Abstract
Patients with chemorefractory non-Hodgkin lymphomas (NHL) generally have a poor prognosis. We used the observational database of the CIBMTR to study the outcome of 533 patients with refractory diffuse large B-cell lymphoma (DLBCL) or grade-III follicular lymphoma (FL-III) who underwent allogeneic transplantation (allo-HCT) using either myeloablative (MA; N=307) or reduced intensity/non-myeloablative conditioning (RIC/NST; N=226), between 1998-2010. We analyzed non-relapse mortality (NRM), relapse/progression, progression-free survival (PFS), and overall survival (OS). Only 45% of the patients at transplant had a Karnofsky performance score of ≥90%. Median follow-up of surviving patients after MA and RIC/NST allo-HCT is 35 months and 30 months, respectively. At 3years, MA allo-HCT was associated with a higher NRM compared to RIC/NST (53% vs. 42%; p=0.03), similar PFS (19% vs. 23%; p=0.40), and lower OS (19% vs. 28%; p=0.02), respectively. On multivariate analysis, FL-III histology was associated with lower NRM (relative-risk [RR]=0.52), reduced risk of relapse/progression (RR=0.42), superior PFS (RR=0.51) and OS (RR=0.53), while MA conditioning was associated with reduced risk of relapse/progression (RR=0.66). Despite a refractory state, a small subset of DLBCL and FL-III patients can attain durable remissions after allo-HCT. Conditioning regimen intensity was not associated with PFS and OS despite a higher risk of relapse/progression with RIC/NST allo-HCT.
Keywords: DLBCL, grade III follicular lymphoma, allogeneic transplantation, refractory, relapsed, graft-versus-host disease
INTRODUCTION
High dose chemo- and/or radiation-therapy and autologous hematopoietic cell transplantation (auto-HCT) is considered a standard therapy for patients with relapsed, chemosensitive diffuse large B-cell lymphoma (DLBCL), and appears to be curative for 40-45% of the patients [1-3]. However, the results of auto-HCT in the high-risk group of patients with relapsed aggressive non-Hodgkin lymphomas (NHL) with chemorefractory disease at the time of autografting have been uniformly disappointing. Allogeneic hematopoietic cell transplantation (allo-HCT) potentially is a curative modality for a variety of hematologic malignancies including indolent and aggressive lymphomas [4-7]. The advantages of an allo-HCT include a tumor-free graft, as well as an allogeneic effect exerted by donor T-cells often referred to as graft-versus-lymphoma (GVL) effect.
Despite the higher risk of transplant-related morbidity and mortality with allo-HCT, select patients with relapsed, aggressive NHL patients, especially the subgroup with chemosensitive disease, can achieve long-term remissions after allo-HCT [6,8-10]. Patients with aggressive NHL refractory to salvage chemotherapy, however, have a poor prognosis and there are only limited data available regarding the outcomes after allo-HCT for this extremely high-risk group. Moreover, the effects of regimen intensity, e.g. myeloablative (MA) conditioning versus reduced-intensity conditioning (RIC) or non-myeloablative conditioning (NST) regimens, are not known. We report herein the outcomes of allo-HCT in patients with chemorefractory, aggressive B-cell NHL relative to the intensity of the transplant conditioning regimens using the observational database of the Center for International Blood and Marrow Transplant Research (CIBMTR). To date, this report represents the largest study of refractory, aggressive NHL patients undergoing allo-HCT.
Subjects and Methods
Data sources
The CIBMTR is a research affiliation of the International Bone Marrow Transplant Registry (IBMTR) and the National Marrow Donor Program (NMDP) established in 2004; both entities had been collecting data for more than one decade prior to the merger. This organization comprises a voluntary working group of more than 450 transplantation centers worldwide that contribute detailed data on consecutive auto- and allo-HCTs to a Statistical Center at the Medical College of Wisconsin in Milwaukee, WI and the NMDP Coordinating Center in Minneapolis, MN. Participating centers are required to report all HCTs consecutively, with compliance monitored by on-site audits. Patients are followed longitudinally, with yearly follow-up. Computerized checks for discrepancies, physicians’ reviews of submitted data, and onsite audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are performed in compliance with the Privacy Rule (HIPAA) as a public health authority and in compliance with all applicable federal regulations pertaining to the protection of human research participants, as determined by continuous review of the Institutional Review Boards of the NMDP and the Medical College of Wisconsin since 1985.
The CIBMTR collects data at two levels: Transplant Essential Data (TED) and Comprehensive Report Form (CRF) data. TED data include disease type, age, gender, pre-transplant disease stage and chemotherapy-responsiveness, date of diagnosis, graft type (bone marrow- and/or blood-derived progenitor cells), conditioning regimen, post-transplant disease progression and survival, development of a new malignancy, and cause of death. All CIBMTR teams contribute TED data. More detailed disease and pre- and post-transplant clinical information are collected on a subset of registered patients selected for CRF data by a weighted randomization scheme. TED and CRF level data are collected pre-transplant, 100 days, and six months post transplant and annually thereafter or until death.
Subjects
The study population included all patients with aggressive, chemo-refractory B-cell NHL receiving an allo-HCT reported to the CIBMTR between 1998 and 2010. Eligible histologies included WHO grade III follicular lymphoma (FL-III), and DLBCL. Subjects included in our analysis with primary refractory disease (i.e. the patients who never achieved a complete remission [CR] or partial remission [PR] in response to any of the pre-HCT therapies) at the time of allo-HCT were labeled as primary induction failure (PIF)-resistant, while the patients with chemorefractory disease at the time allo-HCT, following a relapse from a prior remission were labeled as relapsed (REL)-resistant. Patients with indolent histologies (including grade I-II FL), and ones with transformed large cell lymphomas were not included. Patients with evidence of chemosensitive disease (i.e. patients achieving a CR or PR) in response to the last line of chemotherapy administered before allo-HCT were excluded. Pediatric patients (n=5), and recipients of planned tandem auto-/allo-HCT (n=50), syngeneic-HCT (n=7) and umbilical cord blood transplantation (n=29) were not included in the analysis. The patient- and disease-related variables that are not reported for registration-only patients are indicated at appropriate places in Table 1.
Table 1.
Characteristics of patients that underwent an allogeneic transplant for chemorefractory aggressive FL-III and DLBCL reported to the CIBMTR between 1998 and 2010
| Variable | Myeloablative | RIC/NST | P-value |
|---|---|---|---|
| Number of patients | 307 | 226 | |
| Number of centers | 85 | 59 | |
| Age, median (range), years | 46 (19-66) | 53 (20-70) | <0.001 |
| Sex | 0.293 | ||
| Male | 185 (60) | 126 (56) | |
| Karnofsky score | 0.590 | ||
| <90% | 156 (51) | 135 (60) | |
| Histology | 0.001 | ||
| Follicular Lymphoma (grade 3) | 32 (10) | 48 (21) | |
| DLBCL | 275 (90) | 178 (79) | |
| Prior auto-HCT | 48 (15) | 86 (38) | <0.001 |
| Interval from auto-HCT to allo-HCT, months | 13 (6-136) | 17 (6-198) | 0.149 |
| Interval from diagnosis to transplant, months | 15 (1-238) | 24 (5-340) | <0.001 |
| Disease stage at diagnosis* | 0.940 | ||
| I-II | 30 (34) | 33 (34) | |
| III-IV | 54 (62) | 58 (60) | |
| Missing | 3 ( 4) | 6 ( 6) | |
| B-symptoms at any time prior to transplant* | 0.424 | ||
| A | 41 (47) | 50 (52) | |
| B | 34 (39) | 32 (33) | |
| Missing | 12 (14) | 15 (15) | |
| LDH at any time prior to transplant* | 0.986 | ||
| Normal | 32 (37) | 34 (35) | |
| Abnormal | 53 (61) | 56 (58) | |
| Unknown | 2 ( 2) | 7 ( 7) | |
| Number of prior chemotherapy lines, median (range) | 3 (1-5) | 4 (1-5) | 0.253 |
| Bone marrow involvement at any time prior to transplant* |
0.900 | ||
| No BM involvment | 46 (53) | 46 (47) | |
| BM involvment | 21 (24) | 22 (23) | |
| Missing | 20 (23) | 29 (30) | |
| Extranodal involvement at any time prior to transplant* | 0.507 | ||
| No involvment | 19 (22) | 25 (26) | |
| Involvment | 68 (78) | 71 (73) | |
| Missing | 0 | 1 ( 1) | |
| Conditioning regimens* | NA | ||
| CY/TBI | 103 (50) | 0 | |
| BU/CY | 65 (31) | 0 | |
| TBI Low dose <500cGY-single-TBI<800cGY-fract | 0 | 7 ( 6) | |
| Fludarabine/Melphalan | 0 | 30 (28) | |
| Fludarabine/BU | 0 | 21 (19) | |
| TBI =200cGY | 0 | 14 (13) | |
| Fludarabine+TBI=200cGY | 0 | 9 ( 8) | |
| Fludarabine+CY | 0 | 17 (16) | |
| Fludarabine +Ara-C+ida | 0 | 1 ( 1) | |
| TBI≥500cGY-single-TBI≥800cGY-fract | 17 ( 8) | 0 | |
| Melphalan>150 mg/m^2 | 7 ( 3) | 0 | |
| BU>9 mg/kg | 11 ( 5) | 0 | |
| BU+Melphalan | 5 ( 3) | 0 | |
| CBV/Similar | 0 | 10 ( 9) | |
| CNS involvement at any time prior to transplant* | 0.381 | ||
| No CNS | 84 (97) | 90 (93) | |
| CNS | 3 ( 3) | 6 ( 6) | |
| Missing | 0 | 1 ( 1) | |
| Bulky disease* | 0.571 | ||
| <5 cm | 18 (21) | 22 (23) | |
| ≥5 cm | 26 (30) | 25 (26) | |
| Missing | 43 (49) | 50 (51) | |
| Radiation prior to transplant* | 0.244 | ||
| No | 20 (23) | 19 (20) | |
| Yes | 32 (37) | 48 (49) | |
| Missing | 35 (40) | 30 (31) | |
| Rituximab prior to transplant* | 0.122 | ||
| Rituximab | 57 (59) | 80 (70) | |
| No rituximab | 39 (41) | 35 (30) | |
| Disease status | 0.005 | ||
| PIF-resistant | 159 (52) | 89 (39) | |
| REL-resistant | 148 (48) | 137 (61) | |
| Graft type | 0.008 | ||
| Bone marrow | 65 (21) | 28 (12) | |
| Peripheral blood | 242 (79) | 198 (88) | |
| Type of donor* | 0.090 | ||
| HLA-id sibling | 162 (60) | 94 (47) | |
| Other relative | 16 ( 6) | 13 ( 7) | |
| URD well-matched | 64 (23) | 62 (31) | |
| URD partially matched | 20 ( 7) | 23 (12) | |
| URD mismatched | 10 ( 4) | 8 ( 4) | |
| Year of transplant | 0.002 | ||
| 1998-2001 | 86 (28) | 35 (15) | |
| 2002-2005 | 88 (29) | 82 (36) | |
| 2006-2010 | 133 (43) | 109 (48) | |
| ATG* | <0.001 | ||
| ATG alone | 12 (14) | 24 (25) | |
| Alemtuzumab alone | 0 | 13 (14) | |
| No ATG or alemtuzumab | 75 (26) | 59 (61) | |
| GVHD prophylaxis | 0.107 | ||
| Ex vivo T-cell depletion | 10 ( 3) | 3 ( 1) | |
| Tacrolimus +/− others | 168 (55) | 119 (53) | |
| Cyclosporine +/− others | 105 (34) | 88 (39) | |
| CD34 selection | 6 ( 2) | 0 | |
| Others-not specified | 18 ( 6) | 16 ( 7) | |
| Median FU of survivors (range), months | 35 (3-122) | 30 (3-110) |
Abbreviations: ATG = antithymocyte globulin; BU = busulfan; CMV = cytomegalovirus; CNS = central nervous system; CY = cyclosphosphamide; D = donor; F = female; HLA-id = Human leukocyte antigen-identical; R = recipient; RIC = reduced intensity conditioning; NST = non-myeloablative; PIF = primary induction failure; REL = relapse; GVHD = graft-vs-host disease; M = male; MTX = methotrexate; TBI = total body irradiation; EVAL = evaluable; URD = unrelated donor.
Research level patients only
Definitions
The intensity of conditioning regimens were categorized as MA or RIC/NST using established consensus criteria [11]. Previously established criteria for categorizing the degree of HLA matching were used [12] for unrelated donor transplants (URD). Well-matched patients had either no identified HLA mismatching and informative data at four loci or allele matching at HLA-A, -B, and -DRB1 (6/6). Partially matched pairs had a defined, single-locus mismatch and/or missing HLA data. Mismatched cases had ≥2 allele or antigen mismatches.
Study Endpoints
Primary outcomes were non-relapse mortality (NRM), progression/relapse, progression-free survival (PFS), and overall survival (OS). NRM was defined as death from any cause during the first 28 days after transplantation or death without evidence of lymphoma progression/relapse; relapse was considered a competing risk. Progression/relapse was defined as progressive lymphoma after HCT or lymphoma recurrence after a CR; NRM was considered a competing risk. For PFS, a patient was considered a treatment failure at the time of progression/relapse or death from any cause. For relapse, NRM, and PFS patients alive without evidence of disease relapse or progression were censored at last follow-up. The OS was defined as the interval from the date of transplantation to the date of death or last follow-up. Other outcomes analyzed included acute and chronic graft-versus-host disease (GVHD) and cause of death. Acute GVHD was defined and graded based on the pattern and severity of organ involvement using established criteria [13]. Chronic GVHD was defined as the development of any evidence of chronic GVHD based on clinical criteria [14]. Neutrophil engraftment was defined as first of 3 successive days with absolute neutrophil count (ANC) ≥ 0.5 × 109/L after post-transplantation nadir. Platelet engraftment was considered to have occurred on the first of three consecutive days with platelet count 20 × 109/L or higher, in the absence of platelet transfusion for 7 consecutive days. For engraftment and GVHD, death without the event was considered a competing risk.
Statistical analysis
Probabilities of PFS and OS were calculated using the Kaplan-Meier product limit estimate. Probabilities of NRM, lymphoma progression/relapse, acute and chronic GVHD, and engraftment were calculated using cumulative incidence curves to accommodate for competing risks . Patient-, disease- and transplant-related factors were compared between RIC/NST and MA groups using the Chi-square test for categorical variables and the Wilcoxon two sample test for continuous variables. Associations among patient-, disease, and transplantation-related variables and outcomes of interest were evaluated using multivariate Cox proportional hazards regression. A stepwise selection multivariate model was built to identify covariates that influenced outcomes. Covariates with a P value <0.05 were considered significant. The proportionality assumption for Cox regression was tested by adding a time-dependent covariate for each risk factor and each outcome. Covariates violating the proportional hazards assumption were stratified in the Cox regression model. Results are expressed as relative risk (RR) or the relative rate of occurrence of the event.
The following variables were reported for both registration-level and research-level patients and were considered in multivariate analyses: age at allo-HCT, gender, Karnofsky Performance Score (KPS) at allo-HCT, prior auto-HCT, time interval between diagnosis and allo-HCT, histology (DLBCL vs. FL-III), disease status at allo-HCT, conditioning regimen intensity , donor type, donor–recipient gender match, graft source, year of allo-HCT and type of GVHD prophylaxis.
Results
Patient, Disease-, and Transplant-Related Variables
Between 1998 and 2010, 533 patients received allo-HCT for refractory FL-III (n=80) and DLBCL (n=453); 307 patients received a MA allo-HCT and 226 received a RIC/NST allo-HCT. Twelve DLBCL patients in the MA cohort were included in a prior CIBMTR analysis [15]. Median follow up of survivors for the MA and the RIC/NST groups was 35 months and 30 months, respectively. Completeness of follow up at 3 years was 80% in both groups reflecting good follow up to this time point [16]. Table 1 describes patient-, disease- and transplant related variables of two cohorts (MA vs. RIC/NST) analyzed. The RIC/NST cohort was older compared with MA cohort (median age 53 years vs. 46 years, p<0.001). Only 242 patients (45%) had a pre-transplant KPS of 90 or higher. Median time from diagnosis to transplant was significantly longer in RIC/NST groups compared to MA cohort (24 months vs. 15 months, p-value<0.001).
No significant difference at baseline was observed between the two groups in terms of disease stage at diagnosis, B-symptoms, number of lines of prior therapy, bone marrow or extranodal involvement, disease bulk, central nervous system involvement, prior rituximab use, and donor type. Significantly more patients in the RIC/NST group had a prior history of undergoing an auto-HCT (38% vs. 15%; p-value<0.001) and received a peripheral blood allograft (88% vs. 79%; p-value=0.008). More patients in the MA group had primary refractory disease (PIF-resistant) (52% vs. 39%, p=0.005). The commonest conditioning regimens prior to MA allo-HCT were CY/TBI (cyclophosphamide/total body irradiation) and Bu (busulfan)/CY. The majority of the patients (~90%) in both cohorts received calcineurin inhibitor-based GVHD prophylaxis.
Outcomes
Univariate analysis of patient outcomes after HCT is shown in Table 2, while multivariate analysis for NRM, relapse/progression, PFS and OS are summarized in Table 3.
Table 2.
Univariate outcome probabilities
| Myeloablative |
RIC/NST |
||||
|---|---|---|---|---|---|
| Outcome event | N | Prob (95% CI) | N | Prob (95% CI) | P-value* |
| Time to ANC>0.5 × 109/L | 245 | 189 | |||
| @ 28 days | 85 (80-89) | 90 (85-94) | 0.085 | ||
| @ 100 days | 86 (81-90) | 92 (87-95) | 0.047 | ||
| Platelet recovery ≥ 20 × 109 | 171 | 146 | |||
| @ 28 days | 54 (46-61) | 79 (71-85) | <0.001 | ||
| @ 100 days | 71 (63-77) | 87 (80-92) | <0.001 | ||
| Acute GVHD (II-IV) | 225 | 183 | |||
| @ 100 days | 29 (24-35) | 31 (25-38) | 0.684 | ||
| Chronic GVHD | 229 | 179 | |||
| @ 1 year | 33 (27-39) | 38 (31-45) | 0.274 | ||
| @ 3 years | 37 (31-43) | 39 (32-46) | 0.619 | ||
| NRM | 289 | 218 | |||
| @ 100 days | 38 (32-43) | 25 (20-31) | 0.004 | ||
| @ 1 year | 47 (41-53) | 36 (30-43) | 0.017 | ||
| @ 3 years | 53 (46-59) | 42 (35-49) | 0.034 | ||
| Relapse/Progression | 289 | 218 | |||
| @ 1 year | 27 (22-33) | 32 (26-39) | 0.289 | ||
| @ 3 years | 28 (23-34) | 35 (28-42) | 0.124 | ||
| Progression free survival | 289 | 218 | |||
| @ 1 year | 26 (20-31) | 32 (25-38) | 0.138 | ||
| @ 3 years | 19 (15-25) | 23 (17-30) | 0.408 | ||
| Overall survival | 307 | 226 | |||
| @ 1 year | 31 (25-36) | 41 (34-47) | 0.016 | ||
| @ 3 years | 19 (15-24) | 28 (22-35) | 0.027 | ||
Abbreviations: ANC = neutrophil recovery; NRM = non-relapse mortality; PFS = progression-free survival; PROB = probability; CI = confidence interval.
Probabilities of neutrophil and platelet recovery, platelet recovery, acute GVHD, chronic GVHD, treatment-related mortality and progression/relapse were calculated using the cumulative incidence estimate. Progression-free survival and overall survival was calculated using the Kaplan-Meier product limit estimate.
Table 3.
Multivariate analysis for NRM, progression/relapse, progression free survival and overall survival (follicular/DLBCL)
| Variables | N | RR (95% CI) | P-value |
|---|---|---|---|
| TRM | |||
| Main effect | |||
| RIC/NST | 202 | 1 | 0.113 |
| Myeloablative | 264 | 1.25 (0.95-1.66) | |
| Histology | |||
| DLBL | 393 | 1 | 0.002 |
| follicular | 73 | 0.52 (0.35-0.79) | |
| Donor type | |||
| Unrelated | 217 | 1 | 0.003 |
| Related | 249 | 0.64 (0.48-0.86) | |
| Progression/relapse | |||
| Main effect | |||
| RIC/NST | 202 | 1 | 0.015 |
| Myeloablative | 264 | 0.66 (0.47-0.92) | |
| Histology | |||
| DLBL | 393 | 1 | 0.002 |
| follicular | 73 | 0.42 (0.25-0.73) | |
| Prior autologous transplant | |||
| No prior auto | 365 | 1 | Poverall=0.006 |
| Prior auto, ≤12 m | 39 | 0.90 (0.49-1.65) | 0.742 |
| Prior auto, >12 m | 62 | 0.30 (0.15-0.63) | 0.001 |
| Contrast:≤12 vs. >12 | 2.99 (1.22-7.35) | 0.017 | |
| Progression free survival | |||
| Main effect | |||
| RIC/NST | 202 | 1 | 0.843 |
| Myeloablative | 264 | 1.02 (0.83-1.27) | |
| Histology | |||
| DLBL | 393 | 1 | <0.001 |
| follicular | 73 | 0.51 (0.37-0.70) | |
| Overall survival | |||
| Main effect | |||
| RIC/NST | 226 | 1 | 0.220 |
| Myeloablative | 307 | 1.14 (0.93-1.40) | |
| Histology | |||
| DLBL | 453 | 1 | <0.001 |
| follicular | 80 | 0.53 (0.39-0.72) |
Engraftment and GVHD
The cumulative incidence of neutrophil engraftment at day +28 was 85% in the MA cohort and 90% in the RIC/NST cohort (p-value=0.08) (Table 2). The cumulative incidence of platelet recovery at day +28 was significantly higher after RIC/NST compared to MA conditioning (79% vs. 54%; p-value<0.001). Cumulative incidence of grade II-IV acute GVHD at day +100 was 29% and 31% in MA and RIC/NST groups (p-value = 0.68), respectively (Table 2). Cumulative incidence of chronic GVHD at 1 year post transplantation in similar order was 33% and 38%, respectively (p-value = 0.27).
Non relapse mortality
Cumulative incidence of NRM was significantly lower in RIC/NST cohort vs. MA cohort, both at 100 days (RIC/NST 25% [95% CI 20-31] vs. MA 38% [95% CI 32-43], P-value=0.004) and at 3 years (RIC/NST 42% [95% CI 35-49] vs. MA 53% [95% CI 46-59], P-value=0.03) However, on multivariate analysis conditioning regimen intensity was no longer associated with NRM (MA vs. RIC/NST, RR 1.25, 95% CI 0.94-1.65) (Table 3). FL-III histology (RR=0.52, 95% CI 0.35-0.79; p-value=0.002) and matched related donor (MRD) allo-HCT (RR=0.64, 95% CI 0.48-0.86; p-value=0.003) were associated with a reduced risk of NRM in multivariate analysis. Separate multivariate analysis for patients previously not undergoing an auto-HCT similarly showed reduced risk of NRM with FL-III (RR 0.44, 95% CI 0.27-0.74) and MRD transplantation (RR 0.72, 95% CI 0.51-1.00). Multivariate analysis of DLBCL patients only (by excluding FL-III) also showed association of MRD with reduced risk of NRM (RR=0.65, 95% CI 0.48-0.89).
Relapse/Progression
The one- and three-year probability of relapse/progression were similar in both the MA and the RIC/NST groups (Table 2); at three years it was 28% in the MA cohort (95% CI 23-34) and 35% (95% CI 28-42) in the RIC/NST cohort (p-value=0.12). On multivariate analysis, MA conditioning (RR=0.66, 95% CI 0.47-0.92; p-value=0.02), FL-III histology (RR=0.42, 95% CI 0.25-0.73; p-value=0.002) and a prior auto-HCT more than 12 months before the allo-HCT compared to no prior auto-HCT (RR=0.30, 95% CI 0.15-0.63; p-value=0.001) were associated with a reduced risk of relapse/progression (Table 3). Separate multivariate analysis for patients previously not undergoing an auto-HCT similarly showed reduced risk of relapse/progression with FL-III (R=0.39, p-value=0.002). Multivariate analysis of DLBCL patients only, showed an association of a prior auto-HCT more than 12 months before the allo-HCT, with reduced risk of relapse/progression (RR=0.27, p-value=0.002).
Progression free survival
PFS estimates were not significantly different between MA and RIC/NST groups, neither at one year (26% [95% CI 20-31] vs. 32% [95% CI 25-38], p=0.13) nor at three years (19% [95% CI 15-25] vs. 23% [95% CI 17-30], p=0.40) (Figure 1 and Table 2). On multivariate analysis, FL-III (RR=0.51, 95% CI 0.37-0.70; p-value<0.0001) was associated with a superior PFS compared to DLBCL (Table 3). Separate multivariate analysis for patients previously not undergoing an auto-HCT also showed that only FL-III was associated was superior PFS (RR=0.43, p-value<0.0001), while in the multivariable analysis for DLBCL patients only, none of the variables were significant.
Figure 1.
Kaplan-Meier estimates of adjusted overall survival following allogeneic transplantation for FL-III and DLBCL
Overall survival
In univariate analysis, RIC/NST group had superior OS at one year (41% [95% CI 34-47] vs. 31% [95% CI 25-36], p=0.01) and at three years (28% [95% CI 22-35] vs. 19% [95% CI 15-24], p=0.02) compared to the MA group (Table 2). On multivariate analysis only FL-III (RR=0.53, 95% CI 0.39-0.72; p-value<0.0001) was associated with a reduced risk of death after allo-HCT (Figure 1 and Table 3). Separate multivariate analysis for patients previously not undergoing an auto-HCT also showed that only FL-III was associated was reduced risk of death (RR=0.40, p-value<0.0001), while in the multivariable analysis for DLBCL patients only, none of the variables were significant.
Causes of death
The majority of deaths, 104 in the MA cohort and 77 in the RIC/NST cohort, were attributed to disease relapse and/or progression. Causes of death are summarized in Table 4.
Table 4.
Causes of death
| Cause of death | Myeloablative | RIC/NST |
|---|---|---|
| Total number | 241 | 162 |
| Graft rejection | 0 | 1 ( 1) |
| Infection | 31 (13) | 18 (11) |
| Pulmonary syndrome | 4 ( 2) | 4 ( 2) |
| ARDS | 6 ( 2) | 0 |
| GVHD | 13 ( 5) | 14 ( 9) |
| Primary disease | 104 (43) | 77 (48) |
| Organ failure | 26 (11) | 11 ( 7) |
| Second malignancy | 1 (<1) | 0 |
| Hemorrhage | 4 ( 2) | 2 ( 1) |
| Vascular | 1 (<1) | 1 ( 1) |
| Toxicity | 5 ( 2) | 1 ( 1) |
| Other cause-not specified* | 46 (19) | 33 (20) |
29 cases reported “other HSCT related cause”
Abbreviations: ARDS=adult respiratory distress syndrome; GVHD=graft-versus-host disease.
DISCUSSION
The aims of the present study were to define outcomes of chemorefractory DLBCL and FL-III patients after allo-HCT, relative to the intensity of the conditioning regimens and other variables including graft source and prior use of auto-HCT. This large cohort of refractory aggressive lymphoma patients gleaned from multiple centers provides several important observations. First, despite refractory disease at baseline, approximately a quarter of aggressive NHL patients undergoing allo-HCT are alive and in remission three years post transplantation. Second, although use of MA allo-HCT in this poor prognosis group reduced the relapse risk, OS (on univariate analysis) was inferior likely due to the unacceptably high rates of NRM. Third, histologic subtype emerges as a major predictor of transplantation outcomes, with refractory FL-III consistently showing better outcomes compared to DLBCL; this finding emphasizes the importance of different disease biology of these subtypes. Fourth, high NRM rates after allo-HCT in this high-risk group will continue to be the main barrier towards wider application of this modality.
There is limited published data on the role of allo-HCT in patients with refractory aggressive B-cell NHL. Registry data from EBMT (European Group for Blood and Bone Marrow Transplantation) on outcomes of RIC allo-HCT in patients with NHL (including indolent and aggressive histologies) identified aggressive histology and chemorefractory disease as predictors of inferior outcomes, but included only 13 refractory aggressive lymphoma patients [17]. French [10] and Japanese [8] registry data reported OS rates of ~40% with allografting in aggressive NHL, but these studies included a small number of patients with refractory disease (n=27 in French study and number of B-cell aggressive NHL not reported in Japanese study), whose outcomes were not reported separately. Further, a single institution reported 5 year survival rates of approximately 35% in 46 refractory aggressive NHL patients undergoing allo-HCT; most of these subjects received MA conditioning [18]. Finally, a retrospective analysis from United Kingdom of 17 refractory DLBCL patients receiving RIC allo-HCT showed a disappointing 3 year OS/PFS of only 12% [7]. Our study, the largest report to date, indicates that a small subset of refractory FL-III/DLBCL patients (~25%) can survive long-term post allo-HCT. It is however; important to interpret these numbers in the context of dismal long-term prognosis of these patients with standard chemotherapies, and in light of the fact, that only a minority of patients in our study at transplant had good KPS.
Despite the reduced risk of relapse/progression, we note that MA allo-HCT was not associated with improved survival outcomes, potentially due to high NRM rate of 53% at 3 years. Our report is not a randomized comparison of high versus low intensity conditioning regimens. We cannot discount inherent selection bias, i.e. a tendency of transplant physicians to preferentially offer MA allo-HCT to patients with ‘higher-risk’ or primary-refractory disease. Time interval between diagnosis and allo-HCT was shorter in the MA cohort, compared to RIC/NST cohort, which might be a surrogate marker of more aggressive disease biology of patients included in the former group. However, the distribution of other potentially adverse prognostic factors, e.g. bulky disease, bone marrow involvement, extranodal disease, and lines of prior therapy across these two cohorts, appear balanced at baseline in our study. Hematologic engraftment in RIC/NST cohort appears more robust, likely reflecting more frequent use of peripheral blood rather than bone marrow as an allogeneic graft source in this setting. Our data indicate that in patients with aggressive B-cell NHL who are refractory to conventional therapies, escalating the intensity of conditioning regimens is unlikely to improve patient outcomes. The RIC/NST group in our study is heterogeneous and represents a spectrum of conditioning regimens ranging from truly NST approaches to nearly ablative regimens. However, the relatively small number of patients receiving a truly NST allo-HCT (2Gy-TBI=14, fludarabine/2Gy-TBI=9) in our study, precludes us from assessing the relative risk and benefits of NST versus RIC allo-HCT in refractory B-cell NHL.
The NRM rates we observed for the RIC/NST cohort are higher than the rates generally reported for indolent lymphomas [19,20]; however, in the subset of chemorefractory indolent lymphomas NRM rates approaching 50-60% have been previously reported [21,22]. One-third of the RIC/NST patients in our study previously received an auto-HCT, a potential reason for high NRM. On multivariate analysis, however, a prior auto-HCT was not associated with higher NRM, and other investigators also have not consistently found a prior auto-HCT to significantly influence NRM after the allogeneic procedure in DLBCL [6,7]. Moreover separate multivariable analyses in our study performed by excluding patients who underwent prior auto-HCT, showed findings in line with the multivariable analyses of entire study population. Nonetheless it is clear that mitigating NRM rates via development of novel conditioning approaches in this high-risk group, designed to provide better disease control and acceptable NRM rates, while permitting the GVL effect to emerge post transplantation, are urgently needed. Along these lines Gopal et al. [23] have reported a 30 month 54% survival with a NRM rate of 16% with radioimmunotherapy-based NST in a cohort of mostly refractory B-cell NHL patients, findings similar to those of Bethge and co-workers [24]. Our data also hint that in the context of refractory lymphoma patients, availability of a MRD might be predictive of reduced rates NRM post allo-HCT.
Our report included both registration- and research-level patients reported to CIBMTR. The primary objective of this study was to describe transplantation outcomes of chemorefractory DLBCL and FL-III patients. Missing variables in registration-level patients include disease stage at diagnosis, bulky disease status, B-symptoms, LDH level, bone marrow involvement, and extranodal involvement at any time point before allo-HCT. While some of these variables (e.g. LDH level before allo-HCT) are prognostic [9,25], the significance of their presence at any time point before transplantation (as opposed to their presence at the time of transplantation), in a cohort of exclusively chemotherapy refractory patients is not known. The fact that data about key variables of interest, such as; disease status at transplantation, intensity of conditioning regimens, donor type, graft source, KPS, history of prior autografting and all post-transplantation outcomes of interest (engraftment, GVHD, NRM, OS, PFS etc.) were available on both registration- and research-level patients, supported our decision to include both patient populations. Another possible limitation of our report is the lack of information about functional imaging (i.e. PET or PET/CT scan results) before allo-HCT. Some of the patients included in this study were transplanted in the pre PET-era. Even in patients transplanted in the time period when PET scans became widely available; this imaging modality was likely not uniformly performed in all patients before allo-HCT. Moreover, information about functional imaging is not available for registration-level patients, and the criteria used to uniformly report and interpret response rates using PET scans were not purposed until 2007 [26]. However; wherever available, patients with a negative PET scan before allo-HCT are considered chemosensitive for CIBMTR reporting purposes and were not included in the current study.
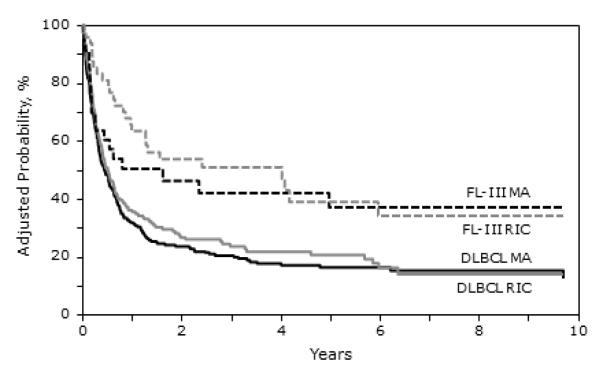
A noteworthy finding of our study is the relatively encouraging outcomes of FL-III patients after allo-HCT despite refractory disease. The 3 year PFS of FL-III patients in our study after MA and RIC/NST was 41% and 40% respectively (Figure 2). The 3 year OS in similar order was 42% and 51% respectively. Vose and colleagues [27] previously have reported that this population is not likely to achieve long-term remissions with auto-HCT. We decided to include FL-III patients in addition to DLBCL in our report, to highlight the fact that while these patients may not achieve durable remissions with auto-HCT, this is not necessarily true following allo-HCT. Our encouraging data support examining the role of allo-HCT in refractory FL-III, prospectively. Whether the outcomes of refractory grade IIIa FL are different from grade IIIb FL following allo-HCT is not known. This data is unfortunately not captured in CIBMTR reporting forms. RIC/NST allo-HCT in aggressive histologies has been associated with a higher risk of relapse compared to more indolent varieties [9]. Within the context of refractory aggressive histologies, the lower relapse rates of FL-III compared to DLBCL is intriguing and illustrates the different disease biologies of these two subtypes.
Figure 2.
Kaplan-Meier estimates of adjusted progression free survival following allogeneic transplantation for FL-III and DLBCL
In order to utilize our data for decision making in the clinic, it is important to interpret these results in the context of outcomes of relapsed DLBCL with other available treatment modalities. In general consolidation with auto-HCT clearly remains the standard option for relapsed and chemosensitive DLBCL, even in the chemoimmunotherapy (CIT)-era [3]. For those DLBCL patients, who relapse following an auto-HCT but remain sensitive to salvage chemotherapies, RIC/NST allo-HCT is routinely offered by many centers based on encouraging outcomes (OS, 45%-50%; PFS, 35%-45%) reported by many groups [5-7,28]. The best therapeutic option for chemorefractory DLBCL patients is more controversial. In the pre CIT-era, van Besien et al [29] initially showed that a small subset of such refractory aggressive NHL patients (~20%) could survive disease-free post allo-HCT. Observations by others [30]; however, suggested that a similar degree of disease control in refractory DLBCL patients could potentially be achieved with auto-HCT (3year PFS=19.4%). It is however important to point out that the outcomes of chemorefractory DLBCL following auto-HCT in a large CIBMTR study were disappointing; with only seven of 52 chemotherapy-resistant patients (13%) surviving disease-free 2 years post HCT [31]. Similarly the outcomes of chemorefractory DLBCL patients in the CIT-era with [3,32], or without [3] auto-HCT have been generally poor (3 year PFS <10-15%). In contrast, the 20-25% 3 year PFS of refractory aggressive NHL patients in our study, has been consistently seen in other reports that included a small subset of refractory patients [7,15,33]. Hence it appears unlikely that in the CIT-era, salvage chemotherapies with or without auto-HCT can provide durable disease control in refractory DLBCL. For such refractory DLBCL patients, especially for the subset with an available MRD consideration of a RIC/NST allo-HCT should be considered a valid option.
In conclusion, our analysis of this large set of registry data show that a subset of chemorefractory FL-III/DLBCL patients can have durable remissions after allo-HCT. MA conditioning does not provide superior survival outcomes, compared to RIC/NST allo-HCT. While refractory, aggressive NHL patients are best managed on a clinical trial, in the absence of a protocol therapy option, consideration of an allograft appears to be a viable option for NHL patients who have disease refractory to conventional therapies.
ACKNOWLEDGEMENTS
We thank Ulrike Bacher, César O Freytes, Miguel-Angel Perales and Sonali Smith for their helpful comments and insights as members of the study committee.
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from Allos, Inc.; Amgen, Inc.; Angioblast; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; Blue Cross and Blue Shield Association; Buchanan Family Foundation; CaridianBCT; Celgene Corporation; CellGenix, GmbH; Children’s Leukemia Research Association; Fresenius-Biotech North America, Inc.; Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.; Genzyme Corporation; GlaxoSmithKline; HistoGenetics, Inc.; Kiadis Pharma; The Leukemia & Lymphoma Society; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; RemedyMD; Sanofi; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; Tarix Pharmaceuticals; Teva Neuroscience, Inc.; THERAKOS, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Footnotes
AUTHOR CONTRIBUTION: M.H., H.M.L, and W.S designed the study and participated in interpretation of data, manuscript preparation and approval of final manuscript. J.C. and K.W.A did the statistical analysis. M.S.C., T.S.F., R.P.G., J.G., G.A.H., P.N.H., J.W.H., D.J.I., R.T.K., A.K., D.M., D.I.M., D.A.R., B.N.S., H.C.S., E.K.W., B.W., G.G.L., S.M., and D.G.M. participated in interpretation of data, manuscript preparation and approval of final manuscript. M.H., H.M.L, and W.S had primary responsibility for manuscript preparation.
The authors have no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFEERENCES
- 1.Philip T, Armitage JO, Spitzer G, et al. High-dose therapy and autologous bone marrow transplantation after failure of conventional chemotherapy in adults with intermediate-grade or high-grade non-Hodgkin’s lymphoma. N.Engl.J.Med. 1987;316:1493–1498. doi: 10.1056/NEJM198706113162401. [DOI] [PubMed] [Google Scholar]
- 2.Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N.Engl.J.Med. 1995;333:1540–1545. doi: 10.1056/NEJM199512073332305. [DOI] [PubMed] [Google Scholar]
- 3.Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J.Clin.Oncol. 2010;28:4184–4190. doi: 10.1200/JCO.2010.28.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khouri IF, Saliba RM, Erwin WD, et al. Nonmyeloablative allogeneic transplantation with or without 90yttrium ibritumomab tiuxetan is potentially curative for relapsed follicular lymphoma: 12-year results. Blood. 2012;119:6373–6378. doi: 10.1182/blood-2012-03-417808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rezvani AR, Norasetthada L, Gooley T, et al. Non-myeloablative allogeneic haematopoietic cell transplantation for relapsed diffuse large B-cell lymphoma: a multicentre experience. Br.J.Haematol. 2008;143:395–403. doi: 10.1111/j.1365-2141.2008.07365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomson KJ, Morris EC, Bloor A, et al. Favorable long-term survival after reduced-intensity allogeneic transplantation for multiple-relapse aggressive non-Hodgkin’s lymphoma. J.Clin.Oncol. 2009;27:426–432. doi: 10.1200/JCO.2008.17.3328. [DOI] [PubMed] [Google Scholar]
- 7.van Kampen RJ, Canals C, Schouten HC, et al. Allogeneic stem-cell transplantation as salvage therapy for patients with diffuse large B-cell non-Hodgkin’s lymphoma relapsing after an autologous stem-cell transplantation: an analysis of the European Group for Blood and Marrow Transplantation Registry. J.Clin.Oncol. 2011;29:1342–1348. doi: 10.1200/JCO.2010.30.2596. [DOI] [PubMed] [Google Scholar]
- 8.Kim SW, Tanimoto TE, Hirabayashi N, et al. Myeloablative allogeneic hematopoietic stem cell transplantation for non-Hodgkin lymphoma: a nationwide survey in Japan. Blood. 2006;108:382–389. doi: 10.1182/blood-2005-02-0596. [DOI] [PubMed] [Google Scholar]
- 9.Armand P, Kim HT, Ho VT, et al. Allogeneic transplantation with reduced-intensity conditioning for Hodgkin and non-Hodgkin lymphoma: importance of histology for outcome. Biol.Blood Marrow Transplant. 2008;14:418–425. doi: 10.1016/j.bbmt.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhedin N, Giraudier S, Gaulard P, et al. Allogeneic bone marrow transplantation in aggressive non-Hodgkin’s lymphoma (excluding Burkitt and lymphoblastic lymphoma): a series of 73 patients from the SFGM database. Societ Francaise de Greffe de Moelle. Br.J.Haematol. 1999;107:154–161. doi: 10.1046/j.1365-2141.1999.01666.x. [DOI] [PubMed] [Google Scholar]
- 11.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol.Blood Marrow Transplant. 2009;15:1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol.Blood Marrow Transplant. 2008;14:748–758. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 14.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am.J.Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 15.Lazarus HM, Zhang MJ, Carreras J, et al. A comparison of HLA-identical sibling allogeneic versus autologous transplantation for diffuse large B cell lymphoma: a report from the CIBMTR. Biol.Blood Marrow Transplant. 2010;16:35–45. doi: 10.1016/j.bbmt.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark TG, Altman DG, De Stavola BL. Quantification of the completeness of follow-up. Lancet. 2002;359:1309–1310. doi: 10.1016/s0140-6736(02)08272-7. [DOI] [PubMed] [Google Scholar]
- 17.Robinson SP, Goldstone AH, Mackinnon S, et al. Chemoresistant or aggressive lymphoma predicts for a poor outcome following reduced-intensity allogeneic progenitor cell transplantation: an analysis from the Lymphoma Working Party of the European Group for Blood and Bone Marrow Transplantation. Blood. 2002;100:4310–4316. doi: 10.1182/blood-2001-11-0107. [DOI] [PubMed] [Google Scholar]
- 18.Hamadani M, Benson DM, Jr, Hofmeister CC, et al. Allogeneic stem cell transplantation for patients with relapsed chemorefractory aggressive non-hodgkin lymphomas. Biol.Blood MarrowTransplant. 2009;15:547–553. doi: 10.1016/j.bbmt.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khouri IF, McLaughlin P, Saliba RM, et al. Eight-year experience with allogeneic stem cell transplantation for relapsed follicular lymphoma after nonmyeloablative conditioning with fludarabine, cyclophosphamide, and rituximab. Blood. 2008;111:5530–5536. doi: 10.1182/blood-2008-01-136242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corradini P, Dodero A, Farina L, et al. Allogeneic stem cell transplantation following reduced-intensity conditioning can induce durable clinical and molecular remissions in relapsed lymphomas: pre-transplant disease status and histotype heavily influence outcome. Leukemia. 2007;21:2316–2323. doi: 10.1038/sj.leu.2404822. [DOI] [PubMed] [Google Scholar]
- 21.Hari P, Carreras J, Zhang MJ, et al. Allogeneic transplants in follicular lymphoma: higher risk of disease progression after reduced-intensity compared to myeloablative conditioning. Biol.Blood Marrow Transplant. 2008;14:236–245. doi: 10.1016/j.bbmt.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vigouroux S, Michallet M, Porcher R, et al. Long-term outcomes after reduced-intensity conditioning allogeneic stem cell transplantation for low-grade lymphoma: a survey by the French Society of Bone Marrow Graft Transplantation and Cellular Therapy (SFGM-TC) Haematologica. 2007;92:627–634. doi: 10.3324/haematol.10924. [DOI] [PubMed] [Google Scholar]
- 23.Gopal AK, Guthrie KA, Rajendran J, et al. 90Y-Ibritumomab tiuxetan, fludarabine, and TBI-based nonmyeloablative allogeneic transplantation conditioning for patients with persistent high-risk B-cell lymphoma. Blood. 2011;118:1132–1139. doi: 10.1182/blood-2010-12-324392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bethge WA, Lange T, Meisner C, et al. Radioimmunotherapy with yttrium-90-ibritumomab tiuxetan as part of a reduced-intensity conditioning regimen for allogeneic hematopoietic cell transplantation in patients with advanced non-Hodgkin lymphoma: results of a phase 2 study. Blood. 2010;116:1795–1802. doi: 10.1182/blood-2010-02-270538. [DOI] [PubMed] [Google Scholar]
- 25.Kenkre VP, Horowitz S, Artz AS, et al. T-cell-depleted allogeneic transplant without donor leukocyte infusions results in excellent long-term survival in patients with multiply relapsed Lymphoma. Predictors for survival after transplant relapse. Leuk.Lymphoma. 2011;52:214–222. doi: 10.3109/10428194.2010.538777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J.Clin.Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 27.Vose JM, Bierman PJ, Loberiza FR, et al. Long-term outcomes of autologous stem cell transplantation for follicular non-Hodgkin lymphoma: effect of histological grade and Follicular International Prognostic Index. Biol.Blood Marrow Transplant. 2008;14:36–42. doi: 10.1016/j.bbmt.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 28.Sirvent A, Dhedin N, Michallet M, et al. Low nonrelapse mortality and prolonged long-term survival after reduced-intensity allogeneic stem cell transplantation for relapsed or refractory diffuse large B cell lymphoma: report of the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire. Biol.Blood Marrow Transplant. 2010;16:78–85. doi: 10.1016/j.bbmt.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 29.van Besien K, Thall P, Korbling M, et al. Allogeneic transplantation for recurrent or refractory non-Hodgkin’s lymphoma with poor prognostic features after conditioning with thiotepa, busulfan, and cyclophosphamide: experience in 44 consecutive patients. Biol.BloodMarrow Transplant. 1997;3:150–156. [PubMed] [Google Scholar]
- 30.Aksentijevich I, Jones RJ, Ambinder RF, Garrett-Mayer E, Flinn IW. Clinical outcome following autologous and allogeneic blood and marrow transplantation for relapsed diffuse large-cell non-Hodgkin’s lymphoma. Biol.Blood Marrow Transplant. 2006;12:965–972. doi: 10.1016/j.bbmt.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 31.Vose JM, Zhang MJ, Rowlings PA, et al. Autologous transplantation for diffuse aggressive non-Hodgkin’s lymphoma in patients never achieving remission: a report from the Autologous Blood and Marrow Transplant Registry. J.Clin.Oncol. 2001;19:406–413. doi: 10.1200/JCO.2001.19.2.406. [DOI] [PubMed] [Google Scholar]
- 32.Alousi AM, Saliba RM, Okoroji GJ, et al. Disease staging with positron emission tomography or gallium scanning and use of rituximab predict outcome for patients with diffuse large B-cell lymphoma treated with autologous stem cell transplantation. Br.J.Haematol. 2008;142:786–792. doi: 10.1111/j.1365-2141.2008.07277.x. [DOI] [PubMed] [Google Scholar]
- 33.Bacher U, Klyuchnikov E, Le-Rademacher J, et al. Conditioning regimens for allotransplants for diffuse large B-cell lymphoma: myeloablative or reduced intensity? Blood. 2012;120:4256–4262. doi: 10.1182/blood-2012-06-436725. [DOI] [PMC free article] [PubMed] [Google Scholar]