evolutionarily Conserved Signaling Intermediate in Toll pathways). In the mitochondria, ECSIT associates with and stabilizes NDUFAF1, a component of complex I. This promotes assembly of complex I of the respiratory chain and enhances mitochondrial ATP production and ROS production in proximity to phagosomes. ECSIT- or TRAF6- deficient macrophages produce less ROS when stimulated by TLRs and have diminished ability to kill Salmonella [60–62].
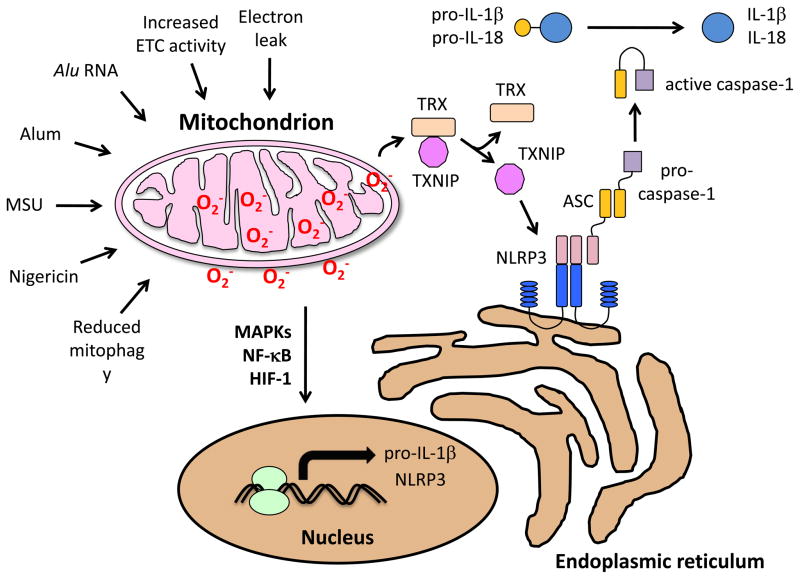
Mitochondrial ROS also play a role sterile inflammation mediated by the cytokine IL-1β(Figure 2). The NLRP3 inflammasome is a cytosolic protein complex which processes IL-1β into a form which can be secreted from the cell and promote acute inflammation. Triggering of the NLRP3 inflammasome is independent of Nox2, as peripheral blood monocytes from patients with CGD actually mount higher production of IL-1β than cells from control subjects [18]. Inhibiting mitophagy with oxidative phosphorylation inhibitors results in increased mitochondrial ROS production as well as increased NLRP3-dependent caspase 1 activation and increased IL-1β and IL-18 secretion in macrophages [63, 64]. Additionally, cells deficient in oxidative phosphorylation or treated with mitochondrial antioxidants show decreased responses to NLRP3 activating stimuli. It has been suggested that because NLRP3 and its adaptor ASC (apoptosis-associated speck-like protein containing a CARD) translocate to mitochondria-associated ER membranes after exposure to inflammasome activators, localization close to mitochondrial ROS may facilitate NLRP3 inflammasome activation [64]. Monosodium urate crystals (MSU), the alum adjuvant, and the ionophore nigericin, all of which are inflammasome activators, cause NLRP3 association with the mitochondria [65]. Alu RNA (noncoding RNAs expressed by the Alu retrotransposon) leads to increased mitochondrial ROS resulting in cytotoxicity through TLR-independent inflammasome activation [66]. Additionally, fatty acids, such as palmitate, both activate the inflammasome and cause mitochondrial ROS accumulation, further suggesting a link between the two [67].
Figure 2. Mitochondrial ROS and priming of the inflammasome.
Different molecular signals and cellular processes can affect the mitochondria, leading to increased ROS production and subsequent priming of the inflammasome. The electron transport chain itself is the main source of mitochondrial ROS, either via increased ETC activity or through electron leak. Alu RNA (noncoding RNAs expressed by the Alu retrotransposon), reduced mitophagy, MSU (monosodium urate crystals), alum (an adjuvant), and nigericin (a microbial toxin) cause NLRP3 association with the mitochondria. Mitochondrial ROS, once produced, leads to inflammasome priming through a variety of pathways, such as sustained MAP kinase activity, increased stability and accumulation of HIF-1, as well as NF-kB activation. Oxidative stress also impacts NLRP3 inflammasome activation through thioredoxin-interacting protein (TXNIP) by causing its release from thioredoxin (TRX), allowing it to bind to NLRP3 and trigger inflammasome assembly.
Mitochondrial ROS, once produced, leads to inflammasome priming through a variety of pathways. One such example is the inactivation of MAPK phosphatases, leading to sustained MAP kinase activity [68]. Increased ROS during hypoxia or stimulation during normoxia can lead to increased stability and accumulation of hypoxia inducible factor 1 (HIF-1) by preventing its degradation [69, 70]. The activation of these proteins, as well as ROS-induced NF-κB activation leads to transcription of pro-IL-1β and NLRP3 [71].
Thioredoxin-interacting protein (TXNIP) may also be able to directly activate the NLRP3 inflammasome. Functional ATP synthesis from oxidative phosphorylation is required for normal TXNIP mRNA and protein levels [72]. Upon oxidative stress, TXNIP was found to be released from oxidized thioredoxin (TRX) and bind to the leucine-rich region of NLRP3, triggering inflammasome assembly [63]. Upregulation of TXNIP by the mitochondrial by ceramide has been shown to lead to induction of p38 and JNK MAPK activation via the TXNIP-ASK1 pathway [73], thus indirectly increasing inflammasome activation. Although it was originally thought that ROS activated NLRP3-dependent processing of IL-1β directly, more recent data suggests that ROS enhance the transcription of genes encoding IL-1β and components of the inflammasome such as caspase-1 and NALP3. Anti-oxidants added after this priming step are much less effective in blocking IL-1 processing [74].
Another line of evidence supporting a role for mitochondrial ROS in acute inflammatory responses comes from studies of the autoinflammatory disease TRAPS (TNFR1-associated periodic syndrome). This autosomal dominant disease is associated with episodes of fevers lasting one week or longer and inflammation in the eyes, skin subcutaneous tissues, joints, and serosal surfaces. As in other chronic inflammatory conditions, inflammation-associated serum proteins such as SAP (serum amyloid P-component) can precipitate in organs and cause damage due to amyloidosis. TRAPS is associated with mutations in TNF-receptor 1 (TNFR1), the receptor that transduces the bulk of the inflammatory effects of the cytokine TNF [75]. The mutant TNFR1 protein in TRAPS is generally retained in the endoplasmic reticulum due to defective folding of the extracellular TNF-binding domain, but it is still able to signal through a functional cytoplasmic domain. Cells from TRAPS patients and knock-in mice harboring TRAPS-associated mutations in TNFR1 have increased activation of p38 and JNK MAP kinases, and increased production of IL-1β, as well as TNF and IL-6, other prototypical inflammatory cytokines, in response to LPS and other TLR-activating stimuli. The enhanced MAP kinase activation seen in cells harboring TRAPS-associated TNFR1 mutations likely depends on ROS. In normal cells, TNF induces sustained JNK and p38 activation, likely because MAP kinase phosphatases are inactivated by ROS [68]. ROS indirectly lead to JNK activation (especially when NF-κB is inactive), and antioxidants have been shown to block this activation. Furthermore, ROS-associated cell death in neurons has been rescued by overexpression of MAPK phosphatases [76]. As with studies of NLRP3 inflammasome activation, production of cytokines in TRAPS was independent of NADPH oxidases, and enhancement was at the level of transcription of cytokine genes, rather than inflammasome-dependent processing of IL-1β. Elevated levels of mitochondrial ROS were seen in cells from TRAPS patients and mice with engineered TRAPS-associated TNFR1 mutations [77]. MitoQ, a mitochondria-targeted antioxidant [78], reduced inflammatory cytokine production in TNFR1 mutant, and to a lesser extent, normal monocytes. Unlike cells in which mitophagy is blocked and ROS leak from a damaged respiratory chain, cells harboring TNFR1 mutations have increased oxygen consumption and respiratory reserve. However, the mechanism by which the increase in respiration could be associated with an increase in ROS production is unclear. Therefore, there is considerable evidence for an increase in mitochondrial ROS production associated with the establishment and maintenance of the inflammatory state that is quite distinct from the utilization of ROS in bacterial killing. This is likely to be a redox signaling modification. However, there remain considerable uncertainties about the mechanisms that regulate this putative redox signal, the cytosolic and nuclear targets that are modified by the mitochondrial ROS, and how these modifications contribute to the pro-inflammatory state.
Interplay between mitochondrial ROS and cellular metabolism and signaling
Mitochondrial ATP production and ROS production are highly dependent on the metabolic state of the cell, and conversely, mitochondrial ROS can influence cellular metabolism. Immune cells are highly adapted to the environments in which they circulate and home to during inflammatory responses, and they differ in their basal metabolic states and responses to activating stimuli. In general, myeloid cells tend to rely on glycolysis to produce ATP, while resting lymphocytes rely on oxidative phosphorylation and switch to aerobic glycolysis upon activation [79]. Myeloid cells, such as neutrophils and monocytes, are recruited from the bloodstream to inflammatory sites by chemotaxis, an energetically demanding process that requires large amounts of ATP hydrolysis and rapid actin turnover [80]. Glycolytic metabolism allows the myeloid cells to perform these functions in the hypoxic environment of inflamed and edematous tissue [81, 82]. Neutrophils are pre-adapted to hypoxic environments, with few mitochondria [83, 84]. In neutrophils, mitochondrial membrane potential is maintained via the glycerol-3-phosphate shuttle in order to regulate aerobic glycolysis instead of producing energy [85]. Macrophages, dendritic cells, and lymphocytes adapt their metabolic activity to environmental cues, including hypoxia.
The hypoxia inducible factor-1 is one of the principal mediators of metabolic adaptation to low concentrations of oxygen. It has emerged as an essential regulator of the survival and functions of myeloid cells in the inflammatory microenvironment [86, 87]. HIF-1 is a transcription factor complex that regulates many genes whose products improve cell survival during oxygen deprivation. Under normoxic conditions, prolyl hydroxylation of HIF-1 by prolyl hydroxylase domain proteins (PHD) leads to its recognition and ubiquitin-mediated degradation via the Von-Hippel Lindau (VHL) protein [88]. However, during hypoxia, HIF-1 is stabilized due to decreased prolyl hydroxylation, and its transcriptional activation is induced [89]. HIF-1 upregulates expression of glycolytic enzymes and downregulates expression of ETC proteins, mediating a physiological glycolytic switch in response to hypoxia. The PHD proteins are also negatively regulated by the TCA cycle intermediates α-ketoglutarate, succinate, fumarate, and malate, with α-ketoglutarate acting as a substrate for the hydroxylase and the other compounds acting as inhibitors, suggesting that HIF-1 activity also constrained by TCA cycle activity [90]. The burst of mitochondrial ROS that is generated at complex III following hypoxia may be important in stabilizing HIF-1, as cells without functional mitochondria (ρ0), treated with ETC inhibitors such as rotenone and myxothiazol, or deficient in ETC components such as cytochrome c fail to stabilize HIF-1, and DNA binding following hypoxia [69, 70].
Macrophages lacking the α subunit of HIF-1 fail to switch to glycolysis after exposure to hypoxic conditions and have metabolic defects even under normoxic conditions, such as reduced ATP and profound deficiencies in aggregation, motility, invasiveness and bacterial killing [86]. These results suggested that HIF-1α can be induced by other stimuli besides hypoxia. Indeed, it has been found that HIF-1α activity is induced by exposure to bacteria [91] and TLR ligands [92] and that it is required for nitric oxide and TNF production in response to these stimuli [91]. LPS stimulation in macrophages can lead to induction of active HIF-1 under normoxic conditions resulting in expression of genes usually only transcribed during hypoxia [93]. This has implications for HIF-1 in inflammation as shown by the increased expression of HIF-1 in macrophages in rheumatoid synovium [94].
Dendritic cells can also modify their metabolism upon activation. It was recently observed that TLR agonists stimulate a metabolic transition to aerobic glycolysis in DCs, a conversion essential for maturation and function [95]. Nitric oxide may be a key player in this switch to glycolysis by acting on components of the ETC to inhibit oxidative phosphorylation. The glycolytic switch acts to maintain ATP levels and cellular survival of DC’s in vivo when oxidative phosphorylation is inhibited by nitric oxide produced as part of the inflammatory response [96].
In contrast to monocytes, naïve resting lymphocytes primarily use oxidative phosphorylation to generate ATP through catabolism of glucose, lipids and amino acids. Without external cues from cytokines or other growth factors, resting T cells internalize and degrade glucose transporter 1 and cannot efficiently use glucose to maintain viability [97]. Upon stimulation by extrinsic signals, e.g. cytokines, hormones, growth factors or TCR stimulation, lymphocyte metabolism switches to glycolysis, even in the presence of oxygen (a phenomenon known as aerobic glycolysis or the Warburg effect). This switch supports the metabolic demand of rapidly dividing cells [98]. Growth factors such as IL-4 and IL-7, as well as CD28 co-stimulation, increase the expression of glucose transporters on the cell surface and regulate the catabolism of glucose [99, 100]. B lymphocytes increase glycolysis through phosphatidylinositol 3-kinase activity following BCR crosslinking while co-engagement of the BCR and FcγRIIB inhibits their glucose utilization [101]. Failure to increase glucose metabolism during lymphocyte activation prevents cell proliferation [102].
Another protein that connects immune cell signal transduction to mitochondrial function is signal transducer and activator of transcription 3 (STAT3). The STAT family proteins are transcription factors involved in many cellular events following stimulation by cytokines, growth factors, and hormones. STAT3 mediates T cell differentiation in response to STAT3-activating cytokines such as IL-6, IL-10 and IL-21 [103], and it is required for development of IL-17-secreting CD4+ T cells in response to IL-6 [104]. In a function distinct from its transcription factor activity, STAT3 has recently been shown to be necessary for normal mitochondrial ETC activity and mitochondrial adaptation to external stimuli. When phosphorylated on serine727, STAT3 translocates to mitochondria and interacts with GRIM-19, a component of ETC complex I. Loss of STAT3 or STAT3 serine phosphorylation led to reduction of cellular ATP and mitochondrial respiration [105, 106]. What functions mitochondrial STAT3 controls in immune cells is not yet clear, but a recent report implicated mitochondrial translocation of STAT3 in the ROS produced during TNF-induced necrotic cell death [107], potentially implicating STAT3 in modulating mitochondrial function in response to pro-inflammatory stimuli.
Mitochondrial ROS as a therapeutic target in inflammatory disorders
Recent findings underscoring the importance of mitochondrial ROS production in inflammation open up opportunities for therapies targeting mitochondrial ROS or the pathways that enhance mitochondrial ROS production. In diseases related to excess NLRP3 activation and IL-1β production, such as genetic syndromes associated with NLRP3 mutations, or more common conditions, such as gout, in which uric acid crystals trigger NLRP3-dependent inflammation, blockade of IL-1β with antibodies or soluble IL-1β receptor antagonist has proved highly successful at rapidly and robustly reducing symptoms and organ damage [108–110]. However, in diseases such as TRAPS and rheumatoid arthritis, where the spectrum of abnormally-produced cytokines is wider, developing therapies designed to decrease mitochondrial ROS may be an important adjunctive therapy in addition to the blockade of TNF or IL-1β, neither of which is fully successful in preventing inflammatory symptoms [111]. Over the past decade, a number of antioxidants targeted to mitochondria have been developed and have proven useful in reducing mitochondrial damage in a wide range of animal models of human pathologies [112, 113]. Among these antioxidants, MitoQ has been shown to reduce organ damage in a range of pathologies associated with inflammation, including LPS-induced septic shock and reduced renal amyloidosis in a model of type I diabetes [114, 115] and has proven protective in an in vitro model of TRAPS [77]. MitoQ has been used safely as an oral therapy in two Phase II human trials and has been well tolerated at an oral dose of 40 mg/day MitoQ for up to at least one year [116] and was also shown to reduce serum liver enzymes, which are surrogates for liver damage, during chronic hepatitis C infection [117]. These studies suggest that testing mitochondria-targeted antioxidants in inflammatory diseases is a worthwhile goal.
Conclusion
The role of ROS in inflammation has expanded considerably from the initial role as a means of killing bacteria to a realization that they may also be important redox signals in maintaining and regulating the inflammatory response. In parallel with this have come multiple findings that point to a role for mitochondria and mitochondrial ROS in the assembly of the inflammasome. This has led to the suggestion that novel therapies designed to decrease mitochondrial ROS production and oxidative damage may provide new opportunities to intervene in the many diseases associated with an elevated or inappropriate inflammatory response. However, there are many major gaps in our knowledge of the mechanisms and targets of mitochondrial ROS production in inflammation, and this is currently an area of intense interest. As the role of mitochondrial ROS in inflammation becomes better understood, it will be particularly interesting to determine whether mitochondrial ROS play a role in inflammatory or inflammation-associated conditions such as multiple sclerosis, rheumatoid arthritis, and premature cardiovascular disease and whether therapies targeted at mitochondrial ROS can impact on them.
Highlights.
In addition to host defense and cellular damage, ROS can act as signaling molecules
Mitochondrial ROS production is dependent on the metabolic state of the cell
Mitochondrial ROS can enhance innate immunity and sterile inflammation
Mitochondrial ROS are potential therapeutic targets in inflammatory disorders.
Footnotes
Conflict of Interest
MPM holds shares in Antipodean Pharmaceuticals Inc., which is developing mitochondria-targeted antioxidants as pharmaceuticals.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murphy MP, Holmgren A, Larsson NG, Halliwell B, Chang CJ, Kalyanaraman B, et al. Unraveling the biological roles of reactive oxygen species. Cell Metab. 2011;13:361–366. doi: 10.1016/j.cmet.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finkel T. Signal transduction by reactive oxygen species. The Journal of cell biology. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janssen-Heininger YM, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, et al. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free radical biology & medicine. 2008;45:1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy MP. Mitochondrial thiols in antioxidant protection and redox signaling: distinct roles for glutathionylation and other thiol modifications. Antioxid Redox Signal. 2012;16:476–495. doi: 10.1089/ars.2011.4289. [DOI] [PubMed] [Google Scholar]
- 5.Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 6.Boivin B, Yang M, Tonks NK. Targeting the reversibly oxidized protein tyrosine phosphatase superfamily. Science signaling. 2010;3:pl2. doi: 10.1126/scisignal.3137pl2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 8.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 9.Roos D, Kuhns DB, Maddalena A, Roesler J, Lopez JA, Ariga T, et al. Hematologically important mutations: X-linked chronic granulomatous disease (third update) Blood Cells Mol Dis. 2010;45:246–265. doi: 10.1016/j.bcmd.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thorp E, Vaisar T, Subramanian M, Mautner L, Blobel C, Tabas I. Shedding of the Mer tyrosine kinase receptor is mediated by ADAM17 protein through a pathway involving reactive oxygen species, protein kinase Cdelta, and p38 mitogen-activated protein kinase (MAPK) J Biol Chem. 2011;286:33335–33344. doi: 10.1074/jbc.M111.263020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Q, Spencer NY, Oakley FD, Buettner GR, Engelhardt JF. Endosomal Nox2 facilitates redox-dependent induction of NF-kappaB by TNF-alpha. Antioxid Redox Signal. 2009;11:1249–1263. doi: 10.1089/ars.2008.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang WJ, Wei H, Tien YT, Frei B. Genetic ablation of phagocytic NADPH oxidase in mice limits TNFalpha-induced inflammation in the lungs but not other tissues. Free Radic Biol Med. 2011;50:1517–1525. doi: 10.1016/j.freeradbiomed.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yazdanpanah B, Wiegmann K, Tchikov V, Krut O, Pongratz C, Schramm M, et al. Riboflavin kinase couples TNF receptor 1 to NADPH oxidase. Nature. 2009;460:1159–1163. doi: 10.1038/nature08206. [DOI] [PubMed] [Google Scholar]
- 14.Pollock JD, Williams DA, Gifford MA, Li LL, Du X, Fisherman J, et al. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 1995;9:202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- 15.Lee K, Won HY, Bae MA, Hong JH, Hwang ES. Spontaneous and aging-dependent development of arthritis in NADPH oxidase 2 deficiency through altered differentiation of CD11b+ and Th/Treg cells. Proc Natl Acad Sci U S A. 2011;108:9548–9553. doi: 10.1073/pnas.1012645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van de Loo FA, Bennink MB, Arntz OJ, Smeets RL, Lubberts E, Joosten LA, et al. Deficiency of NADPH oxidase components p47phox and gp91phox caused granulomatous synovitis and increased connective tissue destruction in experimental arthritis models. Am J Pathol. 2003;163:1525–1537. doi: 10.1016/S0002-9440(10)63509-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hultqvist M, Olofsson P, Holmberg J, Backstrom BT, Tordsson J, Holmdahl R. Enhanced autoimmunity, arthritis, and encephalomyelitis in mice with a reduced oxidative burst due to a mutation in the Ncf1 gene. Proc Natl Acad Sci U S A. 2004;101:12646–12651. doi: 10.1073/pnas.0403831101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van de Veerdonk FL, Smeekens SP, Joosten LA, Kullberg BJ, Dinarello CA, van der Meer JW, et al. Reactive oxygen species-independent activation of the IL-1beta inflammasome in cells from patients with chronic granulomatous disease. Proc Natl Acad Sci U S A. 2010;107:3030–3033. doi: 10.1073/pnas.0914795107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coant N, Ben Mkaddem S, Pedruzzi E, Guichard C, Treton X, Ducroc R, et al. NADPH oxidase 1 modulates WNT and NOTCH1 signaling to control the fate of proliferative progenitor cells in the colon. Mol Cell Biol. 2010;30:2636–2650. doi: 10.1128/MCB.01194-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim GY, Lee JW, Ryu HC, Wei JD, Seong CM, Kim JH. Proinflammatory cytokine IL-1beta stimulates IL-8 synthesis in mast cells via a leukotriene B4 receptor 2-linked pathway, contributing to angiogenesis. J Immunol. 2010;184:3946–3954. doi: 10.4049/jimmunol.0901735. [DOI] [PubMed] [Google Scholar]
- 21.Ibi M, Matsuno K, Shiba D, Katsuyama M, Iwata K, Kakehi T, et al. Reactive oxygen species derived from NOX1/NADPH oxidase enhance inflammatory pain. J Neurosci. 2008;28:9486–9494. doi: 10.1523/JNEUROSCI.1857-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim YS, Morgan MJ, Choksi S, Liu ZG. TNF-induced activation of the Nox1 NADPH oxidase and its role in the induction of necrotic cell death. Mol Cell. 2007;26:675–687. doi: 10.1016/j.molcel.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 23.Lee S, Gharavi NM, Honda H, Chang I, Kim B, Jen N, et al. A role for NADPH oxidase 4 in the activation of vascular endothelial cells by oxidized phospholipids. Free Radic Biol Med. 2009;47:145–151. doi: 10.1016/j.freeradbiomed.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ngkelo A, Meja K, Yeadon M, Adcock I, Kirkham PA. LPS induced inflammatory responses in human peripheral blood mononuclear cells is mediated through NOX4 and Gialpha dependent PI-3kinase signalling. J Inflamm (Lond) 2012;9:1. doi: 10.1186/1476-9255-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rada B, Leto TL. Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. Contributions to microbiology. 2008;15:164–187. doi: 10.1159/000136357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Buul JD, Fernandez-Borja M, Anthony EC, Hordijk PL. Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid Redox Signal. 2005;7:308–317. doi: 10.1089/ars.2005.7.308. [DOI] [PubMed] [Google Scholar]
- 27.Ago T, Kuroda J, Pain J, Fu C, Li H, Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circulation research. 2010;106:1253–1264. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montezano AC, Burger D, Paravicini TM, Chignalia AZ, Yusuf H, Almasri M, et al. Nicotinamide adenine dinucleotide phosphate reduced oxidase 5 (Nox5) regulation by angiotensin II and endothelin-1 is mediated via calcium/calmodulin-dependent, rac-1-independent pathways in human endothelial cells. Circ Res. 2010;106:1363–1373. doi: 10.1161/CIRCRESAHA.109.216036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joo JH, Ryu JH, Kim CH, Kim HJ, Suh MS, Kim JO, et al. Dual oxidase 2 is essential for the toll-like receptor 5-mediated inflammatory response in airway mucosa. Antioxid Redox Signal. 2011;16:57–70. doi: 10.1089/ars.2011.3898. [DOI] [PubMed] [Google Scholar]
- 30.Poss WB, Huecksteadt TP, Panus PC, Freeman BA, Hoidal JR. Regulation of xanthine dehydrogenase and xanthine oxidase activity by hypoxia. The American journal of physiology. 1996;270:L941–946. doi: 10.1152/ajplung.1996.270.6.L941. [DOI] [PubMed] [Google Scholar]
- 31.Zanotto-Filho A, Schroder R, Moreira JC. Differential effects of retinol and retinoic acid on cell proliferation: a role for reactive species and redox-dependent mechanisms in retinol supplementation. Free radical research. 2008;42:778–788. doi: 10.1080/10715760802385702. [DOI] [PubMed] [Google Scholar]
- 32.Papi A, Contoli M, Gasparini P, Bristot L, Edwards MR, Chicca M, et al. Role of xanthine oxidase activation and reduced glutathione depletion in rhinovirus induction of inflammation in respiratory epithelial cells. The Journal of biological chemistry. 2008;283:28595–28606. doi: 10.1074/jbc.M805766200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hartney T, Birari R, Venkataraman S, Villegas L, Martinez M, Black SM, et al. Xanthine oxidase-derived ROS upregulate Egr-1 via ERK1/2 in PA smooth muscle cells; model to test impact of extracellular ROS in chronic hypoxia. PloS one. 2011;6:e27531. doi: 10.1371/journal.pone.0027531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Casero RA., Jr Mammalian polyamine catabolism: a therapeutic target, a pathological problem, or both? J Biochem. 2006;139:17–25. doi: 10.1093/jb/mvj021. [DOI] [PubMed] [Google Scholar]
- 35.Babbar N, Casero RA., Jr Tumor necrosis factor-alpha increases reactive oxygen species by inducing spermine oxidase in human lung epithelial cells: a potential mechanism for inflammation-induced carcinogenesis. Cancer research. 2006;66:11125–11130. doi: 10.1158/0008-5472.CAN-06-3174. [DOI] [PubMed] [Google Scholar]
- 36.Zangar RC, Davydov DR, Verma S. Mechanisms that regulate production of reactive oxygen species by cytochrome P450. Toxicol Appl Pharmacol. 2004;199:316–331. doi: 10.1016/j.taap.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 37.Di Lisa F, Kaludercic N, Carpi A, Menabo R, Giorgio M. Mitochondrial pathways for ROS formation and myocardial injury: the relevance of p66(Shc) and monoamine oxidase. Basic Res Cardiol. 2009;104:131–139. doi: 10.1007/s00395-009-0008-4. [DOI] [PubMed] [Google Scholar]
- 38.Murphy MP. How mitochondria produce reactive oxygen species. The Biochemical journal. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Collins Y, Chouchani ET, James AM, Menger KE, Cocheme HM, Murphy MP. Mitochondrial redox signalling at a glance. Journal of cell science. 2012;125:801–806. doi: 10.1242/jcs.098475. [DOI] [PubMed] [Google Scholar]
- 40.Cao Z, Lindsay JG, Isaacs NW. Mitochondrial peroxiredoxins. Subcell Biochem. 2007;44:295–315. doi: 10.1007/978-1-4020-6051-9_14. [DOI] [PubMed] [Google Scholar]
- 41.Lubos E, Loscalzo J, Handy DE. Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2011;15:1957–1997. doi: 10.1089/ars.2010.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marchi S, Giorgi C, Suski JM, Agnoletto C, Bononi A, Bonora M, et al. Mitochondria-ros crosstalk in the control of cell death and aging. J Signal Transduct. 2012;2012:329635. doi: 10.1155/2012/329635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brand MD. The sites and topology of mitochondrial superoxide production. Exp Gerontol. 2010;45:466–472. doi: 10.1016/j.exger.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. The Journal of biological chemistry. 2002;277:44784–44790. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- 45.Yamagishi SI, Edelstein D, Du XL, Kaneda Y, Guzman M, Brownlee M. Leptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase A. The Journal of biological chemistry. 2001;276:25096–25100. doi: 10.1074/jbc.M007383200. [DOI] [PubMed] [Google Scholar]
- 46.Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA, et al. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002;415:96–99. doi: 10.1038/415096a. [DOI] [PubMed] [Google Scholar]
- 47.West AP, Shadel GS, Ghosh S. Mitochondria in innate immune responses. Nature reviews Immunology. 2011;11:389–402. doi: 10.1038/nri2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naik E, Dixit VM. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. The Journal of Experimental Medicine. 2011;208:417–420. doi: 10.1084/jem.20110367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koshiba T, Yasukawa K, Yanagi Y, Kawabata S. Mitochondrial membrane potential is required for MAVS-mediated antiviral signaling. Science signaling. 2011;4:ra7. doi: 10.1126/scisignal.2001147. [DOI] [PubMed] [Google Scholar]
- 50.Arnoult D, Soares F, Tattoli I, Castanier C, Philpott DJ, Girardin SE. An N-terminal addressing sequence targets NLRX1 to the mitochondrial matrix. Journal of cell science. 2009;122:3161–3168. doi: 10.1242/jcs.051193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moore CB, Bergstralh DT, Duncan JA, Lei Y, Morrison TE, Zimmermann AG, et al. NLRX1 is a regulator of mitochondrial antiviral immunity. Nature. 2008;451:573–577. doi: 10.1038/nature06501. [DOI] [PubMed] [Google Scholar]
- 52.Tattoli I, Carneiro LA, Jehanno M, Magalhaes JG, Shu Y, Philpott DJ, et al. NLRX1 is a mitochondrial NOD-like receptor that amplifies NF-kappaB and JNK pathways by inducing reactive oxygen species production. EMBO Rep. 2008;9:293–300. doi: 10.1038/sj.embor.7401161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rebsamen M, Vazquez J, Tardivel A, Guarda G, Curran J, Tschopp J. NLRX1/NOD5 deficiency does not affect MAVS signalling. Cell death and differentiation. 2011;18:1387. doi: 10.1038/cdd.2011.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu JJ, Quijano C, Chen E, Liu H, Cao L, Fergusson MM, et al. Mitochondrial dysfunction and oxidative stress mediate the physiological impairment induced by the disruption of autophagy. Aging. 2009;1:425–437. doi: 10.18632/aging.100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tal MC, Sasai M, Lee HK, Yordy B, Shadel GS, Iwasaki A. Absence of autophagy results in reactive oxygen species-dependent amplification of RLR signaling. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:2770–2775. doi: 10.1073/pnas.0807694106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jounai N, Takeshita F, Kobiyama K, Sawano A, Miyawaki A, Xin KQ, et al. The Atg5 Atg12 conjugate associates with innate antiviral immune responses. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:14050–14055. doi: 10.1073/pnas.0704014104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rousset S, Emre Y, Join-Lambert O, Hurtaud C, Ricquier D, Cassard-Doulcier AM. The uncoupling protein 2 modulates the cytokine balance in innate immunity. Cytokine. 2006;35:135–142. doi: 10.1016/j.cyto.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 58.Emre Y, Hurtaud C, Nubel T, Criscuolo F, Ricquier D, Cassard-Doulcier AM. Mitochondria contribute to LPS-induced MAPK activation via uncoupling protein UCP2 in macrophages. The Biochemical journal. 2007;402:271–278. doi: 10.1042/BJ20061430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nishio K, Qiao S, Yamashita H. Characterization of the differential expression of uncoupling protein 2 and ROS production in differentiated mouse macrophage-cells (Mm1) and the progenitor cells (M1) J Mol Histol. 2005;36:35–44. doi: 10.1007/s10735-004-2915-x. [DOI] [PubMed] [Google Scholar]
- 60.Kopp E, Medzhitov R, Carothers J, Xiao C, Douglas I, Janeway CA, et al. ECSIT is an evolutionarily conserved intermediate in the Toll/IL-1 signal transduction pathway. Genes & development. 1999;13:2059–2071. doi: 10.1101/gad.13.16.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vogel RO, Janssen RJ, van den Brand MA, Dieteren CE, Verkaart S, Koopman WJ, et al. Cytosolic signaling protein Ecsit also localizes to mitochondria where it interacts with chaperone NDUFAF1 and functions in complex I assembly. Genes & development. 2007;21:615–624. doi: 10.1101/gad.408407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, et al. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472:476–480. doi: 10.1038/nature09973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 64.Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nature Immunology. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 66.Tarallo V, Hirano Y, Gelfand BD, Dridi S, Kerur N, Kim Y, et al. DICER1 loss and Alu RNA induce age-related macular degeneration via the NLRP3 inflammasome and MyD88. Cell. 2012;149:847–859. doi: 10.1016/j.cell.2012.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.De Nardo D, Latz E. NLRP3 inflammasomes link inflammation and metabolic disease. Trends Immunol. 2011;32:373–379. doi: 10.1016/j.it.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 69.Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci U S A. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, et al. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 71.Dagenais M, Skeldon A, Saleh M. The inflammasome: in memory of Dr. Jurg Tschopp. Cell Death Differ. 2012;19:5–12. doi: 10.1038/cdd.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu FX, Chai TF, He H, Hagen T, Luo Y. Thioredoxin-interacting protein (Txnip) gene expression: sensing oxidative phosphorylation status and glycolytic rate. J Biol Chem. 2010;285:25822–25830. doi: 10.1074/jbc.M110.108290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen CL, Lin CF, Chang WT, Huang WC, Teng CF, Lin YS. Ceramide induces p38 MAPK and JNK activation through a mechanism involving a thioredoxin-interacting protein-mediated pathway. Blood. 2008;111:4365–4374. doi: 10.1182/blood-2007-08-106336. [DOI] [PubMed] [Google Scholar]
- 74.Bauernfeind F, Bartok E, Rieger A, Franchi L, Nunez G, Hornung V. Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J Immunol. 2011;187:613–617. doi: 10.4049/jimmunol.1100613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McDermott MF, Aksentijevich I, Galon J, McDermott EM, Ogunkolade BW, Centola M, et al. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell. 1999;97:133–144. doi: 10.1016/s0092-8674(00)80721-7. [DOI] [PubMed] [Google Scholar]
- 76.Chen L, Liu L, Yin J, Luo Y, Huang S. Hydrogen peroxide-induced neuronal apoptosis is associated with inhibition of protein phosphatase 2A and 5, leading to activation of MAPK pathway. Int J Biochem Cell Biol. 2009;41:1284–1295. doi: 10.1016/j.biocel.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 77.Bulua AC, Simon A, Maddipati R, Pelletier M, Park H, Kim KY, et al. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS) The Journal of Experimental Medicine. 2011;208:519–533. doi: 10.1084/jem.20102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smith RA, Kelso GF, James AM, Murphy MP. Targeting coenzyme Q derivatives to mitochondria. Methods Enzymol. 2004;382:45–67. doi: 10.1016/S0076-6879(04)82003-2. [DOI] [PubMed] [Google Scholar]
- 79.Kominsky DJ, Campbell EL, Colgan SP. Metabolic shifts in immunity and inflammation. J Immunol. 2010;184:4062–4068. doi: 10.4049/jimmunol.0903002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cicchetti G, Allen PG, Glogauer M. Chemotactic signaling pathways in neutrophils: from receptor to actin assembly. Crit Rev Oral Biol Med. 2002;13:220–228. doi: 10.1177/154411130201300302. [DOI] [PubMed] [Google Scholar]
- 81.Borregaard N, Herlin T. Energy metabolism of human neutrophils during phagocytosis. J Clin Invest. 1982;70:550–557. doi: 10.1172/JCI110647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lewis JS, Lee JA, Underwood JC, Harris AL, Lewis CE. Macrophage responses to hypoxia: relevance to disease mechanisms. J Leukoc Biol. 1999;66:889–900. doi: 10.1002/jlb.66.6.889. [DOI] [PubMed] [Google Scholar]
- 83.Zucker-Franklin D. Electron microscopic studies of human granulocytes: structural variations related to function. Semin Hematol. 1968;5:109–133. [PubMed] [Google Scholar]
- 84.Maianski NA, Geissler J, Srinivasula SM, Alnemri ES, Roos D, Kuijpers TW. Functional characterization of mitochondria in neutrophils: a role restricted to apoptosis. Cell Death Differ. 2004;11:143–153. doi: 10.1038/sj.cdd.4401320. [DOI] [PubMed] [Google Scholar]
- 85.van Raam BJ, Sluiter W, de Wit E, Roos D, Verhoeven AJ, Kuijpers TW. Mitochondrial membrane potential in human neutrophils is maintained by complex III activity in the absence of supercomplex organisation. PLoS ONE. 2008;3:e2013. doi: 10.1371/journal.pone.0002013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Elks PM, van Eeden FJ, Dixon G, Wang X, Reyes-Aldasoro CC, Ingham PW, et al. Activation of hypoxia-inducible factor-1alpha (Hif-1alpha) delays inflammation resolution by reducing neutrophil apoptosis and reverse migration in a zebrafish inflammation model. Blood. 2011;118:712–722. doi: 10.1182/blood-2010-12-324186. [DOI] [PubMed] [Google Scholar]
- 88.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 89.Semenza GL. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci STKE. 2007:cm8. doi: 10.1126/stke.4072007cm8. [DOI] [PubMed] [Google Scholar]
- 90.Pan Y, Mansfield KD, Bertozzi CC, Rudenko V, Chan DA, Giaccia AJ, et al. Multiple factors affecting cellular redox status and energy metabolism modulate hypoxia-inducible factor prolyl hydroxylase activity in vivo and in vitro. Mol Cell Biol. 2007;27:912–925. doi: 10.1128/MCB.01223-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Peyssonnaux C, Datta V, Cramer T, Doedens A, Theodorakis EA, Gallo RL, et al. HIF-1alpha expression regulates the bactericidal capacity of phagocytes. J Clin Invest. 2005;115:1806–1815. doi: 10.1172/JCI23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, et al. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Blouin CC, Page EL, Soucy GM, Richard DE. Hypoxic gene activation by lipopolysaccharide in macrophages: implication of hypoxia-inducible factor 1alpha. Blood. 2004;103:1124–1130. doi: 10.1182/blood-2003-07-2427. [DOI] [PubMed] [Google Scholar]
- 94.Hollander AP, Corke KP, Freemont AJ, Lewis CE. Expression of hypoxia-inducible factor 1alpha by macrophages in the rheumatoid synovium: implications for targeting of therapeutic genes to the inflamed joint. Arthritis Rheum. 2001;44:1540–1544. doi: 10.1002/1529-0131(200107)44:7<1540::AID-ART277>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 95.Krawczyk CM, Holowka T, Sun J, Blagih J, Amiel E, DeBerardinis RJ, et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115:4742–4749. doi: 10.1182/blood-2009-10-249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Everts B, Amiel E, van der Windt GJ, Freitas TC, Chott R, Yarasheski KE, et al. Commitment to glycolysis sustains survival of NO-producing inflammatory dendritic cells. Blood. 2012;120:1422–1431. doi: 10.1182/blood-2012-03-419747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maciver NJ, Jacobs SR, Wieman HL, Wofford JA, Coloff JL, Rathmell JC. Glucose metabolism in lymphocytes is a regulated process with significant effects on immune cell function and survival. J Leukoc Biol. 2008;84:949–957. doi: 10.1189/jlb.0108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jones RG, Thompson CB. Revving the engine: signal transduction fuels T cell activation. Immunity. 2007;27:173–178. doi: 10.1016/j.immuni.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 99.Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol. 2005;5:844–852. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- 100.Jacobs SR, Herman CE, Maciver NJ, Wofford JA, Wieman HL, Hammen JJ, et al. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J Immunol. 2008;180:4476–4486. doi: 10.4049/jimmunol.180.7.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Doughty CA, Bleiman BF, Wagner DJ, Dufort FJ, Mataraza JM, Roberts MF, et al. Antigen receptor-mediated changes in glucose metabolism in B lymphocytes: role of phosphatidylinositol 3-kinase signaling in the glycolytic control of growth. Blood. 2006;107:4458–4465. doi: 10.1182/blood-2005-12-4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vander Heiden MG, Plas DR, Rathmell JC, Fox CJ, Harris MH, Thompson CB. Growth factors can influence cell growth and survival through effects on glucose metabolism. Mol Cell Biol. 2001;21:5899–5912. doi: 10.1128/MCB.21.17.5899-5912.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Calo V, Migliavacca M, Bazan V, Macaluso M, Buscemi M, Gebbia N, et al. STAT proteins: from normal control of cellular events to tumorigenesis. J Cell Physiol. 2003;197:157–168. doi: 10.1002/jcp.10364. [DOI] [PubMed] [Google Scholar]
- 104.Chen Z, Laurence A, O’Shea JJ. Signal transduction pathways and transcriptional regulation in the control of Th17 differentiation. Semin Immunol. 2007;19:400–408. doi: 10.1016/j.smim.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sarafian TA, Montes C, Imura T, Qi J, Coppola G, Geschwind DH, et al. Disruption of astrocyte STAT3 signaling decreases mitochondrial function and increases oxidative stress in vitro. PLoS ONE. 2010;5:e9532. doi: 10.1371/journal.pone.0009532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wegrzyn J, Potla R, Chwae YJ, Sepuri NB, Zhang Q, Koeck T, et al. Function of mitochondrial Stat3 in cellular respiration. Science. 2009;323:793–797. doi: 10.1126/science.1164551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shulga N, Pastorino JG. GRIM-19-mediated translocation of STAT3 to mitochondria is necessary for TNF-induced necroptosis. J Cell Sci. 2012;125:2995–3003. doi: 10.1242/jcs.103093. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 108.Goldbach-Mansky R. Blocking interleukin-1 in rheumatic diseases. Annals of the New York Academy of Sciences. 2009;1182:111–123. doi: 10.1111/j.1749-6632.2009.05159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sibley CH, Plass N, Snow J, Wiggs EA, Brewer CC, King KA, et al. Sustained response and prevention of damage progression in patients with neonatal-onset multisystem inflammatory disease treated with anakinra: A cohort study to determine three- and five-year outcomes. Arthritis and rheumatism. 2012;64:2375–2386. doi: 10.1002/art.34409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kastner D, Aksentijevich I, Goldbach-Mansky R. Autoinflammatory Disease Reloaded: A Clinical Perspective. Cell. 2010;140:784–790. doi: 10.1016/j.cell.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bulua AC, Mogul DB, Aksentijevich I, Singh H, He D, Muenz L, et al. Efficacy of etanercept in the tumor necrosis factor receptor-associated periodic syndrome (TRAPS) Arthritis and rheumatism. 2012;64:908–913. doi: 10.1002/art.33416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Smith RA, Hartley RC, Cocheme HM, Murphy MP. Mitochondrial pharmacology. Trends in pharmacological sciences. 2012;33:341–352. doi: 10.1016/j.tips.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 113.Smith RA, Hartley RC, Murphy MP. Mitochondria-targeted small molecule therapeutics and probes. Antioxid Redox Signal. 2011;15:3021–3038. doi: 10.1089/ars.2011.3969. [DOI] [PubMed] [Google Scholar]
- 114.Lowes DA, Thottakam BM, Webster NR, Murphy MP, Galley HF. The mitochondria-targeted antioxidant MitoQ protects against organ damage in a lipopolysaccharide-peptidoglycan model of sepsis. Free Radic Biol Med. 2008;45:1559–1565. doi: 10.1016/j.freeradbiomed.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 115.Chacko BK, Reily C, Srivastava A, Johnson MS, Ye Y, Ulasova E, et al. Prevention of diabetic nephropathy in Ins2(+/)(AkitaJ) mice by the mitochondria-targeted therapy MitoQ. Biochem J. 2010;432:9–19. doi: 10.1042/BJ20100308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Snow BJ, Rolfe FL, Lockhart MM, Frampton CM, O’Sullivan JD, Fung V, et al. A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson’s disease. Mov Disord. 2010;25:1670–1674. doi: 10.1002/mds.23148. [DOI] [PubMed] [Google Scholar]
- 117.Gane EJ, Weilert F, Orr DW, Keogh GF, Gibson M, Lockhart MM, et al. The mitochondria-targeted anti-oxidant mitoquinone decreases liver damage in a phase II study of hepatitis C patients. Liver Int. 2010;30:1019–1026. doi: 10.1111/j.1478-3231.2010.02250.x. [DOI] [PubMed] [Google Scholar]