Abstract
Vesicle trafficking plays a crucial role in the establishment of cell polarity in various cellular contexts, including axis-pattern formation in the developing egg chamber of Drosophila. The EGFR ligand, Gurken (Grk), is first localized at the posterior of young oocytes for anterior-posterior axis formation and later in the dorsal-anterior region for induction of the dorsal-ventral (DV) axis, but regulation of Grk localization by membrane trafficking in the oocyte remains poorly understood. Here, we report that Syntaxin-1A (Syx1A) is required for efficient trafficking of Grk protein for DV patterning. We show that Syx1A is associated with the Golgi membrane and is required for the transportation of Grk-containing vesicles along the microtubules to their dorsal anterior destination in the oocyte. Our studies reveal that the Syx1A dependent trafficking of Grk protein is required for efficient EGFR signaling during DV patterning.
Keywords: Drosophila, Syntaxin 1A, Grk trafficking
Introduction
In Drosophila, the anterior-posterior (AP) and dorsal-ventral body axes are determined in the developing oocyte during oogenesis. Establishment of these axes depends on cytoskeletal organization and localization of several important maternal determinants at different subcellular regions of the oocyte (Riechmann and Ephrussi, 2001; van Eeden and St Johnston, 1999). For example, the transforming growth factor α homolog Gurken (Grk) plays two essential roles in these processes. Before stage 7, Grk protein accumulates at the posterior of the oocyte and induces the neighboring follicle cells to adopt the posterior fate (Gonzalez-Reyes et al., 1995; Gonzalez-Reyes and St Johnston, 1998; Roth et al., 1995). Posterior follicle cells then send an unknown signal back to the oocyte that reorients its microtubule cytoskeleton, defining the AP axis of the egg and the future embryo (Grunert and St Johnston, 1996; Ray and Schupbach, 1996). During this process, the posteriorly localized Lgl and Par-1 regulate the microtubule network by preventing microtubule growth from the posterior cortex (Tian and Deng, 2008, 2009). After stage 7, as the oocyte grows, its nucleus moves to an anterior corner, and grk mRNA and protein accumulate around the nucleus. Secreted Grk then activates the epidermal growth factor receptor (EGFR) pathway in overlying follicle cells and induces them to adopt a dorsal cell fate, thus building the foundation for dorsal-ventral axis specification (Nilson and Schupbach, 1999; Van Buskirk and Schupbach, 1999). Failure of Grk to localize to the dorsal anterior corner disrupts dorsoventral patterning of the follicle cells, causing, e.g., loss of dorsal follicle-cell fates (Deng and Bownes, 1997; Queenan et al., 1997), and leads to the disruption of this polarity in the eggshell and embryo (Neuman-Silberberg and Schupbach, 1993, 1994). The dorsally localized Grk protein is believed to be controlled by RNA localization and translation and the secretory pathway (Coutelis and Ephrussi, 2007; Januschke et al., 2007; Murthy and Schwarz, 2004), but how the secretory pathway regulates Grk localization remains largely unknown.
Polarized intracellular transport and vesicular trafficking can be used to create and/or maintain cellular asymmetry during Drosophila development. For example, the establishment and maintenance of epithelial apical-basal polarity depend on directed exocytosis of apical and basolateral transport vesicles to the plasma membrane (Rodriguez-Boulan et al., 2005; Schuck and Simons, 2004). In the oocyte, a small GTPase, Rab6 and an exocyst complex component, Sec5, have been shown to regulate Grk trafficking (Coutelis and Ephrussi, 2007; Januschke et al., 2007; Murthy and Schwarz, 2004), but the mechanism for transportation of Grk protein within the oocyte remains largely unclear. Here, we report the identification of a novel hypomorphic allele of syntaxin 1A (Syx1A), Syx1ASH0113, and its requirement for the second round of Grk-EGFR signaling during oogenesis. Syx1A is a t-SNARE (soluble NSF [N-ethylmaleimide-sensitive fusion protein] attachment protein receptor) protein and acts as a central coordinator of this exocytosis machinery (Chen and Scheller, 2001). In Syx1A germ-line clones, Grk protein was diffused in the ooplasm after stage 7. We also show that Syx1A is required to transport Grk-containing vesicles with Rab6, and loss of Syx1A in the germ-line cells resulted in enlarged vesicles containing Grk, which colocalized with Rab6.
Materials and Methods
Fly strains and genetics
The strains were raised at 25°C on standard media. We used syxΔ229 as the null allele for syx1A (Schulze et al., 1995). The germ-line clones were generated by FLP-FRT–induced mitotic recombination (Xu and Rubin, 1993) from the following strains: yw P[mini-w+, hsFLP]1; P[neoFRT]82B P[Ubi-GFP]83/TM3, Sb, rab6D23D P[neoFRT]40A/Cyo (Coutelis and Ephrussi, 2007), yw P[mini-w+, hsFLP]1; P[Ubi-GFP]33 P[Ubi-GFP]38 P[neoFRT]40A/CyO. We induced germ-line clones by administering 2-h 37°C heat shocks on two consecutive days during the third instar. For syx and rab6 overexpression, we made UASp:syx-GFP and UASp:RFP-syx and obtained ubiRFP-rab6 transgenic fly stocks from A. Guichet (Januschke et al., 2007). Nanos-Gal4-VP16 was used for the overexpression in the germ-line cells. To excise the P-element from FRT-l(3)SH0113 we used P[Δ2–3]99B.
Molecular biology and biochemical analysis
For C-terminal GFP- or N-terminal RFP-tagged Syx1A overexpression, the cDNA of syx1A from DGRC (LD43943) was amplified, sequenced, and subcloned into pUASP:GFP and pUASP:RFP vectors for Drosophila transgenes by means of the primers UASp-Syx1A-GFP (5’-AGATCTATGACTAAAGACAGATTAGC-3’ and 5’-GCGGCCGCCATGAAATAACTGCTAACAT-3’), and UASp-RFP-Syx1A (5’-GCGGCCGCATGACTAAAGACAGATTAGC-3’ and 5’-TCTAGATTACATGAAATAACTGCTA-3’). Transgenic lines of (1) pUASP:syx1A-GFP and (2) pUASP:RFP-syx1A were generated by standard methods at Duke University Model System Genomics and overexpressed by means of germ-line Gal4 drivers (Nanos-Gal4).
To determine the genomic sequence of the syx1A open reading frame (ORF), genomic DNA was extracted from SH0113 homozygous mutant first instar larvae. Using this extracted genomic DNA as a template, syx1A ORF region was amplified and sequenced using the primer set; 5'-ATGACTAAAGACAGATTAGCCG-3' and 5'-TCGAGCCCTAATTTCGTGTG-3'. The resultant sequence of syx1A ORF of SH0113 mutants (876bp) was compared with the wild-type sequences and no mutation in the ORF was identified. The mutation is probably at the regulatory region of the syx1A locus.
Antibody staining, imaging, and analysis
Antibody stainings were performed according to standard procedures. Primary antibodies were diluted as follows: mouse anti-Gurken, 1:20 (Developmental Studies Hybridoma Bank) and mouse anti-Syx1A, 1:50 (Developmental Studies Hybridoma Bank). Secondary antibodies conjugated to Alexa Fluor 546 goat anti-rabbit and anti-mouse (Molecular Probes) were used at 1:400. Fluorescently labeled samples were counterstained with DAPI for visualization of DNA. Images were captured with a Zeiss LSM 510 confocal microscope and assembled in Adobe Photoshop. Signal intensities of Grk antibody staining were plotted with Interactive 3D Surface Plot, an ImageJ plugin.
Colcemid treatments of flies
For the colcemid treatment, flies were fed yeast paste containing 30 µg ml−1 colcemid for 12 h. Subsequent steps were performed as previously described (Tian and Deng, 2009).
Results
Syntaxin 1A regulates Grk localization in the oocyte
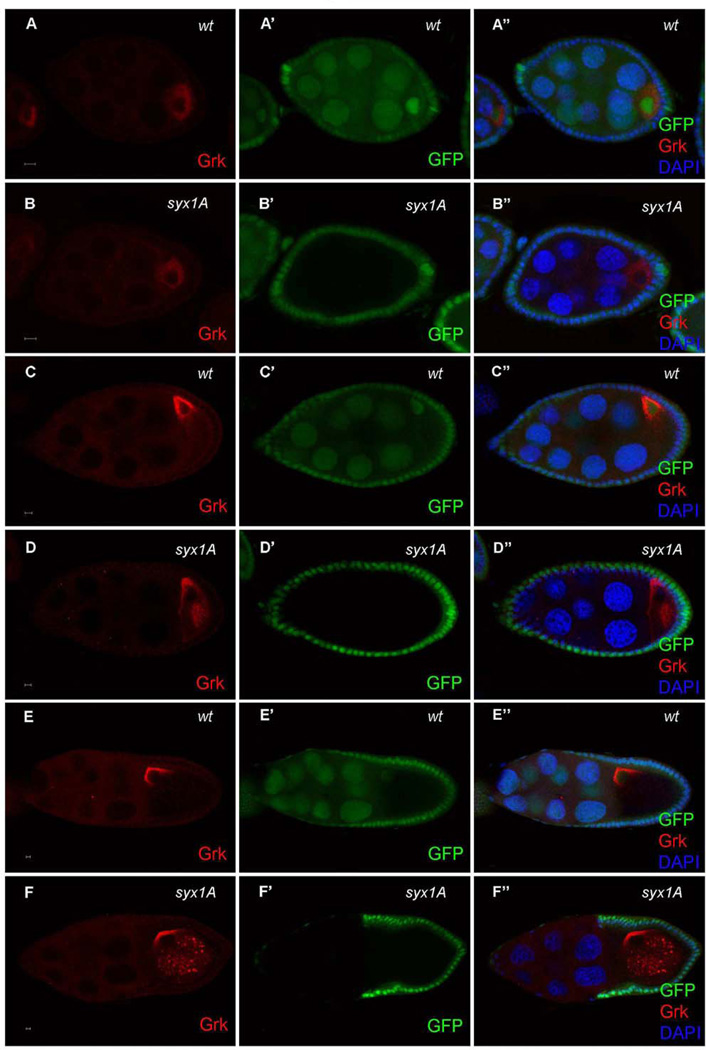
To identify genes involved in oocyte polarity formation, we performed a FLP/FRT mosaic screen for about 400 FRT–P-element insertion lines, from the Szeged Drosophila Stock Center (Bellotto et al, 2002). Staufen-GFP and Grk localization were analyzed in mosaic egg chambers carrying either germ-line or somatic clones of these mutations (Neuman-Silberberg and Schupbach, 1993; Lopez-Schier, et al. 2001). Previously, we have reported the identification of three genes from this screen that are required in either the germline or the somatic follicle cells for oocyte polarity formation (Yu et al.; 2010; Klusza and Deng, 2011; Poulton et al., 2011). Here, we show that Grk is abnormally localized in germ-line clones for one of these stocks, FRT-l(3)SH0113 (hereafter referred to as SH0113) after stage 7 (Fig. 1D and F). Grk, which is normally localized around the oocyte nucleus at the dorsal-anterior corner after stage 7 (Neuman-Silberberg and Schupbach, 1993)(Fig. 1C and E), failed to reach its correct destination in SH0113 germ-line clones and was partially dispersed into the ooplasm (Fig. 1D and F; 100%, n = 110, Fig.S2B and D). To determine whether the Grk localization defect was specific to this stage, we also examined Grk localization in early oocytes. Before stage 7, Grk and the oocyte nucleus are normally localized at the posterior in the wild type. In SH0113 germ-line clones, this localization appeared similar to that in the wild type (100%, n = 50; Fig. 1A and B), suggesting that the target gene of SH0113 is involved in Grk localization and this function is stage specific.
Fig. 1.
Mislocalizations of Grk in Drosophila Syx1A germ-line clones. Grk is normally localized in the wild-type (A–A’’) and syx1A germ-line clone at stage 5 (B–B’’). After stage 7, Grk is normally localized at the anterior-dorsal corner (C–C’’ and E–E’’), but in Syx1A germ-line clones, Grk was partially mislocalized into the cytoplasm of oocytes (D–D’’ and F–F’’). Mutant germ-line clones are marked by the absence of GFP (green) in the nuclei of nurse cells (B’, D’, and F’). Anterior is to the left, and posterior is to the right in all panels. The scale bar is 5 µm.
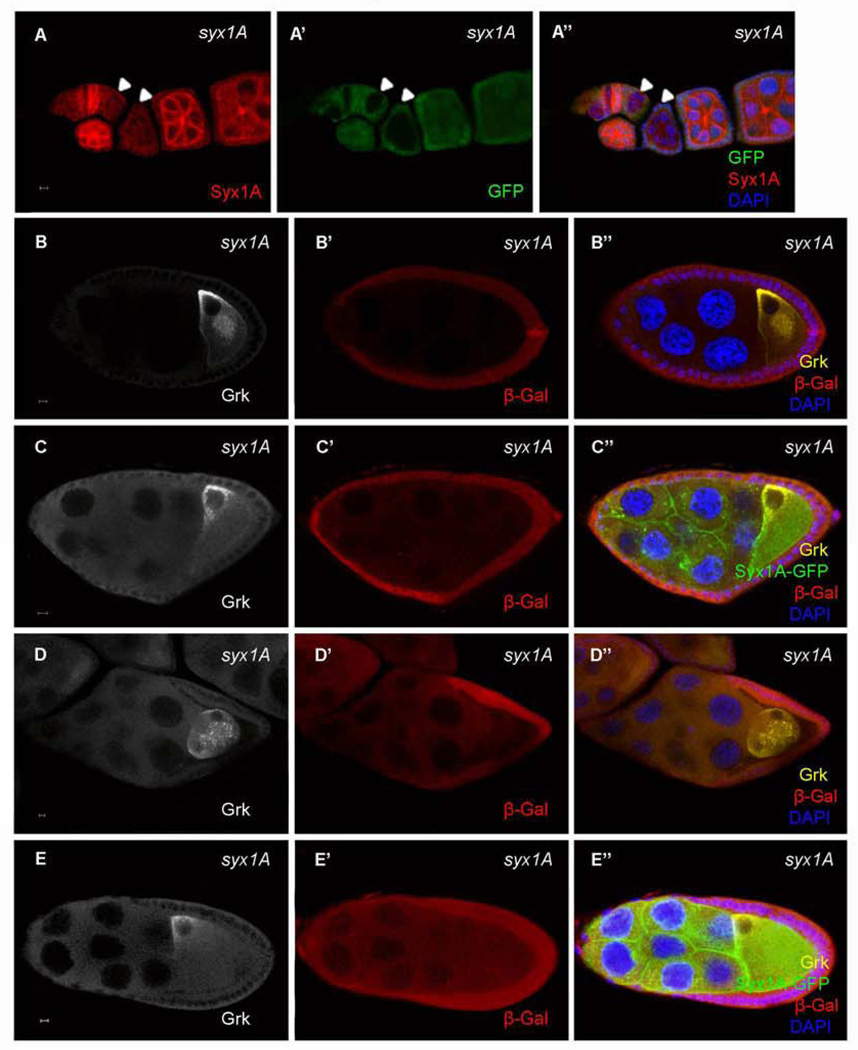
SH0113 is a P-element insertion line close to the CG6954 locus (Oh et al., 2003) and is homozygous lethal. To determine whether the P-element insertion was the cause of the phenotype observed in the SH0113 germ-line clones, we performed a complementation test and found that the deficiency line (Df(3R)ED6096) that covers the CG6954 genomic region did not confer any detectable phenotype when it was transheterozygous with SH0113. A background mutation in SH0113 may therefore be the cause of the phenotypes described above. To identify the gene responsible for the Grk-mislocalization phenotype, we performed a deficiency-mapping assay, using the “deficiency kit” for the right arm of the third chromosome from the Bloomington Drosophila Stock Center, and identified a deficiency line, Df(3R)Exel6197, that failed to complement SH0113. Using the candidate-gene approach, we found that a mutation in syntaxin 1A (Syx1A), Syx1A Δ229, in this deficiency region failed to complement the lethality phenotype of SH0113. To confirm further that the background mutation was associated with Syx1A, we performed three more experiments: First, we examined Syx1A protein level in both the wild type and SH0113 germ-line clones using anti-Syx1A antibody. In the wild type, strong expression of Syx1A was detected in the early stages of oogenesis, and the protein was associated with the membrane (Schulze and Bellen, 1996) (Fig. 2A), but the expression of Syx1A was significantly reduced or lost in the SH0113 germ-line clones (Fig. 2A). Second, we did the rescue experiment by using overexpression of GFP-tagged Syx1A with a UASp vector and found that overexpression of UASp:Syx1A-GFP under the germ-line Gal4 driver Nanos-Gal4 fully alleviated the mislocalization of Grk in the SH0113 germ-line clones (Fig. 2C and E). Third, we removed the P-element from SH0113 using delta 2–3 transposase-mediated P-element excision to determine whether the P-element insertion is involved in the SH0113 mutant phenotypes. The P-element-excised chromosome, FRT SH0113ΔP, complemented CG6954MI02905, a mutant allele of CG6954, but failed to complement Syx1AΔ229 indicating that the P-element insertion does not provide any phenotypes and that the observed mutant phenotypes of SH0113 are solely caused by a mutation in Syx1A. Furthermore, we confirmed that the germ-line clones of SH113ΔP showed Grk mislocalization phenotypes similar to that of the SH0113 germ-line clones. These results confirmed that the Grk mislocalization defect in SH0113 is indeed caused by the mutation in Syx1A (hereafter we refer SH0113 as Syx1ASH0113).
Fig. 2.
Loss of Syx1A protein in the Syx1A mutants and alleviation of the Grk phenotype by overexpression of Syx1A. Syx1A protein detected by anti-Syx1A antibody was lost in the Syx1A germ-line clones without GFP in the nurse cells (A, arrowhead). The Grk-mislocalization phenotype in syx1A germ-line clones without Syx1A overexpression (B and D) can be alleviated by overexpression of Syx1A (UASp:Syx1A-GFP) with Nanos-Gal4 (C and E). Mutant germ-line clones were marked by the absence of LacZ (red) in the nuclei of the nurse cells (B’, C’, D’, and E’). The scale bar is 5 µm.
Syx1A is required for Grk signaling for dorsal follicle-cell differentiation but not for posterior follicle cell patterning
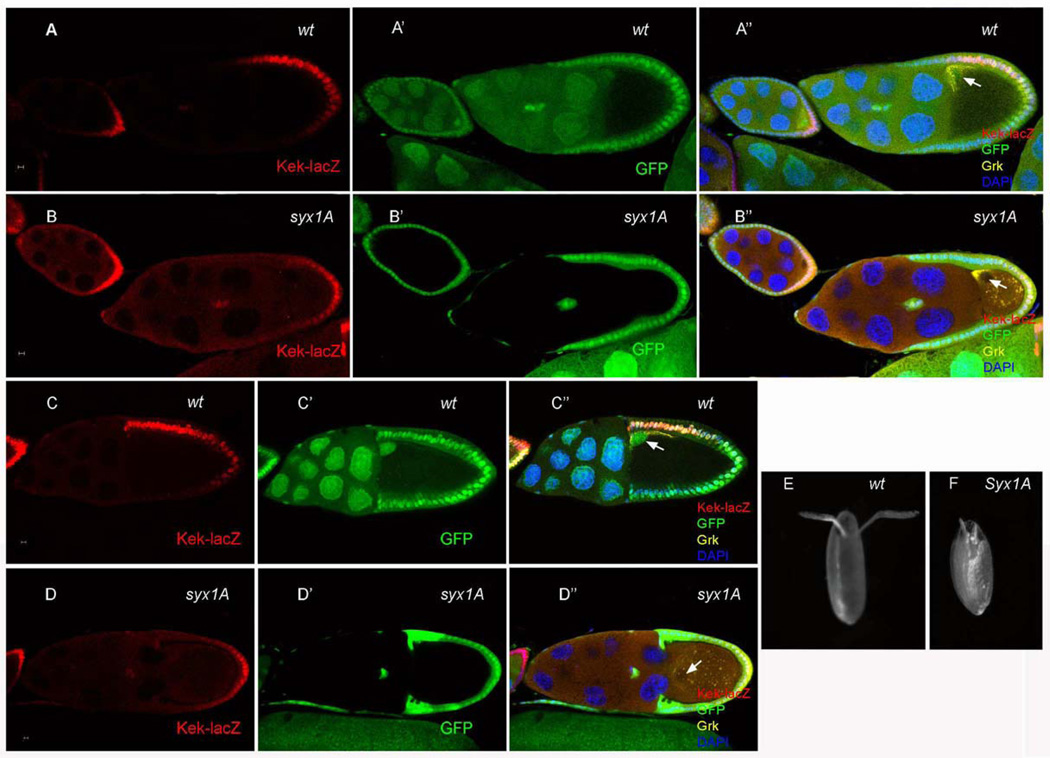
During Drosophila oogenesis, Grk at the oocyte posterior signals to the overlying follicle cells, inducing them to adopt the posterior-follicle-cell fate at stages 6–7. Later, Grk at the dorsal-anterior corner of the oocyte activates EGFR signaling in overlying follicle cells and induces them to adopt a dorsal-cell fate. After EGFR activation, the expression of several genes, such as argos (Zhao and Bownes, 1999), pointed (Morimoto et al., 1996), sprouty (Reich et al., 1999), and kekkon (Ghiglione et al., 1999), are upregulated, and these genes can be used as markers for dorsal follicle-cell differentiation and EGFR activity. To determine whether the mislocalization of Grk protein in Syx1ASH0113 germ-line clones resulted in defects in EGFR signaling and follicle cell differentiation, we examined the expression of kekkon (kek-lacZ) in Syx1ASH0113 mosaic egg chambers (Gonzalez-Reyes and St Johnston, 1998; Poulton and Deng, 2007). As in the wild type, kek-lacZ was expressed in the posterior follicle cells of egg chambers with Syx1A germ-line clones (Fig. 3B), indicating that the Grk-EGFR signaling was normal for posterior-follicle-cell specification. In later stages, however, when the oocyte nucleus and Gurken have been transported to the anterior of the ooctye where the second round of Gurken signaling occurs to specify dorsal follicle cell fate, kek-lacZ was not expressed in the follicle cells adjacent to the oocyte nucleus (Fig. 3D and Fig.S3), indicating that the Grk-EGFR signaling was disrupted at this stage. These findings are consistent with our observations that Grk-protein localization was disrupted in the dorsal region but that the protein localization to the posterior end of the oocyte during early stages appeared normal. The wild-type egg chamber produces two long chorionic appendages at the dorsal anterior end of the mature egg (Fig. 3E). The formation of these dorsal appendages depends on this second round of Grk-EGFR signaling in a dosage-sensitive manner (Deng and Bownes, 1997; Neuman-Silberberg and Schupbach, 1994). In contrast, the absence of Syx1A frequently induced the formation of two small dorsal appendages (Fig. 3F; 79%, n = 101). These results therefore indicate that Syx1A is required for dorsal Grk signaling.
Fig. 3.
Effects on follicle-cell fate markers in Syx1A germ-line clones. kek is expressed in the posterior follicle cells at the early stage and then in the anterior-dorsal follicle cells after stage 7 (A and C). It was expressed at the posterior at the early stage, but it was not expressed at the anterior-dorsal follicle cells after stage 7 in Syx1A germ-line clones (B and D). The length of dorsal appendage was reduced, and the shape was also altered in mutant eggs (F). Mutant germ-line clones are marked by the absence of GFP (green) in the nuclei of the nurse cells (B’ and D’). The arrows show the localization of oocyte nuclei. The scale bar is 5 µm.
Syx1A is associated with Golgi and Rab6-rich particles
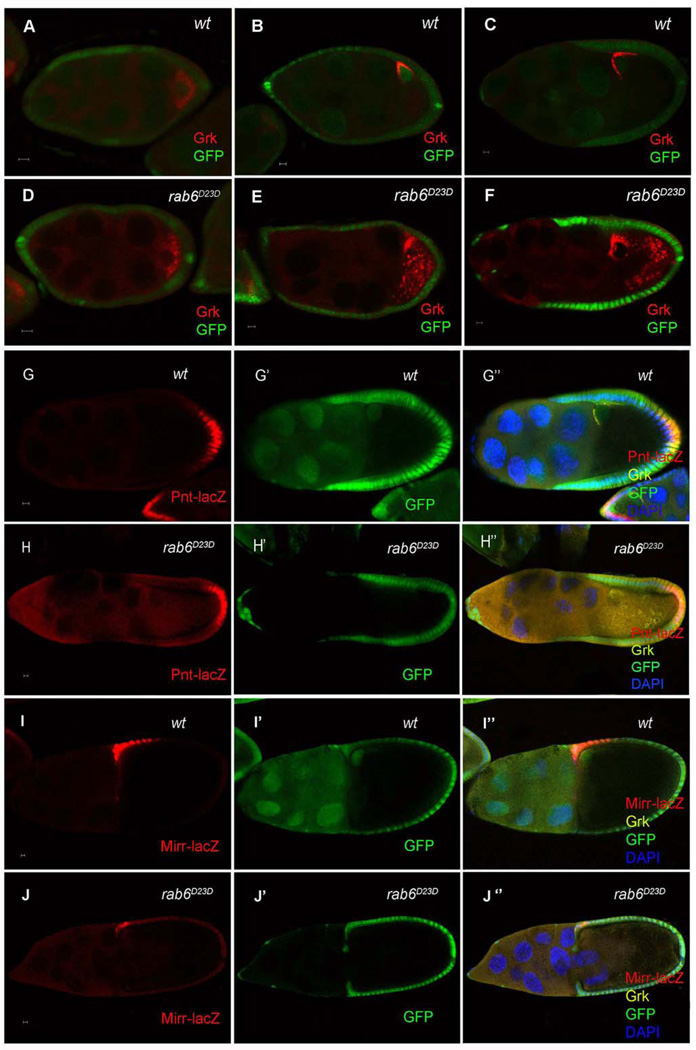
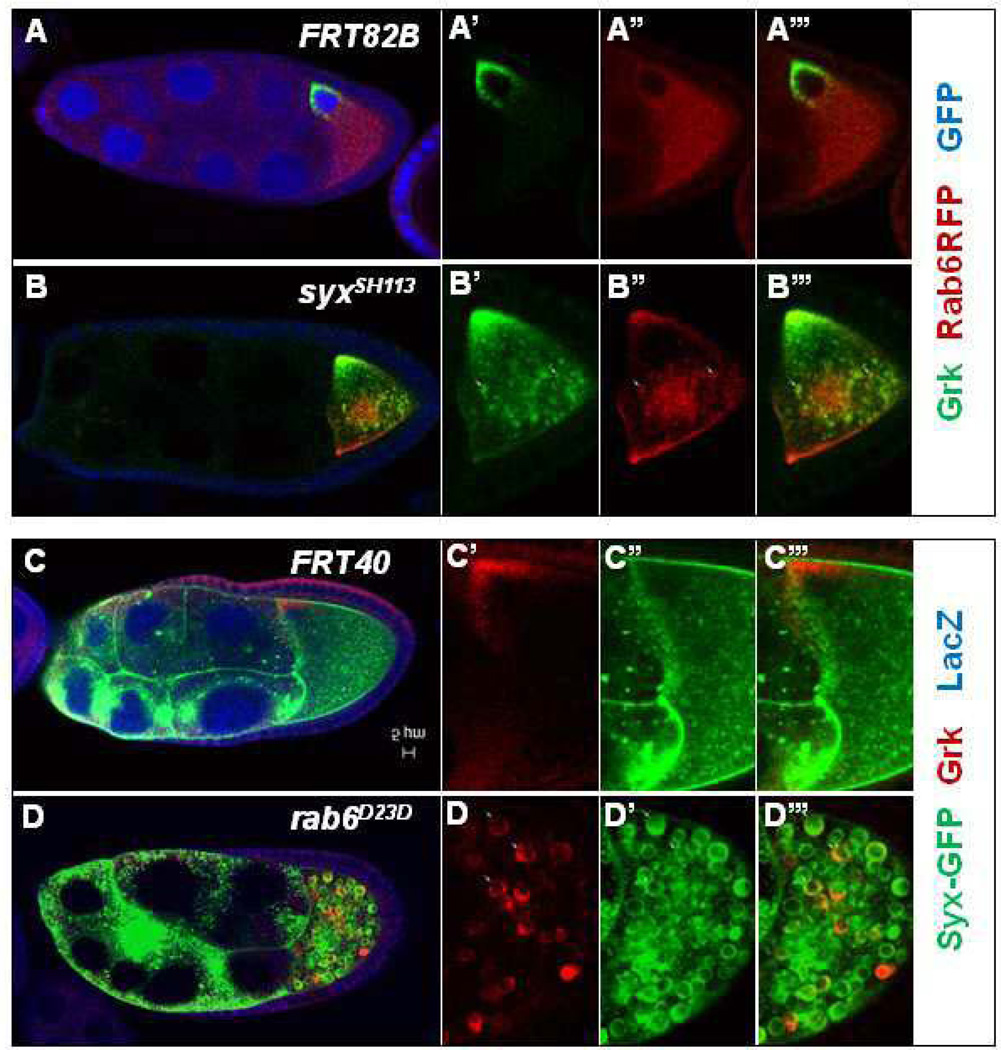
Rab6 GTPase has been shown to regulate Grk localization (Coutelis and Ephrussi, 2007; Januschke et al., 2007), and Januschke et al. (2007) (Januschke et al., 2007) further showed that Rab6 interacts with BicD to transport Grk along microtubules. To determine whether the phenotype in rab6 is similar to that we observed in Syx1A, we compared Grk localization in rab6D23D and Syx1ASH0113 germ-line clones. In the former, Grk protein was localized correctly at the posterior of the oocyte before stage 7 (Fig. 4D), but after stage 7, some was dispersed into the ooplasm (Fig. 4E and F). The timing of the disruption of Grk localization in rab6 germ-line clones is very similar to that in Syx1A. To determine whether this Grk mislocalization also resulted in defects in EGFR signaling in follicle cells, we examined the expression of two EGFR targets in rab6D23D germ-line clones, pointed (pnt) and mirror (mirr). pnt-lacZ was normally expressed in the posterior follicle cells, and its expression was undisrupted in egg chambers bearing the rab6D23D germ-line clones (Fig. 4I). In contrast, the anterior-dorsal follicle-cell marker, mirr-lacZ, showed significantly reduced expression in these egg chambers (Fig. 4J). Rab6, like Syx1A, is therefore specifically required for the second round of Grk-EGFR signaling that specifies the anterior-dorsal follicle cells.
Fig. 4.
Localization of Grk and the follicle cell differentiation in Drosophila rab6D23D mutants. Grk is normally localized in the wild-type (A) and rab6D23D germ-line clone (D) at stage 5. After stage 7, Grk is normally localized at the anterior-dorsal corner (B and C), but Grk was partially mislocalized into the cytoplasm of the oocytes in rab6D23D germ-line clones (E and F). Mutant germ-line clones are marked by the absence of GFP (green) in the nuclei of the nurse cells. The posterior follicle-cell marker pnt was expressed normally in the wild-type and rab6D23 germ-line clones (G and H). The expression of mirr, an anterior-dorsal follicle-cell-fate marker (I), was significantly reduced in rab6D23D germ-line clones (J). Mutant germ-line clones are marked by the absence of GFP (green) in the nuclei of the nurse cells. The scale bar is 5 µm.
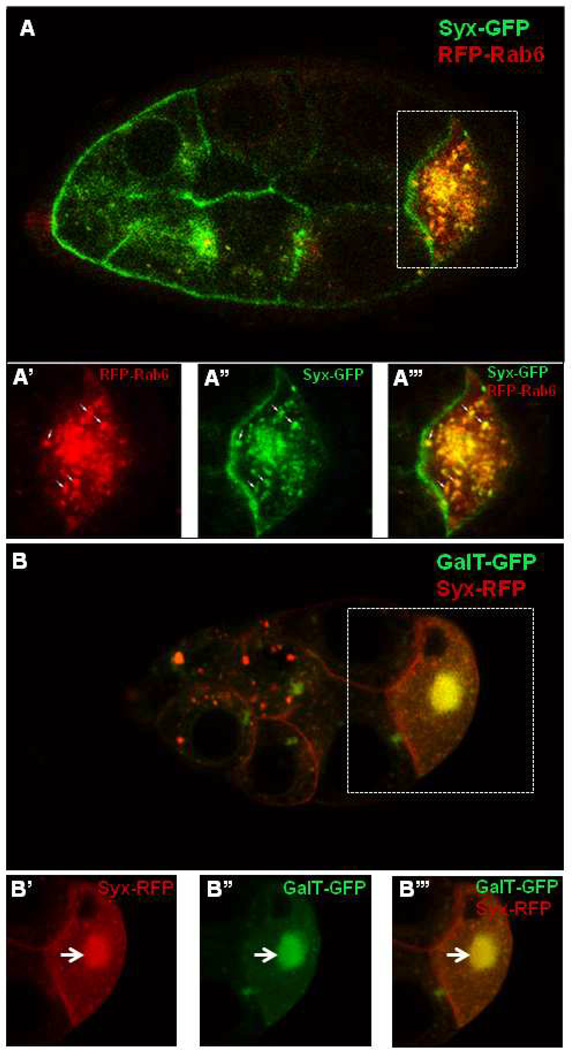
In vertebrate cells, Rab6 is associated with the Golgi and the trans-Golgi network membranes (Del Nery et al., 2006; Mallard et al., 2002; Opdam et al., 2000). In Drosophila oocytes, Rab6 first accumulates at the center of the oocyte at stage 7/8 and then disperses uniformly along the entire oocyte cortex (Januschke et al., 2007). To determine whether Syx1A and Rab6 are localized similarly in the oocyte, we overexpressed a GFP-tagged Syx1A driven by Nanos-Gal4 and RFP-tagged Rab6 with an ubiquitin promoter (ubiRFP-rab6) and found that both Syx1A-GFP and RFP-Rab6 form puncta in the nurse cells and oocytes during oogenesis. Both Rab6 and Syx1A accumulated at the center of the oocyte at stage 7/8 (Fig. 5A–A’’’). In some of these large puncta, RFP-Rab6 and Syx1A-GFP were colocalized (70%, n=250. Fig. 5A–A’’’). After stage 8, Syx1A-GFP was mostly localized at the cortex of the oocyte and the membrane of the nurse cells; some puncta were visible in the nurse cells (data not shown).
Fig. 5.
Syx1A is co-localized with Rab6 and GalT in the oocyte. Syx1A is localized in the membrane and cytoplasm in oocyte and forms puncta in the cytoplasm (A). A–A’’’. At stage 8, Syx1A was accumulated as large puncta and colocalized with Rab6 in the oocyte (arrow). B–B’’’. Syx1A was associated with Golgi membrane marked by GalT in the oocytes, and large puncta included Syx1A and GalT.
Drosophila Rab6 in the nurse cells and oocytes is associated with Golgi membrane and cis-Golgi (Januschke et al., 2007). This localization pattern is similar to that of GalT (UDP-galactose:beta-N-acetylglucosamine beta-1,3-galactosyltransferase) in the center of the oocyte and the cortex and to that of Lava Lamp at the cortex of the oocyte; both are markers for Golgi (Morin et al., 2001; Papoulas et al., 2005). We checked the relationship between Syx1A and Golgi by means of a Golgi-membrane marker (GalT) (Morin et al., 2001). Both GalT and Syx1A accumulated in the center of the oocyte at stage 8, where they were colocalized in some of the puncta (Fig. 5B–B’’’); after stage 8 both were distributed along the cortex of the oocyte (data not shown). The colocalization of both Syx1A and Rab6 with these Golgi proteins indicates that Syx1A and Rab6 were associated with Golgi during Drosophila oogenesis.
Syx1A and Rab6 in Grk trafficking
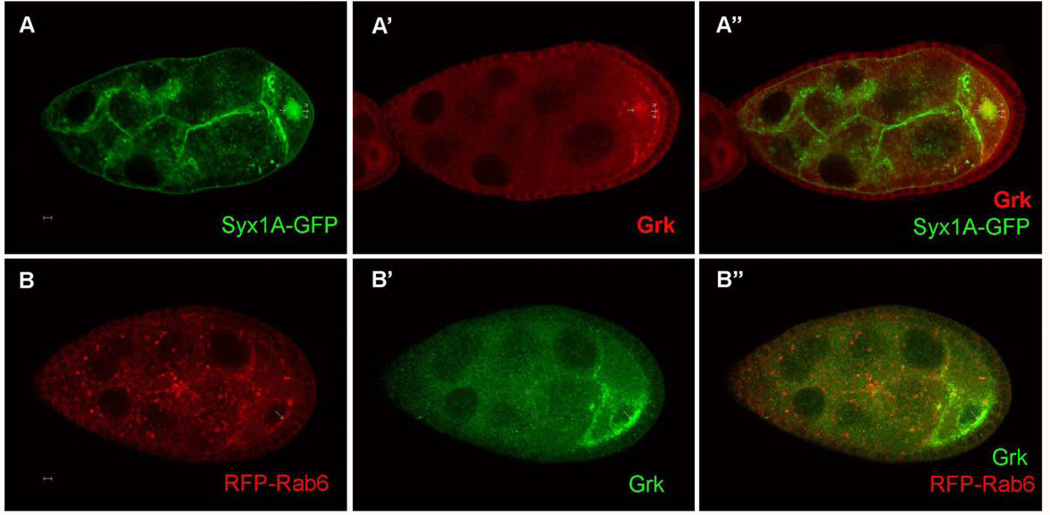
The colocalization in the nurse cells and oocytes and the similarity of Syx1A and Rab6 functions in Grk trafficking indicate that they may act in the similar pathway to regulate Grk trafficking. To analyze further the genetic relationship between Syx1A and Rab6, we expressed RFP-Rab6 in Syx1A germ-line clones and found that both Grk and Rab6 are aggregated into large puncta in the oocyte starting at stage 8, and some mislocalized Grk is colocalized with Rab6 (20%, n=180; Fig. 6B). Since Syx1A is a t-SNARE protein and involved in the target membrane, Syx1A is probably required for the targeting of the vehicle with Grk and Rab6 to the anterior-dorsal corner.
Fig. 6.
Mislocalization of Grk with Rab6 and Syx1A in Drosophila syx1A and rab6 mutants. Grk is normally localized at the anterior-dorsal corner in the wild type after stage 7 (A and C). RFP-Rab6 is localized in the membrane and cytoplasm when overexpressed (A’’). Both Grk and Rab6 were partially accumulated in large vesicles in the cytoplasm of the oocytes in the Syx1A germ-line clones (B–B’’’). The mislocalized Grk and Rab6 were colocalized (B’–B’’’). Syx1A was localized in the membrane and cytoplasm when Syx1A was overexpressed with the Nanos-Gal4 driver (C’). In the rab6D23D germ-line clones, both Grk and Syx1A were partially mislocalized into the cytoplasm of the oocytes and formed large vesicles in the oocyte (D–D’). Mutant germ-line clones are marked by the absence of GFP (green) in the nuclei of the nurse cells (A–B’’’) or by the absence of LacZ (red) in the nuclei of the nurse cell (C–D’’’).
We also made rab6 germ-line clones with Syx1A-GFP expression. In these egg chambers, 90% (n=150) mislocalized Syx1A which form enlarged vesicles in the ooplasm is co-localized with the mislocalized ring-like Grk (Fig. 6D), indicating that Rab6 is probably involved in Grk and Syx1A trafficking to the anterior-dorsal corner. In summary, these results suggest that Syx1A and Rab6 are both required for Grk trafficking to the dorsal anterior corner of the oocyte.
The trafficking of Grk by Syx1A and Rab6 depends on microtubules
The localization of both grk mRNA and Grk protein depends on microtubules (Januschke et al., 2002); we therefore tested the possibility that the movement of Grk with Syx1A and Rab6 also depends on microtubules. We fed the microtubule depolymerizing drug colcemid to Syx1A- or Rab6-overexpressing flies. When Syx1A-GFP-overexpressing flies were treated with colcemid for 12 h, Grk protein was mislocalized to the center and posterior cortex of the oocytes, and some of this mislocalized Grk was colocalized with Syx1A (Fig. 7A). In addition, when ubiRFP-rab6 was expressed, Grk protein was mislocalized along the cortex of the oocyte, and some of it was colocalized with RFP-Rab6 (Fig. 7B). These results indicated that both Syx1A- and Rab6-mediated Grk trafficking depends on the intact microtubule network in the oocyte.
Fig. 7.
Dependence of Syx1A and Rab6-mediated Grk trafficking on microtubules. The microtubule-depolymerizing drug colcemid could disrupt the localization of Syx1A and Rab6, and the mislocalized Syx1A and Rab6 were colocalized with mislocalized Grk protein. The scale bar is 5 µm.
Discussion
The localization of grk mRNA and its protein product in the oocyte is crucial for the establishment of both the AP and dorsal-ventral axes. grk mRNA and protein are localized at the posterior of the oocyte during early oogenesis to activate EGFR signaling in the posterior follicle cells, which in turn send a mysterious signal back to initiate AP axis formation in the oocyte. On the basis of this new AP axis, grk mRNA and protein are subsequently localized at the anterior-dorsal corner of the oocyte to induce dorsal-ventral pattern formation. The localization of grk transcripts depends on the microtubules in the oocyte. These transcripts are transported to the dorsal-anterior destination along the microtubules by Dynein in nonmembranous transport particles and are statically anchored by Dynein within cytoplasmic structures called sponge bodies (Delanoue et al., 2007). Grk protein is believed to be translated from its localized mRNA and is translocated into endoplasmic reticulum; afterward it travels through the Golgi complex and reaches the plasma membrane to be secreted (Bokel et al., 2006; Coutelis and Ephrussi, 2007; Herpers and Rabouille, 2004; Januschke et al., 2007; Kelkar and Dobberstein, 2009). Here, we report our demonstration that localization of Grk protein to the dorsal-anterior region of the oocyte depends on membrane trafficking, differing from the nonmembranous particle transport of its mRNA during this stage (Delanoue et al., 2007). This finding is based on the study of a newly identified hypomorphic allele of Syx1A whose germ-line clones have defective dorsal follicle-cell specification and abnormal Grk protein localization after stage 7. Interestingly, grk mRNA localization is normal until stage 9 in these germ-line clones (100%, n = 20; data not shown). The mislocalization of Grk protein is therefore not a result of its mRNA mislocalization, at least between stages 7 and 9, indicating that the role of Syx1A in Grk protein trafficking is specific.
Using kek-lacZ as a reporter, we found that Syx1ASH0113 germ-line clones strongly disrupted EGFR signaling in the dorsal anterior follicle cells—many egg chambers showed complete loss of dorsal expression of Kek-lacZ—but the majority of mature eggs developed from these clones showed only shortened dorsal appendages, a phenotype indicating disruption of dorsal EGFR signaling but less severe than that of egg chambers with no expression of kek-lacZ in follicle cells adjacent to the oocyte nucleus. This phenotypic discrepancy probably arises because only a small fraction of syx1A germ-line clones develop into mature eggs, and those eggs represent the least marked phenotype. Indeed, we observed many germ-line clones with oocytes smaller than those of wild-type egg chambers at the same developmental stage, perhaps indicating a general role of Syx1A in membrane growth that is essential for oocyte development.
Although no defect was detected in Syx1A clones in Grk posterior localization and signaling to activate EGFR in the posterior follicle cells, we cannot rule out the possibility that Syx1A has no role in the posterior localization of Grk protein. In the germ-line clone of a null allele of syx1A, syx1AΔ229 (Schulze et al., 1995), in contrast, oogenesis is arrested at around stages 5–6, preventing us from examining the effect of Grk-EGFR signaling in the posterior follicle cells (Fig. S1). The new syx1A mutant allele is most likely a hypomorphic allele, sequencing analysis of the open reading frame (ORF) region of the syx1A gene in homozygous syx1AΔ229 mutants did not find any changes in the ORF region, suggesting the mutation is probably at the regulatory region. Nonetheless, our findings suggest that localization of Grk to the dorsal anterior region depends more heavily on Syx1A-dependent membrane trafficking.
Rab6 is known in mammals to promote trafficking at the level of the Golgi apparatus and is colocalized with the Golgi and trans-Golgi markers (Martinez et al., 1994; Opdam et al., 2000; White et al., 1999). Previously, a Rab6-mediated exocytic pathway has been shown to be involved in Grk trafficking in germ-line cells during oogenesis (Coutelis and Ephrussi, 2007; Januschke et al., 2007)(Coutelis and Ephrussi, 2007; Januschke et al., 2007). Our studies suggest that Rab6 has a role similar to that of Syx1A for Grk localization at the dorsal-anterior corner of the oocyte after stage 7. Also, Rab6 appears not to be needed for Grk localization at the posterior before stage 7, a pattern suggesting the functional correlation between Rab6 and Syx1A in Grk trafficking in the oocyte. Consistently, Syx1A and Rab6 can form puncta in the nurse cells and oocytes and are colocalized in some of these puncta, and colocalization of these two proteins with Golgi marker GalT was observed. Furthermore, the mislocalization of Syx1A and Grk in rab6 germ-line clones or of Rab6 and Grk in syx1A germ-line clones indicates that Syx1A and Rab6 can act together for Grk trafficking. We attempted to perform a coimmunoprecipitation study to determine whether these two proteins are physically associated in the oocyte, but no obvious physical interaction between them was detected (data not shown), suggesting that they may not interact directly in the Grk trafficking.
Our study demonstrated the important role vesicle tracking plays in a specific localization of Grk in the oocyte. This process requires the both Syx1A and Rab6, which traffic along the microtubules in the oocyte. About 11 Syntaxin proteins occur in Drosophila (Nakagawa et al., 2011), and they are involved in many different cellular processes. For example, Drosophila Syx5 is required for Golgi reassembly after cell division and for translocation of proteins to the apical membrane (Xu et al., 2002b), and Syx 16 is ubiquitously expressed, appears to be localized to the Golgi apparatus, and may selectively regulate Golgi dynamics (Xu et al., 2002a). Syx 1A is a critical component of the SNARE complex and is essential for synaptic vesicle fusion (Schulze et al., 1995; Wu et al., 1999). Our finding that the mutant for Syx1A can cause such a strong phenotype in the Grk trafficking is exciting. Future studies will determine whether other Syntaxin molecules have similar roles in the oocyte.
Supplementary Material
Highlights.
► Syx1A is required for efficient trafficking of Grk protein for DV patterning ►Syx1A is required for DV patterning ► Syx1A is associated with the Golgi membrane
Acknowledgements
We thank T. Schupbach, J. Poulton, and A. B. Thistle for critically reading the manuscript. We thank A. Guichet, A. Ephrussi, the Bloomington Drosophila Stock Center, The Drosophila Genomics Resource Center, and the Developmental Studies Hybridoma Bank for sending us antibodies, cDNA clones, and fly stocks and the entire staff of the Deng lab for technical help. We also thank K. Riddle and the Biological Science Imaging Facility at FSU for help with confocal microscopy and the Duke University Non-Mammalian Model Systems Flyshop for generating the transgenic flies. A.T. was supported by a Florida/Puerto Rico Affiliate Postdoctoral Fellowship from the American Heart Association. W.M.D. was supported by National Institutes of Health (R01GM072562) and National Science Foundation (IOS-1052333).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bellotto M, Bopp D, Senti KA, Burke R, Deak P, Maroy P, Dickson B, Basler K, Hafen E. Maternal-effect loci involved in Drosophila oogenesis and embryogenesis: P element-induced mutations on the third chromosome. Int J Dev Biol. 2002;46:149–157. [PubMed] [Google Scholar]
- Bokel C, Dass S, Wilsch-Brauninger M, Roth S. Drosophila Cornichon acts as cargo receptor for ER export of the TGFalpha-like growth factor Gurken. Development. 2006;133:459–470. doi: 10.1242/dev.02219. [DOI] [PubMed] [Google Scholar]
- Chen YA, Scheller RH. SNARE-mediated membrane fusion. Nat Rev Mol Cell Biol. 2001;2:98–106. doi: 10.1038/35052017. [DOI] [PubMed] [Google Scholar]
- Coutelis JB, Ephrussi A. Rab6 mediates membrane organization and determinant localization during Drosophila oogenesis. Development. 2007;134:1419–1430. doi: 10.1242/dev.02821. [DOI] [PubMed] [Google Scholar]
- Del Nery E, Miserey-Lenkei S, Falguieres T, Nizak C, Johannes L, Perez F, Goud B. Rab6A and Rab6A' GTPases play non-overlapping roles in membrane trafficking. Traffic. 2006;7:394–407. doi: 10.1111/j.1600-0854.2006.00395.x. [DOI] [PubMed] [Google Scholar]
- Delanoue R, Herpers B, Soetaert J, Davis I, Rabouille C. Drosophila Squid/hnRNP helps Dynein switch from a gurken mRNA transport motor to an ultrastructural static anchor in sponge bodies. Dev Cell. 2007;13:523–538. doi: 10.1016/j.devcel.2007.08.022. [DOI] [PubMed] [Google Scholar]
- Deng WM, Bownes M. Two signalling pathways specify localised expression of the Broad-Complex in Drosophila eggshell patterning and morphogenesis. Development. 1997;124:4639–4647. doi: 10.1242/dev.124.22.4639. [DOI] [PubMed] [Google Scholar]
- Ghiglione C, Carraway KL, 3rd, Amundadottir LT, Boswell RE, Perrimon N, Duffy JB. The transmembrane molecule kekkon 1 acts in a feedback loop to negatively regulate the activity of the Drosophila EGF receptor during oogenesis. Cell. 1999;96:847–856. doi: 10.1016/s0092-8674(00)80594-2. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Reyes A, Elliott H, St Johnston D. Polarization of both major body axes in Drosophila by gurken-torpedo signalling. Nature. 1995;375:654–658. doi: 10.1038/375654a0. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Reyes A, St Johnston D. Patterning of the follicle cell epithelium along the anterior-posterior axis during Drosophila oogenesis. Development. 1998;125:2837–2846. doi: 10.1242/dev.125.15.2837. [DOI] [PubMed] [Google Scholar]
- Grunert S, St Johnston D. RNA localization and the development of asymmetry during Drosophila oogenesis. Curr Opin Genet Dev. 1996;6:395–402. doi: 10.1016/s0959-437x(96)80059-1. [DOI] [PubMed] [Google Scholar]
- Herpers B, Rabouille C. mRNA localization and ER-based protein sorting mechanisms dictate the use of transitional endoplasmic reticulum-golgi units involved in gurken transport in Drosophila oocytes. Mol Biol Cell. 2004;15:5306–5317. doi: 10.1091/mbc.E04-05-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Januschke J, Gervais L, Dass S, Kaltschmidt JA, Lopez-Schier H, St Johnston D, Brand AH, Roth S, Guichet A. Polar transport in the Drosophila oocyte requires Dynein and Kinesin I cooperation. Curr Biol. 2002;12:1971–1981. doi: 10.1016/s0960-9822(02)01302-7. [DOI] [PubMed] [Google Scholar]
- Januschke J, Nicolas E, Compagnon J, Formstecher E, Goud B, Guichet A. Rab6 and the secretory pathway affect oocyte polarity in Drosophila. Development. 2007;134:3419–3425. doi: 10.1242/dev.008078. [DOI] [PubMed] [Google Scholar]
- Kelkar A, Dobberstein B. Sec61beta, a subunit of the Sec61 protein translocation channel at the endoplasmic reticulum, is involved in the transport of Gurken to the plasma membrane. BMC Cell Biol. 2009;10:11. doi: 10.1186/1471-2121-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klusza S, Deng WM. At the crossroads of differentiation and proliferation: precise control of cell-cycle changes by multiple signaling pathways in Drosophila follicle cells. Bioessays. 2011;33:124–134. doi: 10.1002/bies.201000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Schier H, St Johnston D. Delta signaling from the germ line controls the proliferation and differentiation of the somatic follicle cells during Drosophila oogenesis. Genes Dev. 2001;15:1393–1405. doi: 10.1101/gad.200901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallard F, Tang BL, Galli T, Tenza D, Saint-Pol A, Yue X, Antony C, Hong W, Goud B, Johannes L. Early/recycling endosomes-to-TGN transport involves two SNARE complexes and a Rab6 isoform. J Cell Biol. 2002;156:653–664. doi: 10.1083/jcb.200110081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez O, Schmidt A, Salamero J, Hoflack B, Roa M, Goud B. The small GTP-binding protein rab6 functions in intra-Golgi transport. J Cell Biol. 1994;127:1575–1588. doi: 10.1083/jcb.127.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto AM, Jordan KC, Tietze K, Britton JS, O'Neill EM, Ruohola-Baker H. Pointed, an ETS domain transcription factor, negatively regulates the EGF receptor pathway in Drosophila oogenesis. Development. 1996;122:3745–3754. doi: 10.1242/dev.122.12.3745. [DOI] [PubMed] [Google Scholar]
- Morin X, Daneman R, Zavortink M, Chia W. A protein trap strategy to detect GFP-tagged proteins expressed from their endogenous loci in Drosophila. Proc Natl Acad Sci U S A. 2001;98:15050–15055. doi: 10.1073/pnas.261408198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy M, Schwarz TL. The exocyst component Sec5 is required for membrane traffic and polarity in the Drosophila ovary. Development. 2004;131:377–388. doi: 10.1242/dev.00931. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Fujiwara-Fukuta S, Yorimitsu T, Tanaka S, Minami R, Shimooka L, Nakagoshi H. Spatial and temporal requirement of defective proventriculus activity during Drosophila midgut development. Mech Dev. 2011;128:258–267. doi: 10.1016/j.mod.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Neuman-Silberberg FS, Schupbach T. The Drosophila dorsoventral patterning gene gurken produces a dorsally localized RNA and encodes a TGF alpha-like protein. Cell. 1993;75:165–174. [PubMed] [Google Scholar]
- Neuman-Silberberg FS, Schupbach T. Dorsoventral axis formation in Drosophila depends on the correct dosage of the gene gurken. Development. 1994;120:2457–2463. doi: 10.1242/dev.120.9.2457. [DOI] [PubMed] [Google Scholar]
- Nilson LA, Schupbach T. EGF receptor signaling in Drosophila oogenesis. Curr Top Dev Biol. 1999;44:203–243. doi: 10.1016/s0070-2153(08)60471-8. [DOI] [PubMed] [Google Scholar]
- Oh SW, Kingsley T, Shin HH, Zheng Z, Chen HW, Chen X, Wang H, Ruan P, Moody M, Hou SX. A P-element insertion screen identified mutations in 455 novel essential genes in Drosophila. Genetics. 2003;163:195–201. doi: 10.1093/genetics/163.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opdam FJ, Echard A, Croes HJ, van den Hurk JA, van de Vorstenbosch RA, Ginsel LA, Goud B, Fransen JA. The small GTPase Rab6B, a novel Rab6 subfamily member, is cell-type specifically expressed and localised to the Golgi apparatus. J Cell Sci. 2000;113(Pt 15):2725–2735. doi: 10.1242/jcs.113.15.2725. [DOI] [PubMed] [Google Scholar]
- Papoulas O, Hays TS, Sisson JC. The golgin Lava lamp mediates dynein-based Golgi movements during Drosophila cellularization. Nat Cell Biol. 2005;7:612–618. doi: 10.1038/ncb1264. [DOI] [PubMed] [Google Scholar]
- Poulton JS, Deng WM. Cell-cell communication and axis specification in the Drosophila oocyte. Dev Biol. 2007;311:1–10. doi: 10.1016/j.ydbio.2007.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulton JS, Huang YC, Smith L, Sun J, Leake N, Schleede J, Stevens LM, Deng WM. The microRNA pathway regulates the temporal pattern of Notch signaling in Drosophila follicle cells. Development. 2011;138:1737–1745. doi: 10.1242/dev.059352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queenan AM, Ghabrial A, Schupbach T. Ectopic activation of torpedo/Egfr, a Drosophila receptor tyrosine kinase, dorsalizes both the eggshell and the embryo. Development. 1997;124:3871–3880. doi: 10.1242/dev.124.19.3871. [DOI] [PubMed] [Google Scholar]
- Ray RP, Schupbach T. Intercellular signaling and the polarization of body axes during Drosophila oogenesis. Genes Dev. 1996;10:1711–1723. doi: 10.1101/gad.10.14.1711. [DOI] [PubMed] [Google Scholar]
- Reich A, Sapir A, Shilo B. Sprouty is a general inhibitor of receptor tyrosine kinase signaling. Development. 1999;126:4139–4147. doi: 10.1242/dev.126.18.4139. [DOI] [PubMed] [Google Scholar]
- Riechmann V, Ephrussi A. Axis formation during Drosophila oogenesis. Curr Opin Genet Dev. 2001;11:374–383. doi: 10.1016/s0959-437x(00)00207-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Kreitzer G, Musch A. Organization of vesicular trafficking in epithelia. Nat Rev Mol Cell Biol. 2005;6:233–247. doi: 10.1038/nrm1593. [DOI] [PubMed] [Google Scholar]
- Roth S, Neuman-Silberberg FS, Barcelo G, Schupbach T. cornichon and the EGF receptor signaling process are necessary for both anterior-posterior and dorsal-ventral pattern formation in Drosophila. Cell. 1995;81:967–978. doi: 10.1016/0092-8674(95)90016-0. [DOI] [PubMed] [Google Scholar]
- Schuck S, Simons K. Polarized sorting in epithelial cells: raft clustering and the biogenesis of the apical membrane. J Cell Sci. 2004;117:5955–5964. doi: 10.1242/jcs.01596. [DOI] [PubMed] [Google Scholar]
- Schulze KL, Bellen HJ. Drosophila syntaxin is required for cell viability and may function in membrane formation and stabilization. Genetics. 1996;144:1713–1724. doi: 10.1093/genetics/144.4.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze KL, Broadie K, Perin MS, Bellen HJ. Genetic and electrophysiological studies of Drosophila syntaxin-1A demonstrate its role in nonneuronal secretion and neurotransmission. Cell. 1995;80:311–320. doi: 10.1016/0092-8674(95)90414-x. [DOI] [PubMed] [Google Scholar]
- Tian AG, Deng WM. Lgl and its phosphorylation by aPKC regulate oocyte polarity formation in Drosophila. Development. 2008;135:463–471. doi: 10.1242/dev.016253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian AG, Deng WM. Par-1 and Tau regulate the anterior-posterior gradient of microtubules in Drosophila oocytes. Dev Biol. 2009;327:458–464. doi: 10.1016/j.ydbio.2008.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Buskirk C, Schupbach T. Versatility in signalling: multiple responses to EGF receptor activation during Drosophila oogenesis. Trends Cell Biol. 1999;9:1–4. doi: 10.1016/s0962-8924(98)01413-5. [DOI] [PubMed] [Google Scholar]
- van Eeden F, St Johnston D. The polarisation of the anterior-posterior and dorsal-ventral axes during Drosophila oogenesis. Curr Opin Genet Dev. 1999;9:396–404. doi: 10.1016/S0959-437X(99)80060-4. [DOI] [PubMed] [Google Scholar]
- White J, Johannes L, Mallard F, Girod A, Grill S, Reinsch S, Keller P, Tzschaschel B, Echard A, Goud B, Stelzer EH. Rab6 coordinates a novel Golgi to ER retrograde transport pathway in live cells. J Cell Biol. 1999;147:743–760. doi: 10.1083/jcb.147.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MN, Fergestad T, Lloyd TE, He Y, Broadie K, Bellen HJ. Syntaxin 1A interacts with multiple exocytic proteins to regulate neurotransmitter release in vivo. Neuron. 1999;23:593–605. doi: 10.1016/s0896-6273(00)80811-9. [DOI] [PubMed] [Google Scholar]
- Xu H, Boulianne GL, Trimble WS. Drosophila syntaxin 16 is a Q-SNARE implicated in Golgi dynamics. J Cell Sci. 2002a;115:4447–4455. doi: 10.1242/jcs.00139. [DOI] [PubMed] [Google Scholar]
- Xu H, Brill JA, Hsien J, McBride R, Boulianne GL, Trimble WS. Syntaxin 5 is required for cytokinesis and spermatid differentiation in Drosophila. Dev Biol. 2002b;251:294–306. doi: 10.1006/dbio.2002.0830. [DOI] [PubMed] [Google Scholar]
- Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- Yu J, Zheng Y, Dong J, Klusza S, Deng WM, Pan D. Kibra functions as a tumor suppressor protein that regulates Hippo signaling in conjunction with Merlin and Expanded. Dev Cell. 2010;18:288–299. doi: 10.1016/j.devcel.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Bownes M. Misexpression of argos, an inhibitor of EGFR signaling in oogenesis, leads to the production of bicephalic, ventralized, and lateralized Drosophila melanogaster eggs. Dev Genet. 1999;25:375–386. doi: 10.1002/(SICI)1520-6408(1999)25:4<375::AID-DVG11>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.