Abstract
Gas-phase miscible-displacement experiments were conducted using a large weighing lysimeter to evaluate retention processes for volatile organic compounds (VOCs) in water-unsaturated (vadoze-zone) systems, and to test the utility of gas-phase tracers for predicting VOC retardation. Trichloroethene (TCE) served as a model VOC, while trichlorofluoromethane (CFM) and heptane were used as partitioning tracers to independently characterize retention by water and the air-water interface, respectively. Retardation factors for TCE ranged between 1.9 and 3.5, depending on water content. The results indicate that dissolution into the bulk water was the primary retention mechanism for TCE under all conditions studied, contributing approximately two thirds of the total measured retention. Accumulation at the air-water interface comprised a significant fraction of the observed retention for all experiments, with an average contribution of approximately 24%. Sorption to the solid phase contributed approximately 10% to retention. Water contents and air-water interfacial areas estimated based on the CFM and heptane tracer data, respectively, were similar to independently measured values. Retardation factors for TCE predicted using the partitioning-tracer data were in reasonable agreement with the measured values. These results suggest that gas-phase tracer tests hold promise for characterizing the retention and transport of VOCs in the vadose-zone.
Keywords: Gas-Transport, VOCs, Air-Water Interface, Tracers
1. INTRODUCTION
Chlorinated solvents (e.g., trichloroethene, tetrachloroethene, carbon tetrachloride) and hydrocarbon fuels (gasoline, aviation fuel, diesel fuel) are ubiquitous contaminants of subsurface environments for industrialized areas. Characterization and remediation of sites contaminated by these organic immiscible liquids are complicated by a host of issues (see Brusseau et al., 2013, for a recent review). One issue of significance is the presence of contaminant sources located in the vadose zone. There are two primary concerns associated with sites that contain vadose-zone sources. First, discharge of contaminant vapor from the vadose-zone source may impact the underlying groundwater, which could contribute to overall risk posed by the site as well as delay attainment of groundwater cleanup goals. Second, contaminant vapor from the vadose-zone source may migrate to the land surface and transfer into buildings, thereby causing vapor intrusion. The decision to require remediation of a vadose-zone source zone, as well as the development of associated clean-up objectives, is typically based on assessing the potential impact of the vadose-zone source on groundwater or vapor intrusion (e.g., Johnson and Ettinger, 1991; Rosenbloom et al., 1993; DiGiulio et al., 1998, 1999; EPA, 2001; Hers et al., 2002; USACE, 2002; Truex et al., 2009; Carroll et al., 2012).
Acurate assessment of the human-health risks associated with VOC sources in the vadose zone and design of effective mitigation efforts for such contamination require a robust understanding of the transport behavior of VOCs in variably-saturated systems. The transport and fate of volatile organic contaminants in the vadose zone has been studied for a number of years, and as a result, a large body of literature exists regarding the many factors that can influence their behavior (e.g., Brusseau, 1991; EPA, 2001; Rivett et al., 2011). These include gas-phase advection (via pressure gradients and density gradients), diffusion, immiscible-liquid evaporation, immiscible-liquid dissolution, sorption/desorption, air-water mass transfer, accumulation at the air-water interface, and transformation reactions. Most of this prior research has focused on advection, diffusion, and sorption processes. In contrast, there has been much less investigation of the impact of retention by dissolution into water or by accumulation at the air-water interface on VOC transport (Brusseau et al., 1997; Kim et al., 2001). In addition, for systems wherein multiple, coupled processes are operative over a range of temporal and spatial scales, a critical question is the relative impact of each transport and retention process on overall mass flux.
The partitioning-tracer method has been used to aid in characterizing properties of the system that may affect mass distribution among the phases or domains present in the system, as well as in characterizing existing contamination (e.g., organic liquid volume). An advantage of tracer methods is that they can provide information over relatively large scales in a cost-effective manner in comparison to point-sampling measurement methods. Partitioning-tracer tests can be conducted in water-unsaturated systems using gas-phase tracers (e.g., Brusseau et al., 2003a), and they have been used to measure water saturation (Brusseau et al., 1997; Deeds et al., 1999a; Mariner et al., 1999; Nelson et al., 1999; Carlson et al., 2003; Keller et al., 2003; Peng et al., 2005a), organic-liquid saturation (Deeds et al., 1999b; Mariner et al., 1999; Whitley et al., 1999; Brusseau et al., 2003b; Simon and Brusseau, 2007), and air-water interfacial area (Brusseau et al., 1997; Kim et al. 1999; Costanza-Robinson and Brusseau, 2002; Peng et al., 2005b). One potential application for partitioning-tracer tests is to use them to assist in predicting the retention and transport behavior of VOCs. Tracers that partition to bulk water and accumulate at the air-water interface have been used to predict the impact of those domains on VOC retention and transport for laboratory systems (Brusseau et al., 1997; Kim et al., 2001), but apparently have not been employed for larger-scale systems.
There were two objectives for the research reported herein. The first was to examine retention processes for vapor-phase VOC transport in a water-unsaturated system, and the second was to evaluate the utility of gas-phase tracer tests for predicting VOC retardation. Trichloroethene (TCE) is used as a model VOC, while trichlorofluoromethane (CFM) and heptane are used as partitioning tracers to independently characterize retention by water and the air-water interface, respectively. Gas-phase miscible-displacement experiments were conducted using a large weighing lysimeter containing a sandy soil. The lysimeter facility is notable in terms of the experimental control achievable for a system of its size, and provides an opportunity to investigate mass-transfer and retention processes at a scale for which residence times are more representative of field systems. The first objective was addressed by determining the relative contribution of each retention process to overall retardation. The second objective was addressed by comparing the magnitudes of water content, interfacial area, and tracer retardation estimated from the tracer-test results to values measured independently.
2. MATERIALS AND METHODS
Materials
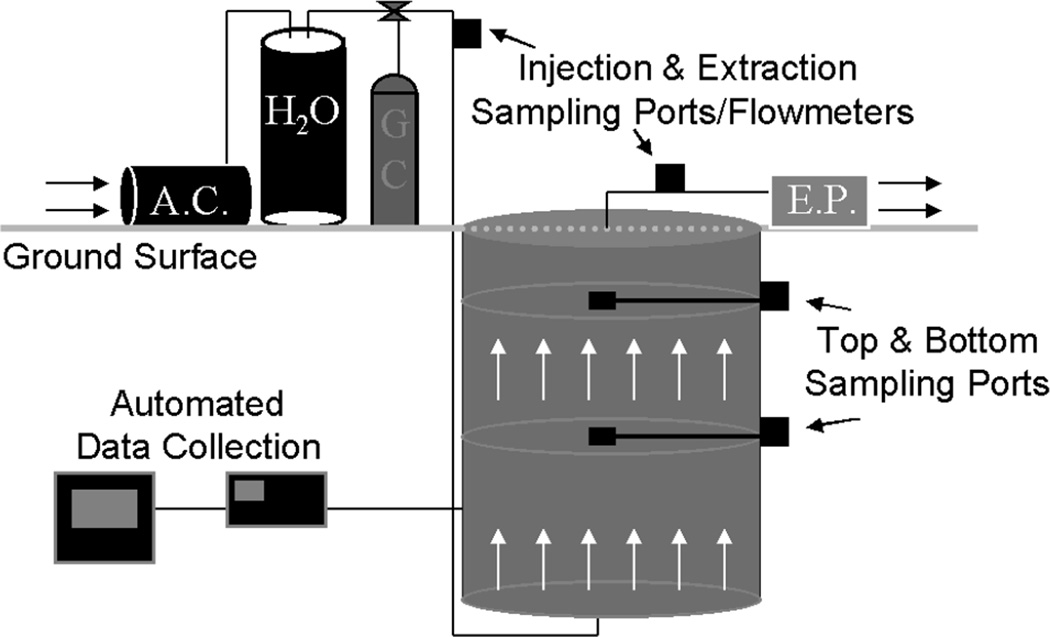
The experiments were conducted using a large weighing lysimeter located at The University of Arizona's Karsten Turf Center for Research. The lysimeter is a 4.0 m-deep, 2.5 m-diameter cylinder, packed homogeneously with Vinton sandy soil (sandy, mixed thermic Typic Torrifluvent). Properties of the porous medium are listed in Table 1. The lysimeter is equipped with 18 tensiometers (Soil Moisture Equipment Corp., Santa Barbara, CA), 21 time domain reflectometry (TDR) probes (Dynamax, Inc., Houston, TX), 3 thermocouples, and 48 porous stainless steel cups for gas injection and sampling (Figure 1). Pressure transducers (model 136PC15G2, Microswitch, Freeport, IL) are installed in the gas-injection and extraction lines, and at 50 cm increments along the axis of flow. All instrumentation is connected to a data logger (Model CR7, Campbell Scientific, Inc., Logan, UT), or directly to a personal computer for data collection and storage. The TDR probes were used to measure water contents at a total of 21 locations before, during, and after the experiments at one-minute intervals. The results showed that water content was relatively uniform throughout the lysimeter for all experiments.
Table 1.
Porous Medium Properties
| Bulk Density, pb (g·cm) | 1.38 |
| Porosity, θT (−) | 0.47 |
| Total Bulk Volume, VT (m3) | 19.63 |
| Specific Surface Area (m2·g−1)a | 3.54 |
| Texture Analysis | 97% Sand; 1.8% Silt; 1.2% Clay |
| Organic Carbon Content (wt/wt %)b | 0.09 |
measured using multi-point Nitrogen BET Method (Micromeritics, Norcross, GA)
Soil, Water, & Plant Analysis Laboratory, Tucson, AZ
Figure 1.
Schematic of the lysimeter system (not to scale). Lysimeter length is 4 m, diameter is 2.5 m. AC = air compressor, EP = extraction pump GC = gas canister, and H2O = humidifier.
Trichloroethene was used as the representative VOC. SF6 and helium were used as conservative tracers. Trichlorofluoromethane was used as the water-partitioning tracer, and heptane was used as the air-water-interface tracer. Relevant physicochemical properties of TCE and the tracers are presented in Table 2. Tracer selection is governed in part by the objective to identify tracers that are retained primarily by a single phase, thereby allowing the tracer to characterize solely that phase (e.g., Brusseau et al., 1997, 2003a). For example, the physicochemical properties of SF6 and helium are such that they reside only in the gas phase, and thus they serve as ideal conservative tracers. Similarly, the properties of heptane are such that accumulation at the air-water interface is the only retention process of significance, and thus, heptane serves as a tracer to characterize the air-water interface.
Table 2.
Summary of Properties for Compounds Useda
| Compound | C0b | KDc (mL·g−1) |
KHd (−) |
KIAh (cm−1) |
Compound Function | |
|---|---|---|---|---|---|---|
| (ppmv) | (µg·L−1) at STP |
|||||
| SF6 | 50 | 215 | - | 70e | - | non-reactive tracer |
| Helium | 1.1·106 | 180 635 | - | - | - | non-reactive tracer |
| TCE | 26 | 137 | 0.014 | 0.4f | 2.64·105 | model VOC |
| Heptane | 37 | 154 | - | 81.6f | 2.33·105 | interfacial-partitioning tracer |
| CFM | 75 | 421 | - | 2.1g | - | water-partitioning tracer |
Temperature ~23 °C
C0 is input concentration of tracer
KD is the sorption coefficient; a dash signifies insignificant
KH is Henry’s coefficient
[Wilson, 1995]
[Schwarzenbach, 1993]
Carlson, 2000]
KIA is the air-water interfacial accumulation coefficient [Hoff, 1993]
Tracer Tests
Two series of experiments were conducted resulting in a total of six vapor-transport experiments. Series 1 was conducted at approximately 6% volumetric water content and series 2 was conducted at approximately 15% water content. Each series consisted of three experiments, Experiment A, B, and C. For both series, Experiments A and B were conducted with TCE at a slower and faster flow rate, respectively (Table 3). Experiment C was conducted at the same higher flow rate as B, with heptane as an interfacial tracer to measure the specific air-water interfacial area of the system. A non-reactive tracer was used for each experiment. The residence times associated with these flow rates, presented in Table 3, are equivalent to approximately 5–12 gas pore volumes per day. These are at the upper end of the wide range of pore-volume throughputs associated with soil vapor extraction systems (e.g., Brusseau et al., 2013), but are substantially lower than those associated with standard laboratory vapor-transport experiments. Table 3 summarizes the experimental conditions for each series and experiment.
Table 3.
Summary of Lysimeter Vapor-Transport Experiments
| Experiment | Mean Qa (L min−1) |
Input Pulse | Trb | Compounds | |
|---|---|---|---|---|---|
| (h) | (PV) | (h) | |||
| 1A | 30.72 | 2.0 | 0.43 | 4.45 | SF6, TCE, CFM |
| 1B | 48.67 | 1.2 | 0.46 | 2.8 | SF6, TCE, CFM |
| 1C | 48.67 | 1.2 | 0.48 | 2.8 | Helium, Heptane |
| 2A | 31.64 | 1.5 | 0.45 | 3.4 | SF6, TCE, CFM |
| 2B | 52.35 | 0.75 | 0.42 | 2.1 | SF6, TCE, CFM |
| 2C | 52.35 | 0.75 | 0.41 | 2.1 | SF6, Heptane |
mean volumetric flow rate equal to the average of measured injection and extraction flow rates
pneumatic residence time
The lysimeter experiments were conducted by first establishing steady-state air flow in the system, then injecting a pulse of gas containing TCE and/or tracers into the system. Upward flow was induced in the lysimeter, as shown in the lysimeter schematic in Figure 1. The TCE and tracer input pulses represented approximately half of the total air-filled pore volume. The pulse was displaced from the system by continual injection of tracer-free air. Air was introduced into the system using a 5-horsepower air compressor and extracted at the same flow rate using a 3/4-horsepower vacuum pump. The air was humidified to greater than 95% RH prior to injection to prevent drying of the soil during the experiment. Humidification was performed by passing the air through a water tower consisting of schedule 40 PVC pipe (0.3 m diameter, 1.8 m length) containing wire mesh to increase mass transfer.
TCE, CFM, and SF6 were contained in a high pressure gas cylinder custom-mixed with a balance of nitrogen to desired concentrations of approximately 25, 75, and 50 ppmv, respectively (Spectra Gases, Alpha, NJ). Pure helium gas was introduced via a high pressure cylinder. Heptane was introduced to the lysimeter using a vapor saturator. Air from the compressor was passed through 4 L of pure liquid heptane contained in schedule 40 PVC pipe (6” o.d.). Due to mass-transfer constraints caused by the simple saturator design, the heptane concentration in the vapor was well below saturation, at <0.001 P/P0. These relatively low concentrations were desirable for these experiments, ensuring heptane concentrations could be considered to be at infinite dilution. The VOC and tracer pulses were not humidified before contacting the soil. This was considered to have minimal impact on water saturation due to the relatively small volume and time-duration of the pulses.
All gases were injected into the soil system via 1/4" stainless steel tubing. The stainless steel tubing was connected via a manifold to 18 lengths of 1/4" Kynar tubing embedded horizontally within a layer of pea gravel at the bottom of the lysimeter. The Kynar tubing led to porous stainless steel cups (40-mm length, 12.5-mm diameter, with an air-entry pressure of 20 cm). The spatial distribution of the cups allowed for uniform injection of the gases across the lysimeter cross-section. Gases were extracted at the top of the lysimeter through four ports drilled into the aluminum lid. The ports were connected to the extraction vacuum pump via 1/4" stainless steel tubing. Injection and extraction flow rates were adjusted using gate-valves and monitored using gas-flow rotameters (Omega Engineering Inc., Stamford, CT).
Sampling and Analysis
Gas samples were collected from the center of the lysimeter at two discrete locations along the axis of flow. These top and bottom sampling ports were located at depths of 90 cm and 225 cm. Samples were obtained via a septum injector nut (Valco Instruments Co., Inc., Houston, TX) and on/off valves (Arizona Valve and Fitting, Phoenix, AZ) connected to 6.4-mm Kynar tubing and stainless steel porous cups embedded horizontally within the soil. Thus, the bottom and top sampling ports are considered point samples. Composite influent and effluent gas samples were also collected via septum injector nuts installed in the stainless steel tubing leading into and out of the lysimeter. Results for these samples are considered flux-averaged composite values. Samples were collected from all ports at time-intervals of approximately 10 minutes, increasing to approximately one hour intervals toward the end of the experiment. This sampling scheme allowed for well-defined breakthrough curves (i.e., plots of concentration versus time) to be constructed.
Gas samples were collected in evacuated 80-mL gas canisters (Tracer Research Corp., Tucson, AZ). SF6, TCE, CFM, and heptane were analyzed using a Varian gas chromatograph with a custom autosampler fitted for the aerosol canisters. SF6, CFM, and TCE were analyzed using a SP1000 (Carbopack B) column (Alltech Associates, Inc., Deerfield, IL) with electron capture detection, while heptane was analyzed using an OV-101 (Chromosorb W) column (Alltech Associates, Inc.) with flame ionization detection. Helium samples were analyzed using a concentric column (Model CTR II, Alltech Associates, Inc.) and thermal conductivity detector.
Data Analysis
The breakthrough curves were analyzed via the method of moments. For some data sets, the elution curve was truncated. A log-linear tail extrapolation was conducted for these cases to enhance the accuracy of moment analysis (e.g., Skopp, 1984; Deeds et al., 1999b). The data were extrapolated to a common point of elution, C/C0 = 10−4. The retardation factor for a tracer undergoing retention was calculated as the ratio of the travel times of the reactive tracer and the non-reactive tracer.
The following total retardation factor can be defined for a system with immobile water and a mobile vapor phase (e.g., Brusseau et al., 1997, 2003a):
| (1) |
where θa, and θw are the volumetric air and water contents, respectively [−]; KH is the Henry’s Law constant [−]; ρb is the bulk density of the porous medium [g·cm−3]; KD is the solid-phase sorption coefficient for a water-solvated sorbent [cm3·g−1]; KIA is the interfacial sorption coefficient [cm]; and AIA is the specific interfacial area [cm2·cm−3 or cm−1; interfacial area per unit bulk volume]. The first term in Equation 1 represents the mobile (vapor) phase; the second term represents sorption to the system solid phase (organic and mineral phases); the third represents retention due to dissolution in (immobile) bulk water; and the fourth term represents interfacial retention. The percent contribution of retention by the solid phase (fS), bulk water-dissolution (fW), and interfacial accumulation (fI) may be calculated as:
| (2) |
| (3) |
| (4) |
Equation 4 involves the mass-balance assumption that interfacial retention accounts for any retention not due to solid-phase sorption or aqueous dissolution.
For a given compound, certain terms in Equation 1 may be reasonably neglected. For example, heptane has been shown to sorb negligibly to soil (Okamura, 1973; Hartkopf, 1973; Dorris, 1981), allowing the second term on the right-hand side to be dropped from the equation. Additionally, the relatively large Henry's Law coefficient for heptane allows the influence of vapor dissolution into bulk water to be neglected. Thus, heptane is expected to serve as an ideal interfacial tracer. The following equation is used for the retention analysis of heptane:
| (5) |
Values for R obtained experimentally for heptane are used in Equation 5 along with the known values of θa and KIA to calculate AIA. Similarly, with minimal solid-phase sorption and accumulation at the air-water interface, CFM serves as a water-partitioning tracer from which estimates of θw can be obtained. The values for AIA and θw derived from the tracer data can then be used in Equation 1 to predict the retardation factor for TCE, with values for all other variables known a priori (Table 2). The efficacy of predicting TCE transport using interfacial tracer data will be assessed by comparing predicted and measured R values.
4. RESULTS
Conservative Tracers
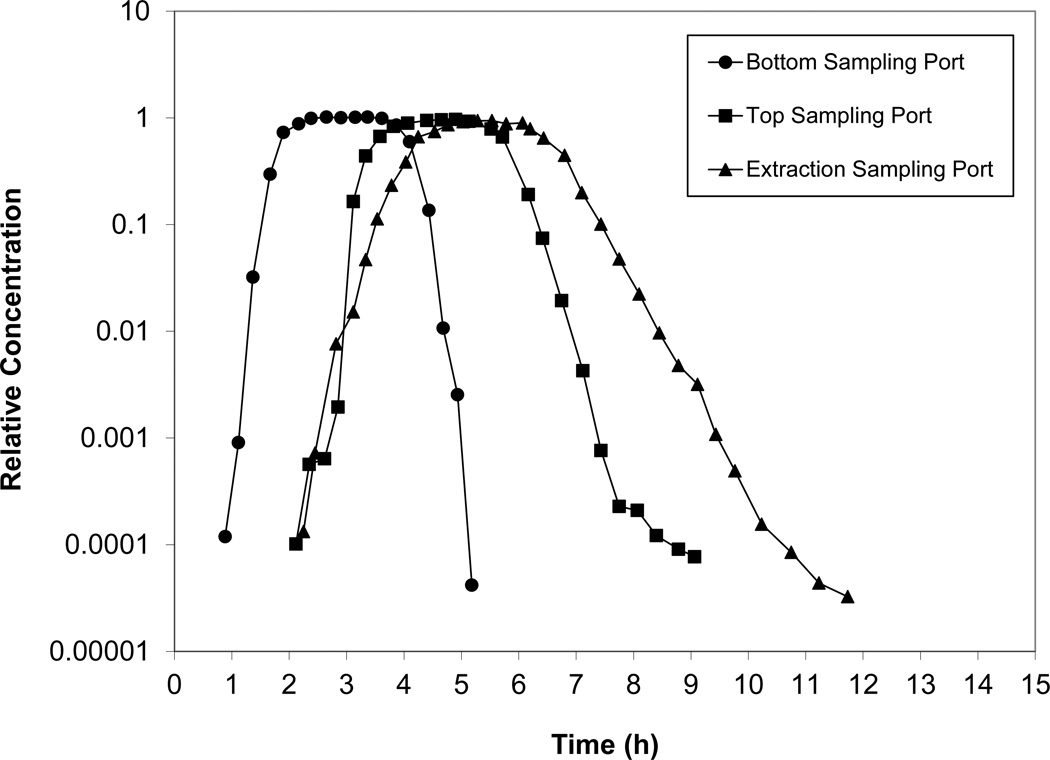
Breakthrough curves for SF6 collected during experiment 1A at all three sampling locations are shown in Figure 2. The BTCs are relatively sharp and symmetrical, indicating ideal transport. The curves show slight broadening and reductions in C/C0 maxima due to increased dispersion at longer residence times in the lysimeter. These curves are typical of the non-reactive tracer transport behavior observed in all experiments. Comparison of replicate breakthrough curves shows good reproducibility, including between SF6 and helium (see Figures 3 and 4). The latter suggests there was minimal impact of density differences in using pure helium versus the mixed gases.
Figure 2.
SF6 breakthrough curves for all sampling ports for experiment 1A. Relative concentration represents the measured concentration (C) normalized by the injection concentration (C0).
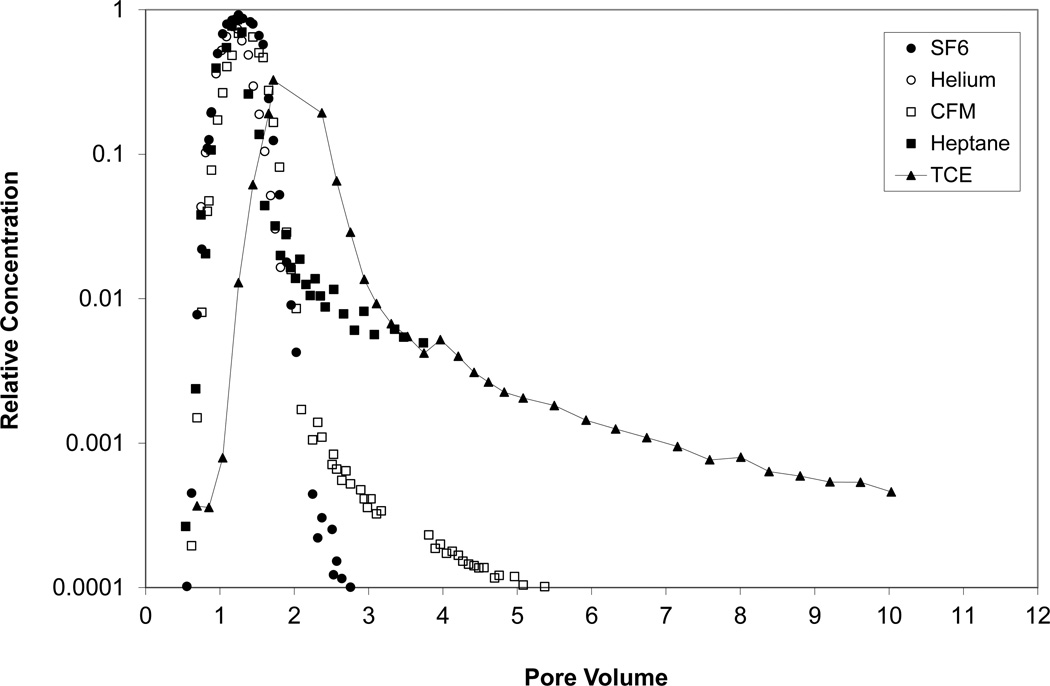
Figure 3.
Breakthrough curves for TCE and all tracers for experiments 1B and 1C; extraction sampling port. Relative concentration represents the measured concentration (C) normalized by the injection concentration (C0).
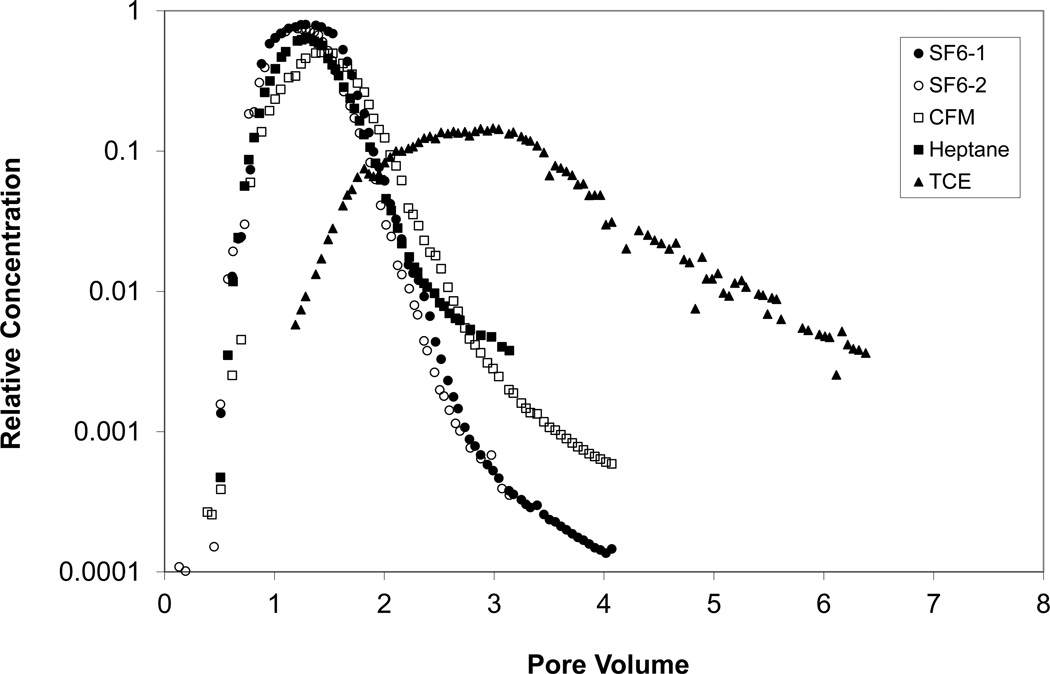
Figure 4.
Breakthrough curves for TCE and all tracers for experiments 2B and 2C; extraction sampling port. Relative concentration represents the measured concentration (C) normalized by the injection concentration (C0).
The air-filled pore volume, Va, of the system can be calculated as the product of the measured flow rates and the travel times calculated for the non-reactive tracer data collected at the extraction port. The mean Va calculated from all Series 1 experiments is 7.8 m3 with a coefficient of variation (COV) of 9%, while for Series 2, the mean Va is 5.9 m3 with a COV of 6%. The relatively low COVs for both series indicate the reproducibility of the experiments. These values are slightly smaller than values of Va, 8.2 (6% error) and 6.5 (9% error), respectively, calculated using the system volume, gravimetrically measured bulk density and associated porosity, and the independently measured water contents.
Partitioning Tracers
Representative breakthrough curves for CFM are presented in Figures 3 and 4. The breakthrough curves exhibit a slight rightward shift of the peak and greater elution tailing compared to the non-reactive tracer, indicative of retention (in this case due to dissolution into water). The retardation factors for CFM ranged between 1.1 and 1.4. The water contents estimated from analysis of the CFM tracer data are reported in Table 4. Values determined independently using TDR are also presented. The tracer-derived values compare well to the TDR values for three of the four cases. The Va value estimated for case 1B based upon the SF6 data exhibited greater disparity from the independently determined value compared to the other experiments, which suggests that the calculated travel time is smaller than expected. This would explain the poor match of water contents observed for case 1B, wherein a smaller-than-expected travel time for the non-reactive tracer leads to an over-estimation of the retardation factor for CFM.
Table 4.
Summary of Tracer Retention Parameters (extraction port data)
| Experiment | θw Tracer | θw Independenta |
AIAb Tracer (cm2·cm−3) |
AIA Laboratoryc (cm2·cm−3) |
|---|---|---|---|---|
| 1A | 0.07 | 0.06 | - | - |
| 1B | 0.12 | 0.06 | - | - |
| 1C | - | - | 6641 | 5230 |
| 2A | 0.17 | 0.15 | - | - |
| 2B | 0.17 | 0.15 | - | - |
| 2C | - | - | 1771 | 1000 |
Measured with time domain reflectometry
AIA is specific air-water interfacial area
Reported by Peng and Brusseau (2005)
Representative breakthrough curves for heptane are also presented in Figures 3 and 4. Similarly to CFM, the breakthrough curves exhibit a slight rightward shift of the peak and greater elution tailing compared to the non-reactive tracer, indicating an impact of retention (in this case due to accumulation at the air-water interface). The retardation factors for heptane ranged between 1.1 and 1.4. The air-water interfacial areas estimated from analysis of the heptane tracer data are reported in Table 4. Values determined independently from interfacial tracer tests conducted for the same soil in the laboratory (Peng and Brusseau, 2005b) are also presented. The tracer-derived values compare reasonably well to the laboratory values. The AIA value estimated for Series-1 conditions is approximately 4 times larger than the value estimated for Series-2 conditions. This is due to the lower water content present for Series 1, wherein airwater interfacial area has been shown to correlate inversely with water content (e.g., Costanza-Robinson and Brusseau, 2002; Kim et al., 1999; Peng and Brusseau, 2005b; Brusseau et al., 2006, 2007).
Trichloroethene
Representative breakthrough curves for TCE are shown in Figures 3 and 4. The TCE curves are shifted significantly to the right with respect to all of the tracers. Measured retardation factors ranged between 1.9 and 2.4 for Series 1 and 2.8 and 3.5 for Series 2 (Table 5). The magnitude of retardation is larger for Series 2 due to the higher water content and the appreciable partitioning of TCE into water. The retardation factors for TCE are larger than those for the partitioning tracers because TCE transport is influenced by all three retention processes (in contrast to the tracers) and because TCE has the smallest Henry’s coefficient (i.e., largest proportional partitioning to water).
Table 5.
Comparison of Tracer-Predicted and Measured TCE Retardation
| Experiment | Port | R Measured |
R Predicted |
% Error |
|---|---|---|---|---|
| 1A | Extraction | 2.0 | 1.94 | 3.0 |
| Top | 2.3 | 2.49 | −8.1 | |
| Bottom | 2.4 | 2.04 | 16 | |
| 1B | Extraction | 2.2 | 2.40 | −12 |
| Top | 1.9 | 2.00 | −5.0 | |
| Bottom | 2.3 | 2.46 | −6.4 | |
| 2A | Extraction | 2.9 | 2.75 | 4.4 |
| Top | 3.2 | 2.41 | 24 | |
| Bottom | 3.4 | 2.91 | 14 | |
| 2B | Extraction | 2.8 | 2.70 | 3.7 |
| Top | 3.3 | 2.79 | 15 | |
| Bottom | 3.5 | 2.82 | 19 |
Equations 2–4 can be used to calculate the contributions of individual retention processes to total retention (RT-1). For all experiments, TCE vapor dissolution into bulk water comprised the largest fraction of total retention, averaging 66%. This is expected due to the low (i.e., <1) Henry’s Law constant for TCE. The specific fractional contribution is a function of water content. Solid-phase sorption contributed the least, approximately 10%, to observed retention. The fractional contribution of solid-phase sorption is relatively constant, as would be expected given that the phase-extent parameter of which it is a function (i.e., ρb) is a constant. The contribution of accumulation at the air-water interface was significant for the experiments, averaging approximately 24%. The fractional contribution for this process is a function of the magnitude of air-water interfacial area, which in turn is a function of water content.
Values for AIA and θw calculated from the tracer data are used, along with other independently determined parameter values presented in Table 2, to predict the retardation factors for TCE (Equation 1). The results are presented in Table 5. Comparison of predicted and measured values results in relative errors ranging from 3 to 24%. The errors for the extraction data, which average approximately 6%, are smaller than for the top and bottom port data. This would be anticipated considering the nature of sample collection for the two sets of data, with the extraction port providing flux-averaged composite data that would typically be less sensitive to local variations in conditions in contrast to the in-situ point samples obtained for the top and bottom ports. The largest error among the extraction-port data is associated with experiment 1B, most likely a function of the larger disparity between tracer-estimated and independently measured water contents noted above for this experiment.
5. CONCLUSIONS
Vapor-phase transport experiments were conducted at an intermediate-scale lysimeter facility to evaluate the relative contribution of individual retention mechanisms to retardation of TCE. The lysimeter facility provided the ability to conduct controlled experiments at a much larger scale than typical laboratory experiments, and thus produce residence times that are more representative of field conditions. This is significant when investigating the impact of mass-transfer processes, given their sensitivity to residence time. Breakthrough curves for non-reactive tracers indicate that relatively ideal flow conditions were achieved in the system. Furthermore, information derived from non-reactive tracer data (e.g. air-filled pore volume) was consistent with independently measured data.
The results of the experiments indicate that vapor-phase transport of TCE was influenced by a combination of retention processes, including, in descending order of contribution, dissolution into water, accumulation at the air-water interface, and solid-phase sorption. These results are consistent with those reported for laboratory experiments (Brusseau et al., 1997; Kim et al., 2001). More generally, the relative contribution of the different retention processes will depend on the specific compound of interest, governed by its’ physicochemical properties, and the properties of the porous medium. The overall magnitude of TCE retardation was primarily a function of water content, both directly through its impact on retention via dissolution into water and indirectly through its impact on air-water interfacial area. The observation that accumulation at the air-water interface was significant for all experiments suggests that prediction of VOC transport in the vadose zone should include consideration of air-water interfacial accumulation, which has generally been ignored.
Water contents and air-water interfacial areas estimated based on the CFM and heptane tracer data, respectively, were similar to independently measured values. This supports the effectiveness of gas-phase tracer tests as a means to estimate water contents and interfacial areas at the field scale. Retardation factors for TCE predicted using the partitioning-tracer data were in reasonable agreement with the measured values, which indicates that the partitioning tracers provided useful information for predicting vapor-phase TCE retention and transport in the system. These results suggest that gas-phase tracer tests holds promise for characterizing the retention and transport of VOCs in the vadose zone.
Highlights.
TCE vapor transport was examined in a large lysimeter
Retention by dissolution into water was significant
Retention at the air-water interface was significant
A suite of tracers were used to predict TCE retardation
ACKKNOWLEDGEMENTS
This research was supported by grants provided by the National Institute of Environmental Health Sciences Superfund Basic Research Program (ES 04940) and by the U.S. Department of Energy. The authors wish to thank Dr. Glenn Thompson and Mr. John Oliver of Tracer Research Corporation (Tucson, AZ) for their generous donation of time and analytical resources. We also thank the reviewers for their constructive comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- Brusseau ML. Transport of Organic Chemicals by Gas Advection in Structured or Heterogeneous Porous Media: Development of a Model and Application to Column Experiments. Water Resources Research. 1991;27((12)):3189–3199. [Google Scholar]
- Brusseau ML, Popovicova J, Silva JAK. Characterizing gas-water-interfacial and bulk-water partitioning for gas-phase transport of organic contaminants in unsaturated porous media. Environmental Science and Technology. 1997;31:1645–1649. [Google Scholar]
- Brusseau ML, Nelson NT, Costanza-Robinson MS. Partitioning Tracer Tests for Characterizing Immiscible-Fluid Saturations and Interfacial Areas in the Vadose Zone. Vadose Zone Journal. 2003a;2((2)):138–147. [Google Scholar]
- Brusseau ML, Bronson KM, Ross S, Nelson NT, Carlson TD. Application of Gas-Phase Partitioning Tracer Tests to Characterize Immiscible-Liquid Contamination in the Vadose Zone Beneath a Fuel Depot. Vadose Zone Journal. 2003b;2((2)):148–153. [Google Scholar]
- Brusseau ML, Peng S, Schnaar G, Costanza-Robinson MS. Relationships among air-water interfacial area, capillary pressure, and water saturation for a sandy porous medium. Water Resour. Res. 2006;42:W03501. [Google Scholar]
- Brusseau ML, Peng S, Schnaar G, Murao A. Measuring air-water interfacial areas with X-ray microtomography and interfacial partitioning tracer tests. Environmental Science and Technology. 2007;41((6)):1956–1961. doi: 10.1021/es061474m. [DOI] [PubMed] [Google Scholar]
- Brusseau ML, Carroll KC, Truex MJ, Becker DJ. Characterization and Remediation of Chlorinated Volatile Organic Contaminants in the Vadose Zone: An Overview of Issues and Approaches. Vadose Zone J. 2013 doi: 10.2136/vzj2012.0137. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson TD, Costanza-Robinson MS, Keller J, Wierenga PJ, Brusseau ML. Intermediate-scale tests of the gas-phase partitioning tracer method for measuring soil-water content. Soil Science Society of America Journal. 2003;67((2)):483–486. [Google Scholar]
- Carroll KC, Oostrom M, Truex MJ, Rohay VJ, Brusseau ML. Assessing Performance and Closure for Soil Vapor Extraction: Integrating Vapor Discharge and Impact to Groundwater Quality. Journal of Contaminant Hydrology. 2012;128:71–82. doi: 10.1016/j.jconhyd.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Costanza-Robinson MS, Brusseau ML. Air-water interfacial areas in unsaturated soils: Evaluation of interfacial domains. Water Resources Research. 2002;38((10)):1195–1211. [Google Scholar]
- Deeds NE, McKinney DC, Pope GA, Whitley GA., Jr Difluoromethane as Partitioning Tracer to Estimate Vadose Water Saturations. Journal of Environmental Engineering, July . 1999a:630–633. [Google Scholar]
- Deeds NE, Pope GA, McKinney DC. Vadose Zone Characterization at a Contaminated Field Site Using Partitioning Interwell Tracer Technology. Environmental Science and Technology. 1999b;33:2745–2751. [Google Scholar]
- DiGiulio DC, Brusseau ML, Ravi V. Use of Diffusion Modeling to Aid Assessment of Rate-Limited Vapor Transport for SVE Closure; Proceedings of the First International Conference on Remediation of Chlorinated and Recalcitrant Compounds; Monterey, CA: 1998. [May 18–21, 1998]. Published in: Physical, Chemical, and Thermal Technologies: Remediation of Chlorinated and Recalcitrant Compounds (C1-5); Wickramanayake, GB and Hinchee, R.E. eds., Battelle, Columbus, OH. [Google Scholar]
- DiGiulio DC, Ravi V, Brusseau ML. Evaluation of Mass Flux to and from Ground Water Using a Vertical Flux Model (VFLUX): Application to the Soil Vacuum Extraction Closure Problem. Ground Water Monit. Remed. 1999;19((2)):96–104. [Google Scholar]
- Dorris GM, Gray DG. Adsorption of Hydrocarbons on Silica-Supported Water Surfaces. Journal of Physical Chemistry. 1981;85:3628–3635. [Google Scholar]
- Environmental Protection Agency (EPA) Development of Recommendations and Methods to Support Assessment of Soil Venting Performance and Closure. EPA/600/R-01/070. 2001 [Google Scholar]
- Hartkopf A, Karger BL. Study of Interfacial Properties of Water by Gas Chromatography. Accounts of Chemical Research. 1973;6:209–216. [Google Scholar]
- Hers I, Zapf-Gilje R, Evans D, Li L. Comparison, Validation, and Use of Models for Predicting Indoor Air Quality from Soil and Groundwater Contamination. Soil Sed. Contamination: An International Journal. 2002;11(4):491–527. [Google Scholar]
- Johnson PC, Ettinger RA. Heuristic Model for Predicting the Intrusion Rate of Contaminant Vapors into Buildings. Environmental Science and Technology. 1991;25:1445–1452. doi: 10.1021/acs.est.8b01106. [DOI] [PubMed] [Google Scholar]
- Keller JM, Brusseau ML. In-situ characterization of soil-water content using gas-phase partitioning tracer tests: Field-scale evaluation. Environmental Science and Technology. 2003;37((14)):3141–3144. doi: 10.1021/es0340329. [DOI] [PubMed] [Google Scholar]
- Kim H, Rao PSC, Annable MD. Gaseous tracer technique for estimating air-water interfacial areas and interface mobility. Soil Science Society of America Journal. 1999;63((6)):1554–1560. [Google Scholar]
- Kim H, Annable MD, Rao PSC. Gaseous transport of volatile organic chemicals in unsaturated porous media: Effect of water-partitioning and air-water interfacial adsorption. Environmental Science and Technology. 2001;35((22)):4457–4462. doi: 10.1021/es001965l. [DOI] [PubMed] [Google Scholar]
- Mariner PE, Jin M, Studer JE, Pope GA. The First Vadose Zone Partitioning Interwell Tracer Test for Nonaqueous Phase Liquid and Water Residual. Environmental Science and Technology. 1999;33((16)):2825–2828. [Google Scholar]
- Nelson NT, Brusseau ML, Carlson TD, Costanza MS, Young MH, Johnson GR, Wierenga PJ. A gas-phase partitioning tracer method for the in situ measurement of soil-water content. Water Resources Research. 1999;35((12)):3699–3707. [Google Scholar]
- Okamura JP, Sawyer DT. Gas chromatographic studies of sorptive interactions of normal and halogenated hydrocarbons with water-modified soil, silica, and Chromosorb W. Analytical Chemistry. 1973;45:80–84. [Google Scholar]
- Peng S, Brusseau ML. Gas-phase partitioning tracer test method for water content measurement: Evaluating efficacy for a range of porous-medium textures. Vadose Zone Journal. 2005a;4((3)):881–884. [Google Scholar]
- Peng S, Brusseau ML. The impact of soil texture on air-water interfacial areas in unsaturated sandy porous media. Water Resour. Res. 2005b;Vol. 41(No. 3):W03021. [Google Scholar]
- Rivett MO, Wealthall GP, Dearden RA, McAlary TA. Review of unsaturated-zone transport and attenuation of volatile organic compound (VOC) plumes leached from shallow source zones. Journal of Contaminant Hydrology. 2011;123:130–156. doi: 10.1016/j.jconhyd.2010.12.013. [DOI] [PubMed] [Google Scholar]
- Rosenbloom J, Mock P, Lawsan P, Brown J, Turin HJ. Application of VLEACH to an Arizona superfund site. Ground Water Monitor, Remed. 1993 Summer;:159–169. [Google Scholar]
- Simon MA, Brusseau ML. Analysis of a gas-phase partitioning tracer test conducted in an unsaturated fractured-clay formation. Journal of Contaminant Hydrology. 2007;90((3–4)):146–158. doi: 10.1016/j.jconhyd.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Skopp J. Estimation of true moments from truncated data. Amer. Instit. Chem. Engin. J. 1984;30:151–155. [Google Scholar]
- Strategic Environmental Research and Development Program (SERDP) Final Report: SERDP/ESTCP Expert Panel Workshop on Reducing the Uncertainty of DNAPL Source Zone Remediation. 2006 [Google Scholar]
- Truex MJ, Oostrom M, Brusseau ML. Estimating Persistent Mass Flux of Volatile Contaminants from the Vadose Zone to Ground Water. Ground Water Monitoring and Remediation. 2009;29:63–72. [Google Scholar]
- USACE, US Army Corps of Engineers. Engineering and Design: Soil Vapor Extraction and Bioventing. 2002 EM 1110-1-4001. [Google Scholar]
- Whitley GAJ, McKinney DC, Pope GA, Rouse BA, Deeds NE. Contaminated Vadose Zone Characterization Using Partitioning Gas Tracers. Journal of Environmental Engineering (June) 1999:574–582. [Google Scholar]