Abstract
Chronic opioid exposure leads to changes in gene expression (functional changes), resulting in structural changes in neural circuits that are linked to eventually behavioral changes. Little is known about the cellular and molecular mechanisms of how such changes occur. In this study, we found that mu-opioid [D-Ala2, N-Me-Phe4, Gly5-ol]-enkephalin (DAMGO) and morphine exposure led to dynamic changes in neural differentiation- and growth-associated genes, IκBα and NTRK2 (TrkB), in differentiating and differentiated human neuroblastoma SH-SY5Y cells. Chromatin immunoprecipitation-polymerase chain reaction (ChIP-PCR) analysis revealed that binding of NF-κB/p65 to the IκBα promoter in living cells was temporally altered when the cells were exposed to morphine. The changes in gene expression correlated with the changes in neurite length of the RA-differentiating and RA-differentiated neuron-like cells. Our findings for the first time showed that TrkB signaling and NF-κB/IκBα signaling temporally correlated with each other in response to single-dose and repeated mu-opioid treatment in differentiating and differentiated human neuron-like cells. The findings from this human cell study in vitro indicate that both relatively high single-dose and chronic opioid exposure may induce the structural changes in the developing human brain and the adult brain by altering the expression of neuronal differentiation- and neurite outgrowth-related genes IκBa and TrkB in vivo.
Keywords: SH-SY5Y cell model, NF-κB, IκBα, Morphine, BDNF, TrkB
Introduction
A generally accepted hypothesis for the development of opioid addiction is that chronic opioid exposure leads to altered gene expression that results in neuroadaptations that are eventually linked to addictive behavioral changes [1]. Thus, a better understanding of the molecular mechanisms of chronic opioid-induced gene expression will provide us insights into how opioid tolerance and addiction develop and may eventually provide new molecular targets for the prevention and treatment of opioid tolerance and dependence. We previously elucidated the detailed molecular mechanisms of the nerve growth factor (NGF)-mediated delta opioid receptor (OPRD1 or DOR) gene expression during NGF-induced differentiation [2, 3, 4, 5], showing that sustained activation of phosphoinositol-3-kinase (PI3K)/Akt/nuclear factor κB (NF-κB) signaling is required for NGF-modulated OPRD1 gene expression [5]. We hypothesized that neurotrophic factor-mediated PI3K/Akt/NF-κB signaling might be important for neuronal differentiation and the development of opioid tolerance and dependence [5, 6]. This hypothesis was further supported by recent behavioral studies by others that have shown that both Akt and NF-κB signaling are important for opioid action in animal models [7, 8]. However, little is known about the molecular mechanisms of the involvement of both neurotrophic factor signaling and NF-κB signaling in opioid function in humans.
NF-κB is a master transcription factor, which plays a critical role in immune responses, neural plasticity, and long-term memory. NF-κB signaling is required in synaptic signaling and various behavior alterations [9]. NF-κB signaling regulates many opioid action-related genes, including three opioid receptor genes ([6], references therein), brain-derived neurotrophic factor (BDNF) [10], inhibitor of NF-κB (IκBα) [11]. Chronic morphine treatment and naloxone-precipitated withdrawal increase the mRNA level of NF-κB-dependent gene IκBα in the frontal cortex [12]. Inhibition of NF-κB activity led to the reduction of naloxone-precipitated withdrawal signs in vitro [13] and in vivo [8]. Chronic morphine induced neuronal structure changes in nucleus accumbens (ventral striatum) [14], which are associated with motivational withdrawal. Moreover, neurotrophic tyrosine kinase receptor 2 (NTRK2 or TrkB) signaling and NF-κB signaling are known to be involved in synaptic plasticity [9, 15]. Thus, we hypothesized that chronic morphine might act through regulation of NF-κB signaling-dependent and neuronal growth- and differentiation-associated genes such IκBα and TrkB to induce changes in the morphology of human neurons, which may be eventually involved in morphine-induce behavioral changes.
In this study, we examined the effects of mu-opioid DAMGO and morphine on the expression of neuronal activity-regulated TrkB gene and NF-κB-dependent gene IκBα. We also evaluated the correlation between changes in gene expression and structural changes in differentiating and differentiated human neuroblastoma cells that express both functional delta and mu opioid receptors (OPRM1) that are often co-expressed in various neurons in the human nervous system.
Materials and Methods
Cell culture and reagents
SH-SY5Y cells were purchased from American Type Tissue Culture (ATCC), cultured and maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with nutrient mixture F12 (Ham) (1:1) (Invitrogen, Grand Island, NY) and 10% fetal bovine serum (FBS) (Atlanta Biologicals, USA) (Medium A). Morphine sulfate, [D-Ala2, N-Me-Phe4, Gly5-ol]-enkephalin acetate salt (DAMGO), naloxone, and retinoic acid (RA) were purchased from Sigma-Aldrich (St. Louis, MO).
Opioid treatment
Cells were harvested using 0.05% trypsin-EDTA (Invitrogen) and plated at a concentration of 1 or 2 ×106 cells/4ml in 60mm dishes. Cells were treated with or without 10 μM RA for varied times (1 or 4, or 5 days). For 4- and 5-day RA treatment, cells were re-fed with fresh 10 μM RA and the medium at day 2 or day 3 during the treatment, respectively. After RA treatment, medium A (see above) was changed to DMEM+1%ITS-X (Invitrogen) (medium B) and cells were treated with 10 μM DAMGO and morphine at varied times.
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA was isolated according to the Tri Reagent® protocol from Molecular Research Center (Cincinnati, OH) as previously described [5]. cDNA was synthesized using High Capacity cDNA Reverse Transcription Kit (Invitrogen). Sybr® Green Supermix (Bio-Rad, Hercules, CA) was used for real time PCR analysis on IQ5® Multicolor Real-time PCR Detection System (Bio-Rad). PCR primers used were as follows: human IκBα sense, 5′-cgcccaagcacccggataca-3′; antisense, 5′-agggcagctcgtcctctgtga-3′; human TrkB sense, 5′-ccaagaatgagtatgggaagg-3′, antisense, 5′-gcatagaccgagagatgttcc-3′ (TrkB primers were designed by Aaron Perey in the lab). All PCR primers were synthesized by Integrated DNA Technology, Inc. (Coralville, Iowa). Real time PCR conditions: preheated at 95 °C for 3 min; at 94 °C for 20 sec and at 60 °C for 1 min for 50 cycles. PCR reactions were carried out in triplicate or more replicates to determine the gene mRNA levels. Melting curve was used to verify PCR products. The fold changes in mRNA levels relative to β-actin were determined by the equation: fold change = 2−ΔΔCT where ΔCT = CT(gene of interest) − CT(β-actin) and ΔΔCT = ΔCT(treated)−ΔCT(untreated) for each experiment.
Chromatin immunoprecipitation (ChIP)-PCR assays
ChIP assays were performed according to the published protocol [2]. Briefly, 2x106 SH-SY5Y cells were treated on a 60-mm dish with or without DAMGO or Morphine for varied times. Chromatin in live cells was cross-linked with 1% formaldehyde. DNA was sheared by sonication after the cells were washed and lysed in lysis buffer. Cross-linked DNA/protein complexes were isolated using the ChIP assay kit according to the manufacturer’s protocol (Millipore, Boston, MA, USA). Chromatin was immunoprecipitated using anti-NF-κB/p65 antibody (Millipore). The primers for the IκBα promoter 5′-upstream region of the transcription initial site are: sense, 5′-GACGACCCCAATTCAAATCG-3′; antisense, 5′-TCAGGCTCGGGGAATTTCC-3′ (Millipore). qPCR was carried out to detect and quantify DNA as described above. The fold change in DNA binding to the IκBα gene relative to input DNA was determined as described previously [2].
Determination of neurite length
Images of drug-treated cells were obtained using the VistaVision™ Phase Contrast Microscope USB camera (VWR, Radnor, PA). Neurite length was measured using Neuron J plugin with the NIH Image J program [16]. The drug treatment of the images was unknown to the investigator who carried out the measurement at the time. All neurites within the image frame were counted. No threshold was set for neurite length. Any neurites that were not entirely within the frame were excluded. Once all neurites had been traced, the measure tracings tool was used to measure tracings.
Statistical analysis
Student’s t test was used to determine whether or not the sample is significantly different from the control. Statistically significant differences between samples and controls were indicated by single * (p < 0.05), double **(p < 0.01), and triple asterisks ***(p < 0.001).
Results
Single-dose morphine down-regulated the expression of both IκBα and TrkB genes
Previously, we showed that NGF-sustained activation of PI3K/Akt and NF-κB signaling pathways play a key role in the epigenetic upregulation of the OPRD1 gene expression [3]. Both PI3K/Akt and NF-κB signaling pathways are required for RA-induced differentiation of SH-SY5Y cells and are constitutively activated in the RA-differentiated cells [17, 18]. In addition, PI3K/Akt and NF-κB signaling pathways are “constitutively” activated in diverse neurons [19, 20]. Thus, the differentiating and differentiated human SH-SY5Y cells may mimics the developing and mature neurons in humans, respectively. We examined whether single-dose morphine would affect the expression of the IκBα gene in differentiated SH-SY5Y cells. Morphine treatment for 24h led to a decrease of 16% in the IκBα mRNA level (Fig 1B). Since BDNF signaling to NF-κB could be also through TrkB [21] that is important for maintaining neuronal morphology and adult neurogenesis [22], we further examined the effects of morphine on TrkB gene expression (Fig. 1B). Morphine exposure resulted in a reduction of 17% in the TrKB mRNA level. DAMGO treatment for 24h also decreased both IκBα and TrkB mRNA levels in the RA-differentiated cells (data not shown). Since both BDNF/TrkB/PI3K/Akt and NF-κB signaling are important for neurotrophin-induced neuronal differentiation and development [23, 24], we further examine the effect of morphine on expression of IκBα and TrkB in differentiating SH-SY5Y cells. We found that morphine treatment resulted in a decrease of 11% and 20%, respectively, in both IκBα and TrkB mRNA levels (Fig. 1A) during RA-induced differentiation of 24 h.
Figure 1. Single-dose morphine down regulated the expression of both IκBα and TrkB genes in differentiating and differentiated human neuroblastoma cells.
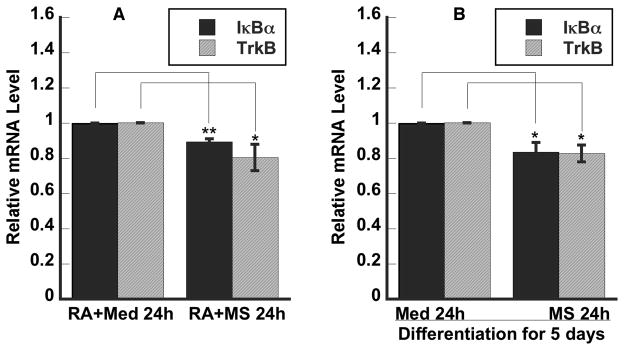
RA, retinoic acid; Med, culture medium; MS, morphine sulfate. A. RA in medium A with or without MS for 24 h. B. RA for 5 days and then with or without MS for 24 h in medium B. Relative mRNA levels were determined using qPCR. Data are presented as mean ± S.E.M. (n= 3–5; *, p<0.05; **, p<0.01) from 3–5 independent experiments.
NF-κB/p65 bound to the IκBα promoter in morphine-treated human neuroblastoma cells
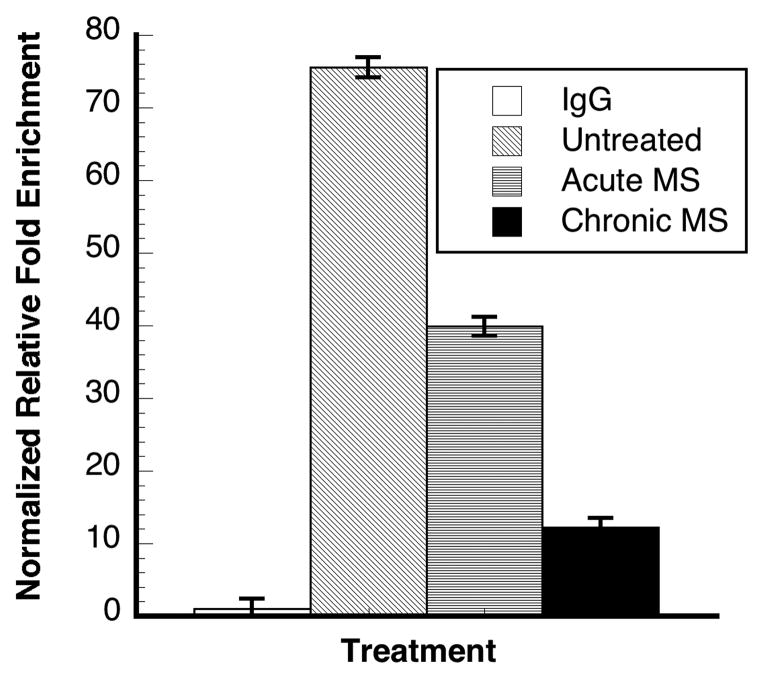
To evaluate whether the morphine-mediated IκBα gene expression is indeed NF-κB/p65-dependent, we examine the association of NF-κB/p65 protein with the NF-κB binding cis-element region of the IκBα promoter using the ChIP analysis method according to the previous reported protocol [4]. The extent of the binding of p65 to the promoter of IκBα at 24h (Fig. 2) was time-dependent and consistent with that of morphine-mediated IκBα gene expression at the same time point (Fig. 1). This result strongly suggests that morphine-mediated IκBα gene expression under the current conditions is likely NF-κB/p65-dependent.
Figure 2. NF-κB/p65 bound to the IκBα promoter in morphine-treated human neuroblastoma cells.
IgG, mouse immunoglobin G; acute MS, MS for 30 min; chronic MS, MS for 24h. Y-axis presents the relative fold chromatin enrichment by anti-NF-κB/p65 in comparison with IgG enrichment. Data are presented in mean ± S.D. from three determinations of representative of two experiments.
Mu opioid-mediated structural changes in differentiating and differentiated human neuroblastoma cells
To examine the effects of the mu-opioid agonist DAMGO and morphine on morphological changes in differentiated neuroblastoma cells, we carried out the measurement of neurite length in DAMGO- and morphine-treated and untreated cells. DAMGO treatment for 24h led to a significant decrease in neurite length of differentiated SH-SY5Y cells (Fig. 3A). Morphine treatment also resulted in an appreciable decrease in neurite length in differentiating and differentiated SH-SY5Y cells (Fig. 3B, C). The extent of decrease in neurite length correlated with that of the decrease in the relative IκBα and TrkB mRNA levels that were down-regulated by DAMGO (data not shown) and morphine (Fig. 1), respectively.
Figure 3. Mu opioids mediated neurite length in differentiating and differentiated human neuroblastoma cells.
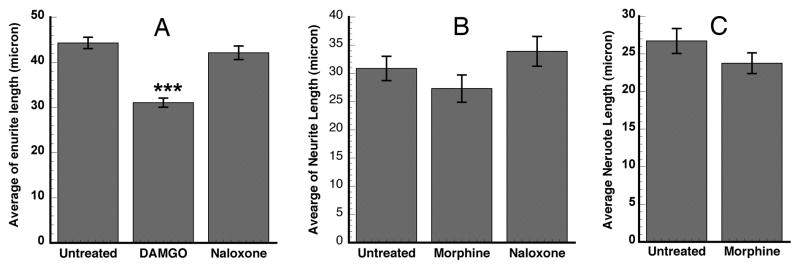
A and B: Cells were differentiated by RA-induction for 5 days in medium A and then treated with 10 μM DAMGO (Fig. 3A) or 10 μM morphine (Fig. 3B) or 10 μM universal opioid antagonist naloxone in medium B for 24h. C. Treated with both 10 μM RA and 10 μM morphine at the same time for 24 h in medium A (Fig. 3C). The average of neurite length was determined by dividing the total neurite length of all measurements by the total number of measured neurites. Data are presented as mean ± S.E.M. (n= 279–416 for A, ***, p<0.001; 107–156 for B; and 182–190 for C).
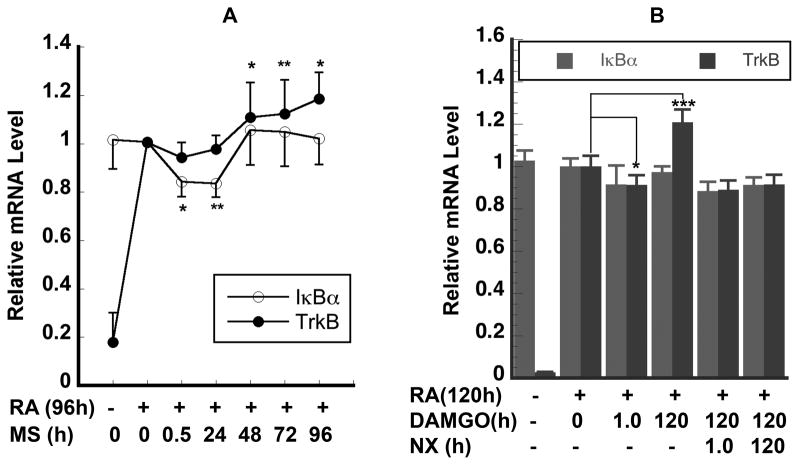
Repeated DAMGO and morphine exposure induced biphasic modulation of the TrkB and IκBα mRNA levels
To further evaluate if differentiated SH-SY5Y cells might mimic the responses of the adult dopamine neurons to chronic morphine exposure, we examined the effect of repeated morphine exposure on the expression of these two critical genes IκBα and TrkB. The cells were exposed to 10 μM DAMGO or morphine daily and the medium was also changed daily. Under such experimental conditions, we found that both morphine (Fig. 4A) and DAMGO (Fig. 4B) treatments resulted in the biphasic expression of IκBα and TrkB genes. First, morphine treatment induced a decrease in the IκBα and TrkB mRNA levels at 30 min (acute stage) (Fig. 4A). After 48h, repeated morphine exposure resulted in an appreciable increase of 6% in the IκBα mRNA level and increased the TrkB mRNA level by 11–18% higher than that of the untreated cells (Fig. 4A). Similar biphasic TrkB mRNA responses were also observed in DAMGO treated cells at 1h and 120h (Fig. 4B). Such biphasic response is very similar to the pattern of changes in the cAMP level in the classic mouse neuroblastoma x rat glioma NG-108-15 hybrid cell model [25] and in the rat brain [26] after chronic morphine exposure. Thus, both IκBα and TrkB transcripts should be considered as transcriptional biomarkers of morphine actions in human neurons.
Figure 4. Repeated DAMGO and morphine exposure induced biphasic modulation of the TrkB and IκBα mRNA levels.
A. Cells were RA-differentiated for 4 days and then daily treated with morphine to give a total morphine exposure time of 0.5, 24, 48, 72, and 96h respectively. B. Cells were RA-differentiated for 5 days and then daily treated with 10 μM DAMGO or 10 μM naloxone to give a total DAMGO or naloxone exposure time of 1.0 and 120h, respectively. In Fig 4A, data are presented as mean ± S.E.M. obtained from 5–7 independent experiments; in Fig 4B, data are represented as mean ± S.D. of 3–6 replicates of two separate samples (*, p< 0.05; **, p<0.01; *** p<0.001).
Naloxone blocked DAMGO-induced TrkB mRNA overshoot
To evaluate whether the effects of morphine and DAMGO were through opioid receptor signaling, we examine the effect of naloxone on DAMGO-induced TrkB mRNA overshoot. After cells were RA-differentiated for 5 days and then daily re-fed with medium B for 5 days, acute 10 μM DAMGO treatment for 1h led to a decrease of 9% in the TrkB mRNA level (Fig 4B). On the other hand, repeated DAMGO treatment daily for 5 days led to an increase of 21% in the TrkB mRNA level (Fig. 4B) after 5 day-RA treatment. An overall rebound of 30% in TrkB mRNA from the acute DAMGO stage to the 5-day DAMGO stage was observed. Naloxone treatment for 1h reversed the DAMGO-sensitized expression of TrkB gene to the level reached by acute DAMGO exposure. Naloxone treatment for 120 h also brought the IκBα mRNA level to that reached by acute DAMGO-treatment (Fig. 4B). Furthermore, a similar naloxone-induced TrkB mRNA level reverse was also observed in morphine-treated cells (data not shown). Taken together, these results show that mu-opioid mediated expression of the TrkB gene may be involved in opioid-induced sensitization in humans.
Discussion
A comprehensive understanding of the molecular mechanisms of opioid actions in humans will eventually provide novel molecular targets for pain management and for the prevention and treatment of opioid tolerance and addiction. With limited availability and lack of rigorous controls, the postmortem human tissues have limitations for detailed mechanistic studies. A good human cell model that can mimic tissue-specific neurons that are involved in drug actions would be a valuable tool to unravel the molecular basis of action of opioids in humans. Taken together with our studies of the rat PC12 cell model [5] and others’ observations on the key role of NF-κB [13] and neurotrophins in opioid actions in rodent models [27, 28], we previously hypothesized that neurotrophin-mediated PI3K/Akt and NF-κB signaling might work together to regulate opioid actions in vivo [5]. Our rat PC12 cell model studies show that NGF/TrkA/PI3K/Akt/NF-κB signaling can crosstalk with OPRD1 signaling to modulate the morphology of differentiating neurons (Sen et al. and Chen, manuscript in preparation). In this study, we examined the expression of TrkB and IκBα genes in differentiating and differentiated human SH-SH5Y cells. Single dose DAMGO or morphine suppressed the expression of both TrkB and IκBα genes (Fig. 1). The reduction in the IκBα mRNA level correlates well with the extent of binding of p65 to the IκBα promoter (Fig. 2). Our data have shown that reduction in the TrkB mRNA and IκBα mRNA levels correlates well with the reduction in neurite length (Fig. 3). Furthermore, repeated DAMGO or morphine exposure up-regulated the TrkB gene (Fig. 4). Such uprgulation is also naloxone-dependent (Fig. 4), indicating the action is opioid receptor-mediated, which is also consistent with morphine-mediated reduction in OPRM1 mRNA in the same cell model [29]. Although the SH-SH5Y cell line has been used as a human cell model for studying cellular opioid actions for many years [30], to our knowledge, we are the first to clearly show that both acute and repeated mu-opioid DAMGO and morphine exposure can temporarily modulate the expression of both TrkB and IκBα genes in both differentiating and differentiated SYH-SY5Y cells. These results together suggest that a crosstalk between TrkB signaling and NF-κB/p65/IκBα signaling may be involved in morphine actions in humans.
It has been well documented that single-high dose morphine induced long lasting behavioral and neurochemical sensitization in rats [31]. Our results from both acute and chronic morphine studies together with the results from others indicate that modulation of both BNDF/TrkB signaling and NF-κB/IκBα signaling may play a critical role in the sensitization of morphine actions in humans. This implication has been further supported by our morphological result that single dose DAMGO and morphine decreased neurite outgrowth within 24h (Fig. 3). Moreover, during the final stage of preparing this manuscript3, an independent study [32] has shown that chronic morphine down-regulates the BDNF mRNA level in VTA in a conditioned place preference mouse model, suggesting that BDNF or TrkB may be a negative regulator for morphine reward. The result of TrkB gene expression from our single-dose (10 μM) DAMGO or morphine experiment in the human cell model appears to be consistent with that from the mouse model study [32]. On the other hand, our repeated morphine-induced upregulation of Trkb mRNA (Fig. 4) is consistent with the observation from the study of chronic morphine-treated naïve rat locus coeruleus (LC) [28]. We have yet to find a report on study of either TrkB or IκBα on opioid actions in humans. Interestingly, a recent report shows that there is a positive correction between the serum levels of BDNF, the endogenous TrkB ligand, and craving for drug in opiate-dependent patients [33]. Moreover, a postmortem human brain tissue study shows that NF-κB signaling is involved in human alcoholism [34]. The endogenous opioid system is likely associated with the development of alcoholism [35]. Although there are reports on opioid-mediated NF-κB signaling in undifferentiated SH-SY5Y cells [36, 37], our study is the first to show the effects of both acute and repeated mu-opioid DAMGO and morphine on expression of both TrkB and IκBα genes in differentiating and differentiated SH-SY5Y cells. Taken together, it is very tempting to hypothesize that a crosstalk between TrkB signaling and NF-κB/IκBα signaling may be the key to regulate varied opioid actions including opioid-induced sensitization in the human nervous system.
In conclusion, we have identified two potential biomarkers TrkB and IκBα in response to both single-dose and repeated morphine exposure in differentiating and differentiated human neuroblastoma neuron-like cells. These two molecules may crosstalk each other to be involved in opioid actions in humans. The detailed molecular mechanisms of how these two molecules-mediated signaling pathways crosstalk each other to mediate opioid functions in human cells are under investigation.
Highlights.
Single-dose morphine down-regulated the expression of both IκBα and TrkB genes
Single-dose morphine reduced binding of p65 to the IκBα promoter
Mu-opioids reduced neurite length in differentiating and differentiated cells
Repeated μ-opioids induced biphasic expression of the IκBα and TrkB genes
TrkB and IκBα crosstalk may be a key step to regulate opioid actions in humans
Acknowledgments
This work was supported by NIH grants 1R21DA029430 and 3R21DA029430-02S1. Abra Guo was an undergraduate intern of the Summer Research with NIDA Program.
Footnotes
Part of the results in this manuscript were presented in the International Conference and Exhibition on Addiction Research & Therapy, August 20–22, 2012 Embassy Suites, Las Vegas, USA.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Comb MJ, Kobierski L, Chu HM, Tan Y, Borsook D, Herrup K, Hyman SE. Regulation of opioid gene expression: a model to understand neural plasticity. NIDA Res Monogr. 1992;126:98–112. [PubMed] [Google Scholar]
- 2.Chen YL, Monteith N, Law PY, Loh HH. Dynamic association of p300 with the promoter of the G protein-coupled rat delta opioid receptor gene during NGF-induced neuronal differentiation. Biochem Biophys Res Commun. 2010;396:294–8. doi: 10.1016/j.bbrc.2010.04.083. [DOI] [PubMed] [Google Scholar]
- 3.Chen YL, Law PY, Loh HH. NGF/PI3K signaling-mediated epigenetic regulation of delta opioid receptor gene expression. Biochem Biophys Res Commun. 2008;368:755–60. doi: 10.1016/j.bbrc.2008.01.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen YL, Law PY, Loh HH. Action of NF-kappaB on the delta opioid receptor gene promoter. Biochem Biophys Res Commun. 2007;352:818–22. doi: 10.1016/j.bbrc.2006.11.103. [DOI] [PubMed] [Google Scholar]
- 5.Chen YL, Law PY, Loh HH. Sustained Activation of Phosphatidylinositol 3-Kinase/Akt/Nuclear Factor {kappa}B Signaling Mediates G Protein-coupled {delta}-Opioid Receptor Gene Expression. J Biol Chem. 2006;281:3067–74. doi: 10.1074/jbc.M506721200. [DOI] [PubMed] [Google Scholar]
- 6.Chen YL, Law PY, Loh HH. Nuclear Factor kappaB Signaling in Opioid Functions and Receptor Gene Expression. J Neuroimmune Pharmacol. 2006;1:270–279. doi: 10.1007/s11481-006-9028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russo SJ, Bolanos CA, Theobald DE, DeCarolis NA, Renthal W, Kumar A, Winstanley CA, Renthal NE, Wiley MD, Self DW, Russell DS, Neve RL, Eisch AJ, Nestler EJ. IRS2-Akt pathway in midbrain dopamine neurons regulates behavioral and cellular responses to opiates. Nat Neurosci. 2007;10:93–9. doi: 10.1038/nn1812. [DOI] [PubMed] [Google Scholar]
- 8.Rehni AK, Bhateja P, Singh TG, Singh N. Nuclear factor-kappa-B inhibitor modulates the development of opioid dependence in a mouse model of naloxone-induced opioid withdrawal syndrome. Behav Pharmacol. 2008;19:265–9. doi: 10.1097/FBP.0b013e3282febcd9. [DOI] [PubMed] [Google Scholar]
- 9.Meffert MK, Chang JM, Wiltgen BJ, Fanselow MS, Baltimore D. NF-kappa B functions in synaptic signaling and behavior. Nat Neurosci. 2003;6:1072–8. doi: 10.1038/nn1110. [DOI] [PubMed] [Google Scholar]
- 10.Jiang X, Tian F, Du Y, Copeland NG, Jenkins NA, Tessarollo L, Wu X, Pan H, Hu XZ, Xu K, Kenney H, Egan SE, Turley H, Harris AL, Marini AM, Lipsky RH. BHLHB2 controls Bdnf promoter 4 activity and neuronal excitability. J Neurosci. 2008;28:1118–30. doi: 10.1523/JNEUROSCI.2262-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown K, Park S, Kanno T, Franzoso G, Siebenlist U. Mutual regulation of the transcriptional activator NF-kappa B and its inhibitor, I kappa B-alpha. Proc Natl Acad Sci U S A. 1993;90:2532–6. doi: 10.1073/pnas.90.6.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ammon S, Mayer P, Riechert U, Tischmeyer H, Hollt V. Microarray analysis of genes expressed in the frontal cortex of rats chronically treated with morphine and after naloxone precipitated withdrawal. Brain Res Mol Brain Res. 2003;112:113–25. doi: 10.1016/s0169-328x(03)00057-3. [DOI] [PubMed] [Google Scholar]
- 13.Capasso A. Involvement of nuclear factor-kB in the expression of opiate withdrawal. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:1259–68. doi: 10.1016/s0278-5846(01)00178-6. [DOI] [PubMed] [Google Scholar]
- 14.Robinson TE, Kolb B. Morphine alters the structure of neurons in the nucleus accumbens and neocortex of rats. Synapse. 1999;33:160–2. doi: 10.1002/(SICI)1098-2396(199908)33:2<160::AID-SYN6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 15.Bergami M, Rimondini R, Santi S, Blum R, Gotz M, Canossa M. Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proc Natl Acad Sci U S A. 2008;105:15570–5. doi: 10.1073/pnas.0803702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meijering E, Jacob M, Sarria JC, Steiner P, Hirling H, Unser M. Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry A. 2004;58:167–76. doi: 10.1002/cyto.a.20022. [DOI] [PubMed] [Google Scholar]
- 17.Lopez-Carballo G, Moreno L, Masia S, Perez P, Barettino D. Activation of the phosphatidylinositol 3-kinase/Akt signaling pathway by retinoic acid is required for neural differentiation of SH-SY5Y human neuroblastoma cells. J Biol Chem. 2002;277:25297–304. doi: 10.1074/jbc.M201869200. [DOI] [PubMed] [Google Scholar]
- 18.Feng Z, Porter AG. NF-kappaB/Rel proteins are required for neuronal differentiation of SH-SY5Y neuroblastoma cells. J Biol Chem. 1999;274:30341–4. doi: 10.1074/jbc.274.43.30341. [DOI] [PubMed] [Google Scholar]
- 19.Bhakar AL, Tannis LL, Zeindler C, Russo MP, Jobin C, Park DS, MacPherson S, Barker PA. Constitutive nuclear factor-kappa B activity is required for central neuron survival. J Neurosci. 2002;22:8466–75. doi: 10.1523/JNEUROSCI.22-19-08466.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi TJ, Huang P, Mulder J, Ceccatelli S, Hokfelt T. Expression of p-Akt in sensory neurons and spinal cord after peripheral nerve injury. Neurosignals. 2009;17:203–12. doi: 10.1159/000210400. [DOI] [PubMed] [Google Scholar]
- 21.Sniderhan LF, Stout A, Lu Y, Chao MV, Maggirwar SB. Ankyrin-rich membrane spanning protein plays a critical role in nuclear factor-kappa B signaling. Mol Cell Neurosci. 2008;38:404–16. doi: 10.1016/j.mcn.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahashi J, Palmer TD, Gage FH. Retinoic acid and neurotrophins collaborate to regulate neurogenesis in adult-derived neural stem cell cultures. J Neurobiol. 1999;38:65–81. [PubMed] [Google Scholar]
- 23.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–64. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gavalda N, Gutierrez H, Davies AM. Developmental switch in NF-kappaB signalling required for neurite growth. Development. 2009;136:3405–12. doi: 10.1242/dev.035295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma SK, Klee WA, Nirenberg M. Dual regulation of adenylate cyclase accounts for narcotic dependence and tolerance. Proc Natl Acad Sci U S A. 1975;72:3092–6. doi: 10.1073/pnas.72.8.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salles KS, Colasanti BK, Craig CR, Thomas JA. Involvement of brain cyclic AMP in the acute and chronic effects of morphine in the rat. Pharmacology. 1978;17:128–37. doi: 10.1159/000136846. [DOI] [PubMed] [Google Scholar]
- 27.Smith DJ, Leil TA, Liu X. Neurotrophin-4 is required for tolerance to morphine in the mouse. Neurosci Lett. 2003;340:103–6. doi: 10.1016/s0304-3940(03)00084-3. [DOI] [PubMed] [Google Scholar]
- 28.Numan S, Lane-Ladd SB, Zhang L, Lundgren KH, Russell DS, Seroogy KB, Nestler EJ. Differential regulation of neurotrophin and trk receptor mRNAs in catecholaminergic nuclei during chronic opiate treatment and withdrawal. J Neurosci. 1998;18:10700–8. doi: 10.1523/JNEUROSCI.18-24-10700.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu X, Mao X, Blake AD, Li WX, Chang SL. Morphine and endomorphins differentially regulate micro-opioid receptor mRNA in SHSY-5Y human neuroblastoma cells. J Pharmacol Exp Ther. 2003;306:447–54. doi: 10.1124/jpet.103.048694. [DOI] [PubMed] [Google Scholar]
- 30.Yu VC, Sadee W. Efficacy and tolerance of narcotic analgesics at the mu opioid receptor in differentiated human neuroblastoma cells. J Pharmacol Exp Ther. 1988;245:350–5. [PubMed] [Google Scholar]
- 31.Vanderschuren LJ, De Vries TJ, Wardeh G, Hogenboom FA, Schoffelmeer AN. A single exposure to morphine induces long-lasting behavioural and neurochemical sensitization in rats. Eur J Neurosci. 2001;14:1533–8. doi: 10.1046/j.0953-816x.2001.01775.x. [DOI] [PubMed] [Google Scholar]
- 32.Koo JW, Mazei-Robison MS, Chaudhury D, Juarez B, LaPlant Q, Ferguson D, Feng J, Sun H, Scobie KN, Damez-Werno D, Crumiller M, Ohnishi YN, Ohnishi YH, Mouzon E, Dietz DM, Lobo MK, Neve RL, Russo SJ, Han MH, Nestler EJ. BDNF is a negative modulator of morphine action. Science. 2012;338:124–8. doi: 10.1126/science.1222265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heberlein A, Dursteler-MacFarland KM, Lenz B, Frieling H, Grosch M, Bonsch D, Kornhuber J, Wiesbeck GA, Bleich S, Hillemacher T. Serum levels of BDNF are associated with craving in opiate-dependent patients. J Psychopharmacol. 2011;25:1480–4. doi: 10.1177/0269881111411332. [DOI] [PubMed] [Google Scholar]
- 34.Okvist A, Johansson S, Kuzmin A, Bazov I, Merino-Martinez R, Ponomarev I, Mayfield RD, Harris RA, Sheedy D, Garrick T, Harper C, Hurd YL, Terenius L, Ekstrom TJ, Bakalkin G, Yakovleva T. Neuroadaptations in human chronic alcoholics: dysregulation of the NF-kappaB system. PLoS One. 2007;2:e930. doi: 10.1371/journal.pone.0000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oswald LM, Wand GS. Opioids and alcoholism. Physiol Behav. 2004;81:339–58. doi: 10.1016/j.physbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 36.Borner C, Hollt V, Kraus J. Mechanisms of the inhibition of nuclear factor-kappaB by morphine in neuronal cells. Mol Pharmacol. 2012;81:587–97. doi: 10.1124/mol.111.076620. [DOI] [PubMed] [Google Scholar]
- 37.Liu AM, Wong YH. Mu-opioid receptor-mediated phosphorylation of IkappaB kinase in human neuroblastoma SH-SY5Y cells. Neurosignals. 2005;14:136–42. doi: 10.1159/000086296. [DOI] [PubMed] [Google Scholar]