Abstract
Background
Colorectal cancer (CRC) screening rates remain low among low-income and minority populations. The purpose of this study was to determine whether providing patients with screening information, activating them to ask for a screening test, and telephone barriers counseling improves CRC screening rates compared with providing screening information only.
Methods
Patients were randomized to CRC screening information plus patient activation and barriers counseling (n = 138) or CRC screening information (n = 132). Barriers counseling was attempted among activated patients if screening was not completed after one month. CRC screening test completion was determined by medical record review at two months after the medical visit. Logistic regression was used to determine whether activated patients were more likely to complete CRC screening, after adjustment for confounding factors (e.g., demographic characteristics and CRC knowledge).
Results
Patients were African American (72.2%), female (63.7%), had annual household incomes less than $20,000 (60.7%), no health insurance (57.0%), and limited health literacy skills (53.7%). In adjusted analyses, more patients randomized to the activation group completed a screening test (19.6% vs. 9.9%; OR = 2.35, 95% CI: 1.14–5.56; P = 0.020). In addition, more activated patients reported discussing screening with their provider (54.4% vs. 27.5%, OR = 3.29, 95% CI: 1.95–5.56; P < 0.001) and had more screening tests ordered (39.1% vs. 17.6%; OR = 3.40, 95% CI: 1.88–6.15; P < 0.001) compared with those in the control group.
Conclusion
Patient activation increased CRC screening rates among low-income minority patients.
Impact
Innovative strategies are still needed to increase CRC screening discussions, motivate providers to recommend screening to patients, as well as assist patients to complete ordered screening tests.
Introduction
Colorectal cancer (CRC) incidence and mortality rates have decreased over the past 2 decades in the United States because of increased screening rates and advances in treatment (1–4). Nonetheless, CRC remains a leading cause of cancer mortality in the U.S. Certain segments of the population, namely African Americans, have not benefitted equally from screening and still have elevated CRC incidence and mortality rates (1, 2). Reasons for CRC disparities are numerous, complex, and occur at multiple levels (patient, provider, health system, society; refs. 5, 6). One reason for CRC disparities is that lower CRC screening rates occur among African Americans and among lower socioeconomic (SES) populations (1,2, 7–9).
Because CRC screening tests are available and CRC screening has been shown to be cost effective (10–12), the increased CRC mortality rates in these populations indicate the need for programs to increase the use of these tests to help reduce disparities. More than a decade of cancer behavioral research has provided insight to barriers to and facilitators for CRC screening (5, 13, 14). In the past, interventions directed at the individual patient level have usually provided CRC and CRC screening information to patients in expectation that increased knowledge would improve CRC screening rates. Prior research also suggests that a healthcare provider’s recommendation to undergo screening has been one of the strongest predicators of an individual completing a CRC screening test (13, 14).
Previously, to improve patient-provider discussions about health-related issues, communication training has mostly centered on the physician half of the patientprovider dyad with little attention given to improving patients’ communication skills (15). Existing literature on patient communication skills training, however, supports its value in improving patients’ participation in medical interviews, recall of treatment information and recommendations, and patient outcomes (16–19). The goal of this study was to evaluate whether average-risk patients provided with CRC screening information, activated to ask their healthcare provider for a CRC screening test, and given telephone barriers counseling would complete more CRC screening tests compared with patients provided with CRC screening information only. In addition, secondary outcomes were to evaluate whether activated patients also (i) show greater information seeking about CRC screening and (ii) have more CRC screening tests ordered by their providers compared with patients provided with CRC screening information only.
Materials and Methods
Setting and study participants
The study was conducted from November 2007 to May 2010 in one Federally Qualified Health Center (FQHC) that serves a mostly minority and low SES population in Columbus, Ohio. On average, the health center addresses the medical needs of approximately 6,000 patients annually; 30% of patients are 50+ years old and 54% are African American. Healthcare providers at the center were aware that 2 CRC screening educational programs were being tested; however, they were not aware of the purpose of the study.
To be eligible for this study, men and women had to be 50 years or older, average risk for CRC, not within CRC screening guidelines, able to speak and understand English, and have a working telephone. In addition, patients had to have a scheduled appointment with a provider for a nonacute medical reason and be able to come to the health center 1 hour prior to their scheduled appointment. Eligibility of patients was determined after medical record review and a brief telephone screening interview. Informed consent procedures and study protocols were approved by the Institution Review Board of The Ohio State University.
Randomization and intervention design
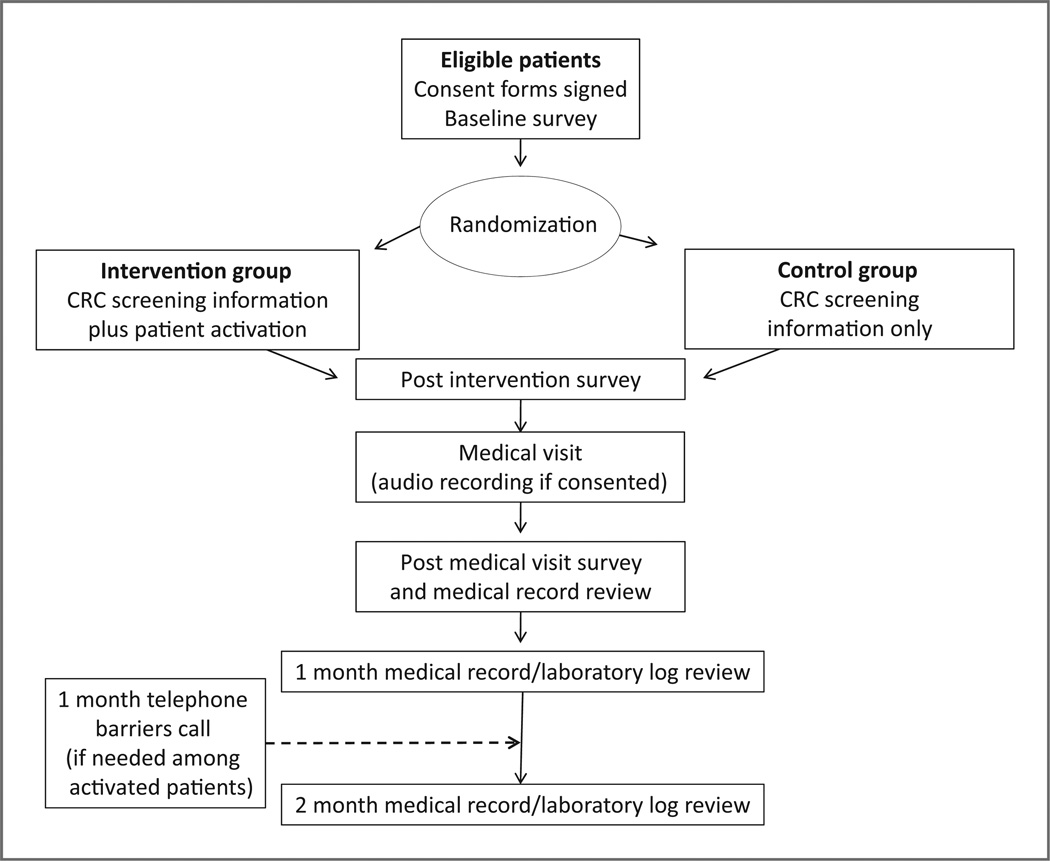
After signing a consent form, HIPAA form, and a medical record release form, patients completed a face to face baseline interview conducted by a research assistant (Fig. 1). Patients were then randomized into the intervention arm (patient activation plus CRC screening information and barriers counseling) or control arm (CRC screening information only) via a computerized permuted randomization using a block size of 8. A second research assistant delivered the intervention to the patients. The intervention group watched a 12-minute video entitled “Ask your doctor about colon cancer screening,” received a brochure that supplemented the video and focused on asking their provider for a CRC screening test, and received a second brochure on tips to prevent CRC (e.g., the importance of daily exercise). A description of the video content and production has been previously reported (20).
Figure 1.
Study design.
The intervention was based on the Protection Motivation Theory (PMT) (21, 22). According to the PMT, the contradictory impact of threatening information (threat appraisal) followed by coping appraisal influences an individual’s decision to react to health information. In addition, the intervention included the PACE (Presenting information, Asking questions, Checking for understanding, Expressing concerns) communication system which focused communication training for the patient to ask their healthcare provider about CRC screening (16, 18). The control group watched a 10-minute video entitled “Colon cancer screening.” The video for the participants in the control group was the same as shown to the intervention group except the patient activation section was not included in this video. In addition, the participants in the control group received the brochure focused on tips to prevent CRC. Following the educational session, all patients completed a brief face to face interview to document changes in CRC and CRC screening knowledge, attitudes, and intention to complete CRC screening. Subsequent medical visits were audio taped, if the patient and provider agreed and consented to taping.
Following the medical visit and before leaving the health center, patients completed a short face to face interview that addressed: whether CRC screening was discussed with the provider; who initiated the CRC screening discussion if it occurred; and what CRC screening test was ordered or why a screening test was not ordered. All patients received a $25 gift card in appreciation of their time. Medical record reviews were conducted to collect information about any CRC screening test ordered by the provider and completion of any CRC screening test on all patients as soon as the medical chart became available after the visit and at 1 and 2 months following the medical visit.
One month after the medical visit, if a patient in the intervention group had a CRC screening test ordered and did not complete the test, telephone barriers counseling to address patient identified CRC screening barriers was conducted. If a patient in the intervention group did not have a screening test ordered, telephone barriers counseling focused on activating them to ask for a CRC screening test by calling their provider or asking their provider at their next medical visit. Several attempts were made to contact each activated patient on different days and times. In addition, calls were made to patients who completed a fecal occult blood test (FOBT) to assess what components of the intervention motivated them to complete the screening test.
Measures
Baseline information collected was based on the constructs included in PMT including CRC susceptibility, self-efficacy and response efficacy for CRC screening, etc. Survey items included demographic characteristics (age, gender, race, ethnicity, marital status, education, employment status, annual household income, and health insurance); medical history; past cancer screening behaviors; CRC and CRC screening knowledge (10 true and false questions), CRC screening attitudes, barriers, and intention measured by a validated instrument (Likert scale: strongly agree to strongly disagree; refs. 23, 24); health literacy (REALM; ref. 25); and 1 item measuring shared decision making preference (26).
The primary outcome in this study was whether or not a participant completed a CRC screening test by review of the medical record and laboratory log book at 2 months following randomization. Two months were considered an adequate follow-up period for this study because the CRC screening test recommended most frequently at the health center was the FOBT. Secondary outcomes were whether patients discussed CRC screening with their providers based on self-report in the post medical visit interview and whether patients had CRC tests ordered as found in the medical record review.
Statistical analysis
Descriptive statistics were used to provide overall characteristics by treatment arm and to ensure balancing of covariates after randomization. An intention to treat analysis, based on random assignment to the intervention or control arm, was used to determine the effect of the intervention on completing a CRC screening test in the 2 months following the medical visit. Logistic regression models were constructed to evaluate the intervention effect on the primary outcome (completion of CRC screening), and 2 secondary outcomes defined a priori (a CRC screening test ordered, and a patient-provider CRC screening discussion) and to control confounding by factors measured at baseline. Confounding was controlled by inclusion in the regression model when removal of the confounding factor from the model resulted in at least a 10% change in the intervention effect. For logistic regression analyses, likelihood ratio χ2 tests were used to determine improved statistical fit. All statistical analyses were conducted by SAS (version 9.2; SAS Institute Inc.).
Results
Study participants
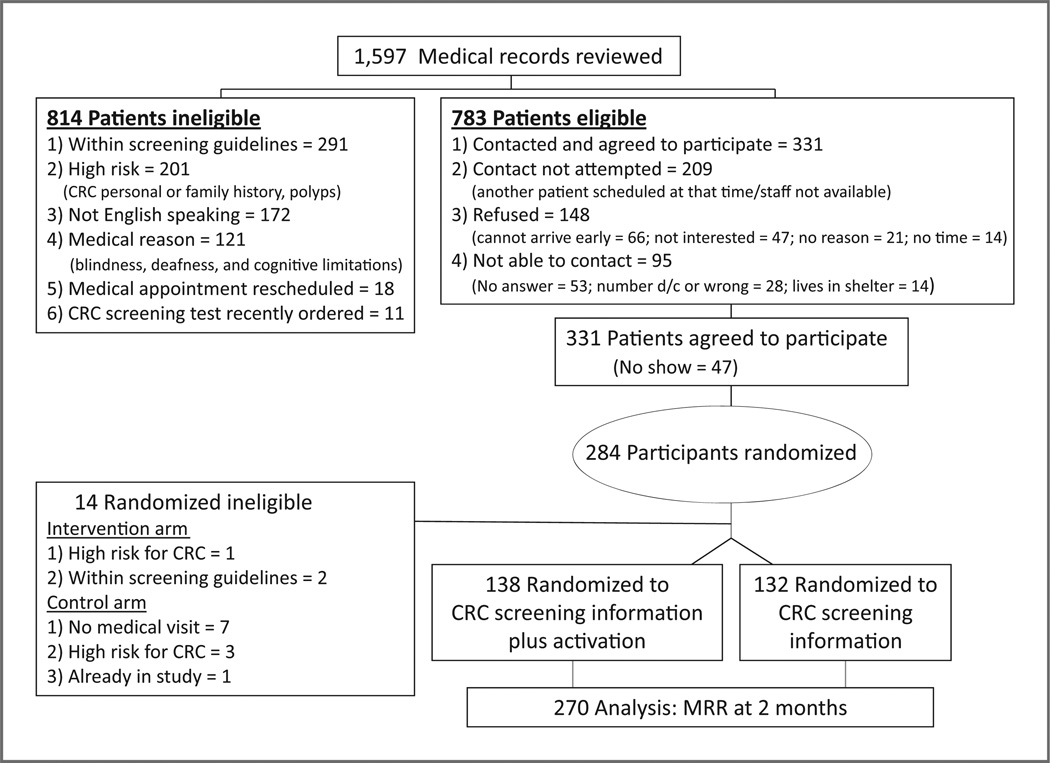
Figure 2 displays the number of patients selected, assessed for eligibility, accrued, randomized, and assessed for the primary outcome. The main reasons patients were ineligible were: within screening guidelines (n = 291), high risk (CRC personal or family history; (n = 201), did not speak English (n = 172), medical reasons (n = 121), medical appointment rescheduled (n = 18), or a CRC screening test was recently ordered by medical record review (n = 11). There were 148 patients who refused to participate because they were not able to come to the health center early (n = 66), were not interested (n = 47), provided no reason (n = 21), or had no time (n = 14). The patients who refused participation in the study were older (mean = 60.5 years) and more were males (39.6%) than the participants (mean age = 56.0 years, 36.4% males; P < 0.05).
Figure 2.
Study flow of participants, CONSORT diagram.
Among the 331 patients who agreed to participate, 47 did not arrive at the health center in time to participate in the study. Of the 284 patients who met all eligibility criteria and consented, 141 were randomized to the patient activation intervention group and 143 patients were randomized to the education only group. Fourteen patients were found to be ineligible after randomization (intervention group: 1 patient was high risk for CRC and not eligible for the FOBT and 2 patients were found to be within CRC screening guidelines; control group: 7 patients did not have a medical visit because of co-pay issues, 3 patients were determined to be high-risk patients not eligible for the FOBT, and 1 patient was randomized previously). The overall response rate was 58.1% (270 of 465).
Baseline characteristics of intervention and control groups are shown in Table 1. Participants (n = 270) were predominantly female (63.7%), African American (72.2%), had a high school education (72.6%), had annual household income less than $20,000 (60.7%), no health insurance (57.0%), and limited health literacy skills (53.7%). Although most participants were not married (85.9%), more participants in the control arm of the study (17.4%) were married/living together compared with participants in the intervention arm (10.9%; P < 0.05). There were no other statistically significant differences in characteristics at baseline between the intervention and control groups. Among the 270 patients, 83 (30.7%) reported having 2 or more comorbidities, 65 (24.1%) reported having completed CRC screening in the past, and 206 (76.3%) preferred some degree of shared decision making. There were no significant differences between participant groups for baseline CRC screening knowledge and CRC screening attitudes and beliefs (Table 2).
Table 1.
Baseline participant demographics by treatment arm (N = 270)
| Characteristic | CRC screening information plus patient activation n = 138 |
CRC screening information only n = 132 |
|---|---|---|
| Age (y, median) | 55.7 | 56.3 |
| Gender (% female) | 92 (66.7) | 80 (60.6) |
| Race (% African American) | 104 (75.4) | 91 (68.9) |
| Marital statusa (% married/living as married) | 15 (10.9) | 23 (17.4) |
| Education (% less than high school) | 40 (29.0) | 34 (25.8) |
| Household income (% with income <$20,000) | 87 (63.0) | 77 (58.3) |
| Health insurance (% with no insurance) | 82 (59.4) | 72 (54.5) |
| Health literacy: less than high school reading level (% with REALM score <60) | 80 (58.0) | 65 (49.2) |
| Comorbid conditions (% with 2+ conditions) | 46 (33.3) | 37 (28.0) |
| Previous CRC screening (% reporting previous test completion) | 34 (24.6) | 31 (23.5) |
| Shared decision making (% prefer involvement with medical decisions) | 101 (73.2) | 105 (79.6) |
P < 0.05.
Table 2.
Baseline CRC screening characteristics of participants by treatment arm (N = 270)
| CRC screening information plus patient activation (n = 138) |
CRC screening information only (n = 132) |
|
|---|---|---|
| CRC screening knowledgea (mean ± SD) | 6.1 ± 1.8 | 6.3 ± 1.7 |
| CRC screening attitudes and beliefsb (mean + SD) | ||
| Salience and coherence | 2.9 ± 0.9 | 2.8 ± 0.8 |
| Self-efficacy | 2.5 ± 0.8 | 2.5 ± 0.8 |
| Perceived susceptibility | 2.5 ± 0.7 | 2.3 ± 0.8 |
| Worries and fears (including barriers) | 3.0 ± 1.0 | 2.9 ± 0.9 |
| Intention | 2.4 ± 0.8 | 2.6 ± 0.7 |
CRC screening knowledge (correct number from 10 true/false questions).
CRC screening attitudes and beliefs (strongly agree = 1 to strongly disagree = 4; ref. 33).
Intervention effectiveness
Medical record review pertaining to CRC screening status was completed for all 270 patients. Overall, CRC screening completion was documented in 40 patients (27 in the intervention group and 13 in the control group). Thirty-five patients completed FOBT tests and 5 patients completed a colonoscopy within the 2-month follow-up period. Logistic regression modeling (Table 3) showed that patients randomized to the intervention arm were 2.35 (95% CI: 1.14–5.56) times as likely to complete CRC screening based on medical record review at 2 months after the medical visit. In addition, patients in the intervention arm were more likely to self-report discussing CRC screening with their provider (OR = 3.29; 95% CI: 1.95–5.56) and had more CRC screening tests ordered as determined by medical record review (OR 3.40; 95% CI: 1.88–6.15). There was no evidence of confounding or effect modification by any of the prespecified variables collected at baseline.
Table 3.
ORs and 95% CIs from adjusted logistic regression models for discussing CRC screening with a healthcare provider, having a CRC screening test ordered, and completing CRC screening
| CRC screening | CRC screening information plus patient activation (n = 138) |
CRC screening information only (n = 132) |
OR (95% CI)c | P |
|---|---|---|---|---|
| Discussiona | 75 (54.4) | 36 (27.5) | 3.29 (1.95–5.56) | <0.001 |
| Orderedb | 54 (39.1) | 23 (17.6) | 3.40 (1.88–6.15) | <0.001 |
| Completionb | 27 (19.6) | 13 (9.9) | 2.35 (1.14–5.56) | 0.020 |
Self-report.
Medical record review.
Adjusted for age, gender, race, and provider.
Process evaluation
Process evaluation documented that: (i) 2 patients in the intervention group watched most (90%) but not the entire video because the health care provider requested to see the patient (post intervention survey was completed); (ii) 5 patients in the intervention group completed the education session but had it interrupted for a phone call, to use the rest room, or because a nurse needed to talk to the patient; (iii) all patients but one in the intervention group received the brochures; (iv) among the 54 patients in the intervention group who had a CRC screening test ordered, 18 patients returned an FOBT within a month and did not need telephone barriers counseling; 12 patients could not be reached for barriers counseling and we were able to contact 24 patients for telephone barriers counseling at 1 month after their medical visit (1 person completed the FOBT and 2 patients completed their scheduled colonoscopy); (v) among the 84 patients in the intervention group who did not have a CRC screening test ordered, 2 patients returned an FOBT within a month and did not need barriers counseling; 25 patients could not be reached for telephone barriers counseling; and we reached 57 patients at 1 month and reminded them to call the doctor’s office or to ask their provider for a CRC screening test at their next medical visit (1 patient completed screening after being activated during the 1 month telephone counseling call); and (vi) 43.5% (103 of 237) of patients agreed to have their medical visit audio taped (near the end of the study, one provider (33 patients) refused to have medical visits recorded).
The most frequent CRC screening barriers reported by the participants who had a CRC screening test ordered were dealing with other medical issues, keep putting it off, too busy, or waiting for a scheduled colonoscopy. Among patients who did not have a CRC screening test ordered, the most frequent comment made was that providers did not mention CRC screening to them. In addition, among patients reached after completing the FOBT, most patients stated that the educational video made them realize how important CRC screening was and that the video showed them how to complete the FOBT.
Discussion
Improving CRC screening rates among minority and low SES populations is critical to reduce CRC disparities in the United States. Previous studies have shown that a provider’s recommendation for a CRC screening test is the strongest facilitator to get patients to complete a CRC screening test (13, 14, 27). More recently, however, studies have shown that patient-provider discussions about CRC screening often do not occur or patient-provider conversations about CRC screening are limited in the information that is exchanged (e.g., lack discussion of different screening test options; refs. 28–30).
In this randomized trial, we tested the efficacy of providing CRC screening information, activating patients to discuss CRC screening with their provider, and telephone barriers counseling to improve CRC screening rates among average-risk patients in need of a CRC screening test who were recruited from one FQHC. The intervention was hypothesized to improve CRC screening knowledge and attitudes, and empower patients to initiate CRC screening discussions with providers. Subsequent to more patient-provider CRC screening discussions, there would be an increase in CRC screening tests ordered, and thus, an increase in CRC screening test completion rates. The impact of the intervention on CRC screening knowledge, barriers, attitudes, and intention is the topic of another manuscript, as is a content analysis of the CRC screening discussions.
Overall, the results show an increase in CRC screening completion rates among activated patients by medical record review. Self-report of CRC screening discussions and CRC screening tests ordered by medical record review were also found among the patients who received the patient activation intervention. In this study, the control group received CRC screening information, thus the intervention effect, although significant, may have been greater if we used a true control group. In this study, a true control was not used because we thought it was unethical not to provide information about CRC screening to minority and low-income patients.
Telephone CRC screening barriers counseling among this population was not successful due to the fact that only 54 (39.1%) patients in the intervention group received a CRC screening recommendation, 37 (31.4%) of the 118 patients needing CRC screening barriers counseling were not reached, and just 4 (4.9%) of the 81 patients completed screening after telephone barriers counseling (2 patients completed FOBT and 2 patients completed scheduled colonoscopy). The main barriers reported by patients (other medical issues, too busy, etc.) in this study are similar to previous reports of patient-level CRC screening barriers (14, 31, 32). In addition, it must be noted that even when CRC screening was reported as being discussed during a medical visit (n = 111), a CRC screening test was not ordered for 34 (30.6%) patients. Reasons for not ordering a CRC screening test following a CRC screening discussion in this study include forgetfulness, concentration on a different medical issue, and not ordering an FOBT for patients without health insurance and who could not afford a colonoscopy.
Although the difference between study arms was significant in this study, the findings also identified that patient-provider CRC screening discussions may not always lead to a CRC screening test being ordered and a test being ordered may not always lead to completion of the recommended CRC screening test. Only 14.8% (40 of 270) of the patients in this study completed CRC screening. This is similar to national data which reports a 19.5% CRC screening rate among individuals without health insurance (1).
Even though CRC screening rates have increased in the past decade, there remains a trend of lower CRC screening rates within recommended guidelines among minority and underserved populations (9). Because health care providers are aware of the importance of screening to reduce CRC mortality, there seem to be problems along the steps (encounters) and interfaces (transfer of information) of the screening process (5, 33). Innovative strategies at the patient, provider, and system levels to improve CRC screening rates among minority and low SES populations are still needed.
In this study, the intervention focused on activating patients to ask their health care provider for a CRC screening test. The results of this study are similar to other patient-level interventions reported recently that used different content, intensity, tailoring, and delivery channels to increase CRC screening rates (34–40). Overall, modest increases in CRC screening have been documented among patients randomized to a CRC screening intervention delivered via brochures, physician letters, videos, DVDs, decision aids, automated telephone calls, motivational interviewing, or patient navigation. For example, Miller and colleagues randomized predominantly minority and low-income patients to a CRC screening decision aid that encouraged patients to discuss CRC screening with their provider versus a control group (34). Patients randomized to the CRC screening intervention had more screening tests ordered (30% vs. 21%) and completed (19% vs. 14%) than the patients in the control group, although the differences were not statistically significant.
Even though these interventions have increased CRC screening, they have not improved screening rates to the level of other cancer screening rates (e.g., breast cancer), especially among minority and low-income populations. It is time to recognize that interventions focused only at the patient-level have limited improvements in CRC screening rates. The results of our study and others support evidence that future interventions to increase CRC screening rates need to consider contextual factors at multiple levels (41, 42).
Our study should be interpreted with several limitations. First, we were unable to contact many potentially eligible patients and many patients were not able to arrive 1 hour early for their appointments to participate in the study. To minimize this problem, we tried to contact patients numerous times on different days and times. Still, the refusal rate for this study was 42%. Although these issues may have caused a selection bias, the internal validity of this study was likely protected by randomization and statistical control for potential confounding by measured baseline characteristics. The generalizability of the results is limited by conducting the study in one FQHC. It is possible that the minority and low-income patients using this health center could be significantly different than other minority and low-income patients using other FQHCs. In addition, patient agreement for audio taping their medical visits varied by the research assistant (24%–83%) and we were not able to contact almost one third of participants in the intervention group for CRC screening barriers counseling, largely because of disconnected telephone numbers or unanswered calls. Finally, we believe a longer follow-up time is needed for CRC screening intervention studies because 6 additional activated patients who had a colonoscopy ordered at the time of the medical visit completed the test within 6 months instead of 2 months, thus outside of the follow-up date set a priori in this study.
In spite of limitations, this study was able to recruit a mostly African American and low SES population in need of CRC screening. In addition, we captured information on participant characteristics, such as health literacy, to assess factors associated with both the intervention and outcome. Audio-taped medical visits provided insight into why screening tests were not recommended in several cases. Furthermore, CRC screening completion was determined by medical record and laboratory log book review. Results, however, may not be generalizable to all FQHCs and to populations outside of Ohio.
In conclusion, activating patients to ask healthcare providers for CRC screening tests improves CRC screening rates compared with providing patients with CRC screening information only. Because the addition of activating patients was successful among minority and low-income patients, this study speaks to the added importance of activating patients in the CRC screening process. Future studies to improve CRC screening among minority and low SES populations should include innovative strategies to motivate providers to recommend screening tests to patients, as well as to assist patients to complete ordered CRC screening tests (but not telephone barriers counseling).
Acknowledgments
Grant Support
This work was supported by the following grants: K07 CA107079 (M.L. Katz) and P30 CA016058 (Behavioral Measurement Shared Resource at The Ohio State University).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.American Cancer Society. Colorectal Cancer Facts and Figures 2011–2013. Atlanta: American Cancer Society; 2011. [Google Scholar]
- 2.American Cancer Society. Cancer Facts and Figures 2011. Atlanta: American Cancer Society; 2011. [Google Scholar]
- 3.Vital signs: colorectal cancer screening, incidence, and mortality— United States, 2002–2010. MMWR Morb Mortal Wkly Rep. 2011;60:884–889. [PubMed] [Google Scholar]
- 4.Naishadham D, Lansdorp-Vogelaar I, Siegel R, Cokkinides V, Jemal A. State disparities in colorectal cancer mortality patterns in the United States. Cancer Epidemiol Biomarkers Prev. 2011;20:1296–1302. doi: 10.1158/1055-9965.EPI-11-0250. [DOI] [PubMed] [Google Scholar]
- 5.Zapka J. Innovative provider- and health system-directed approaches to improving colorectal cancer screening delivery. Med Care. 2008;46:S62–S67. doi: 10.1097/MLR.0b013e31817fdf57. [DOI] [PubMed] [Google Scholar]
- 6.Klabunde CN, Lanier D, Breslau ES, Zapka JG, Fletcher RH, Ransohoff DF, et al. Improving colorectal cancer screening in primary care practice: innovative strategies and future directions. J Gen Intern Med. 2007;22:1195–1205. doi: 10.1007/s11606-007-0231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vital signs: colorectal cancer screening among adults aged 50–75 years—United States. MMWR Morb Mortal Wkly Rep. 2008;59:808–812. [PubMed] [Google Scholar]
- 8.Irby K, Anderson WF, Henson DE, Devesa SS. Emerging and widening colorectal carcinoma disparities between Blacks and Whites in the United States (1975–2002) Cancer Epidemiol Biomarkers Prev. 2006;15:792–797. doi: 10.1158/1055-9965.EPI-05-0879. [DOI] [PubMed] [Google Scholar]
- 9.Klabunde CN, Cronin KA, Breen N, Waldron WR, Ambs AH, Nadel MR. Trends in colorectal cancer test use among vulnerable populations in the United States. Cancer Epidemiol Biomarkers Prev. 2011;20:1611–1621. doi: 10.1158/1055-9965.EPI-11-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maciosek MV, Solberg LI, Coffield AB, Edwards NM, Goodman MJ. Colorectal cancer screening: health impact and cost effectiveness. Am J Prev Med. 2006;31:80–99. doi: 10.1016/j.amepre.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Pignone M, Saha S, Hoerger T, Mandelblatt J. Cost-effectiveness analyses of colorectal cancer screening: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137:96–104. doi: 10.7326/0003-4819-137-2-200207160-00007. [DOI] [PubMed] [Google Scholar]
- 12.Bradley CJ, Lansdorp-Volgelaar I, Yabroff KR, Dahman B, Mariotto A, Feuer EJ, et al. Productivity savings from colorectal cancer prevention and control strategies. Am J Prev Med. 2011;41:e5–e14. doi: 10.1016/j.amepre.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klabunde CN, Vernon SW, Nadel MR, Breen N, Seeff LC, Brown ML. Barriers to colorectal cancer screening: a comparison of reports from primary care physicians and average-risk adults. Med Care. 2005;43:939–944. doi: 10.1097/01.mlr.0000173599.67470.ba. [DOI] [PubMed] [Google Scholar]
- 14.Vernon SW. Participation in colorectal cancer screening: a review. J Natl Cancer Inst. 1997;89:1406–1422. doi: 10.1093/jnci/89.19.1406. [DOI] [PubMed] [Google Scholar]
- 15.Cegala DJ, Lenzmeier Broz S. Physician communication skills training: a review of theoretical backgrounds, objectives and skills. Med Educ. 2002;36:1004–1016. doi: 10.1046/j.1365-2923.2002.01331.x. [DOI] [PubMed] [Google Scholar]
- 16.Cegala DJ. Patient communication skills training: a review with implications for cancer patients. Patient Educ Couns. 2003;50:91–94. doi: 10.1016/s0738-3991(03)00087-9. [DOI] [PubMed] [Google Scholar]
- 17.Cegala DJ, Marinelli T, Post D. The effects of patient communication skills training on compliance. Arch Fam Med. 2000;9:57–64. doi: 10.1001/archfami.9.1.57. [DOI] [PubMed] [Google Scholar]
- 18.Cegala DJ, McClure L, Marinelli TM, Post D. The effects of communication skills training on patients' participation during medical interviews. Patient Educ Couns. 2000;41:209–222. doi: 10.1016/s0738-3991(00)00093-8. [DOI] [PubMed] [Google Scholar]
- 19.Cegala DJ, Post DM, McClure L. The effects of patient communication skills training on the discourse of older patients during a primary care interview. J Am Geriatr Soc. 2001;49:1505–1511. doi: 10.1046/j.1532-5415.2001.4911244.x. [DOI] [PubMed] [Google Scholar]
- 20.Katz ML, Heaner S, Reiter P, van Putten J, Murray L, McDougle L, et al. Development of an educational video to improve patient knowledge and communication with their healthcare providers about colorectal cancer screening. Am J Health Educ. 2009;40:220–228. doi: 10.1901/jaba.2009.40-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rogers RW, Prentice-Dunn S. Protection motivation theory, in handbook of health behavior research. In: gochman d., editor. Determinants of health behavior: personal and social. New York: Plenum; 1997. [Google Scholar]
- 22.Rogers RW. Cognitive and physiological processes in fear appeals and attitude change: a revised theory of protection motivation. In: Cacioppo J, Petty R, editors. Social Psychophysiology. New York: Guilford Press; 1983. [Google Scholar]
- 23.Vernon SW, Meissner H, Klabunde C, Rimer BK, Ahnen DJ, Bastani R, et al. Measures for ascertaining use of colorectal cancer screening in behavioral, health services, and epidemiologic research. Cancer Epidemiol Biomarkers Prev. 2004;13:898–905. [PubMed] [Google Scholar]
- 24.Vernon SW, Myers RE, Tilley BC. Development and validation of an instrument to measure factors related to colorectal cancer screening adherence. Cancer Epidemiol Biomarkers Prev. 1997;6:825–832. [PubMed] [Google Scholar]
- 25.Murphy PW, Davis TC, Long SW, Jackson RH, Decker BC. Rapid estimate of adult literacy in medicine (REALM): a quick reading test for patients. Journal of Reading. 1993;37:124–130. [Google Scholar]
- 26.Degner LF, Sloan JA, Venkatesh P. The control preferences scale. Can J Nurs Res. 1997;29:21–43. [PubMed] [Google Scholar]
- 27.Klabunde CN, Lanier D, Nadel MR, McLeod C, Yaun G, Vernon SW. Colorectal cancer screening by primary care physicians: recommendations and practices, 2006–2007. Am J Prev Med. 2009;37:8–16. doi: 10.1016/j.amepre.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ling BS, Trauth JM, Fine MJ, Mor MK, Resnick A, Braddock CH, et al. Informed decision-making and colorectal cancer screening: is it occurring in primary care? Med Care. 2008;46:S23–S29. doi: 10.1097/MLR.0b013e31817dc496. [DOI] [PubMed] [Google Scholar]
- 29.Zapka JM, Klabunde CN, Arora NK, Yean G, Smith JL, Kobrin SC. Physicians’ colorectal cancer screening discussion and recommendation patterns. Cancer Epidemiol Biomarkers Prev. 2011;20:509–521. doi: 10.1158/1055-9965.EPI-10-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McQueen A, Bartholomew LK, Greisinger AJ, Medina GG, Hawley ST, Haidet P, et al. Behind closed doors: physician-patient discussions about colorectal cancer screening. J Gen Intern Med. 2009;24:1228–1235. doi: 10.1007/s11606-009-1108-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Worthley DL, Cole SR, Esterman A, Mehaffey S, Roosa NM, Smith A, et al. Screening for colorectal cancer by faecal occult blood test: why people choose to refuse. Intern Med J. 2006;36:607–610. doi: 10.1111/j.1445-5994.2006.01155.x. [DOI] [PubMed] [Google Scholar]
- 32.Jones RM, Woolf SH, Cunningham TD, Johnson RE, Krist AH, Rothemich SF, et al. The relative importance of patient-reported barriers to colorectal cancer screening. Am J Prev Med. 2010;38:499–507. doi: 10.1016/j.amepre.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zapka JG, Taplin SH, Solberg LI, Manos MM. A framework for improving the quality of cancer care: the case of breast and cervical cancer screening. Cancer Epidemiol Biomarkers Prev. 2003;12:4–13. [PubMed] [Google Scholar]
- 34.Miller DP, Spangler JG, Case D, Goff DC, Singh S, Pignone MP. Effectiveness of a web-based colorectal cancer screening patient decision aid. A randomized controlled trial in a mixed-literacy population. Am J Prev Med. 2011;40:608–615. doi: 10.1016/j.amepre.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vernon SW, Bartholomew LK, McQueen A, Bettencourt JL, Greisinger A, Coan SP, et al. A randomized controlled trial of a tailored interactive computer-delivered intervention to promote colorectal cancer screening: sometimes more is just the same. Ann Behav Med. 2011;41:284–299. doi: 10.1007/s12160-010-9258-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lasser KE, Murillo J, Lisboa S, Casimir AN, Valley-Shah L, Emmons KM, et al. Colorectal cancer screening among ethnically diverse, low-income patients. Arch Intern Med. 2011;171:906–912. doi: 10.1001/archinternmed.2011.201. [DOI] [PubMed] [Google Scholar]
- 37.Menon U, Belue R, Wahab S, Rugen K, Kinney AY, Maramaldi P, et al. A randomized trial comparing the effect of two phone-based interventions on colorectal cancer screening adherence. Ann Behav Med. 2011;42:294–303. doi: 10.1007/s12160-011-9291-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mosen DM, Feldstein AC, Perrin N, Rosales AG, Smith DH, Liles EG, et al. Automated telephone calls improved completion of fecal occult blood testing. Med Care. 2010;48:604–610. doi: 10.1097/MLR.0b013e3181dbdce7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schroy PC, Emmons K, Peters E, Glick JT, Robinson PA, Lydotes MA, et al. The impact of a novel computer-based decision aid on shared decision making for colorectal cancer screening: a randomized trial. Med Decis Making. 2011;31:93–107. doi: 10.1177/0272989X10369007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sequist TD, Zaslavsky AM, Colditz GA, Ayanian JZ. Electronic patient messages to promote colorectal cancer screening. Arch Intern Med. 2011;171:636–641. doi: 10.1001/archinternmed.2010.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pasick RJ, Barker JC, Otero-Sabogal R, Burke NJ, Joseph G, Guerra C. Intention, subjective norms, and cancer screening in the context of relational culture. Health Educ Behav. 2009;36:91S–110S. doi: 10.1177/1090198109338919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anhang Price R, Zapka J, Edwards H, Taplin SH. Organizational factors and the cancer screening process. J Natl Cancer Inst Monogr. 2010;40:38–57. doi: 10.1093/jncimonographs/lgq008. [DOI] [PMC free article] [PubMed] [Google Scholar]