Abstract
A major goal of animal research is to identify interventions that can promote successful aging and delay or reverse age-related cognitive decline in humans. Recent advances in standardizing cognitive assessment tools for humans have the potential to bring preclinical work closer to human research in aging and Alzheimer’s disease. The National Institute of Health (NIH) has led an initiative to develop a comprehensive Toolbox for Neurologic Behavioral Function (NIH Toolbox) to evaluate cognitive, motor, sensory and emotional function for use in epidemiologic and clinical studies spanning 3 to 85 years of age. This paper aims to analyze the strengths and limitations of animal behavioral tests that can be used to parallel those in the NIH Toolbox. We conclude that there are several paradigms available to define a preclinical battery that parallels the NIH Toolbox. We also suggest areas in which new tests may benefit the development of a comprehensive preclinical test battery for assessment of cognitive function in animal models of aging and Alzheimer’s disease.
Keywords: NIH Toolbox, Animal models, Executive function, Working memory, Episodic memory, Processing speed, Attention
1. Introduction
There has been little uniformity among measures used in human neuropsychological assessment to assess cognitive deficits associated with aging and Alzheimer’s disease (AD). This has had a negative impact on the pace of discovery in research on aging and dementia in clinical trials, and confounds the utility of preclinical assessment. Accordingly, the National Institutes of Health supported the development of a comprehensive assessment tool, the NIH Toolbox For Assessment Of Neurological And Behavioral Function, for use in longitudinal, epidemiological, and intervention studies. The entire NIH Toolbox covers emotion, cognition, motor, and sensory function, whereas the cognition section of the NIH Toolbox provides a specific neuropsychological instrument battery (NIH Toolbox Cognitive Function Battery [CFB]), to probe several cognitive domains (working memory, episodic memory, attention, executive function, processing speed, language, and reading). To move the field of translational research forward, there is an urgent need to develop a comparable preclinical test battery using animal models.
Use of animal models for evaluation of cognitive dysfunction involves simulating specific behaviors or symptoms associated with human cognition. Three important validation criteria for evaluation of such model systems are face, construct, and predictive validity. Although construct validity relies on a match between the proposed pathophysiology of a condition and that of the model species, predictive validity focuses on a match with clinical studies in its response to interventions. The third criterion used to define validity of a model is “face validity,” which relies on a match between the behavioral effects observed in a model and those exhibited by the species being modeled. Although it is used in initial design of tests, it is a less stringent criterion compared with construct and predictive validity. Two types of models that are traditionally used in brain aging and AD research are discussed here.
1.1. Natural or spontaneous models
These include species that show a natural deposition of amyloid and tau proteins, both hallmarks of the disease condition in humans, together with cognitive decline. Nonhuman primates (Gearing et al., 1997; Martin et al., 1994; Price et al., 1991) and dogs (Cotman and Head, 2008; Head and Torp, 2002; Pugliese et al., 2006) are 2 of the most common species used as spontaneous models. These animals have a well-developed prefrontal cortex and a relatively long lifespan. This ensures that the animals can perform higher-order cognitive tasks and exhibit age-induced behavioral abnormalities that parallel cognitive deficits shown by aged humans.
The rhesus monkey, for instance, has a lifespan of more than 30 years. This is equivalent to about 90 human years (Price et al., 1991). These monkeys share a 92% to 95% genetic homology with humans and, similar to humans, show age-related cognitive impairments relative to their young counterparts (Herndon et al., 1997; Smith et al., 2004). Aged monkeys also show loss of cholinergic activity (as seen in human AD patients) and deposition of amyloid plaques that can be visualized by using human anti-Aβ protein antibodies (Summers et al., 1997; Voytko et al., 2001). Furthermore, pharmacological interventions that increase acetylcholine release have been successfully shown to enhance cognitive performance in aged monkeys (Terry et al., 1993).
The aged dog, which is considered to be 1 of the best and most accessible animal models of brain aging, also shows many of the key features of human brain aging, mild cognitive impairment (MCI), and early AD (Cotman and Head, 2008, Sarasa and Pesini, 2009). Like the aged human brain, the canine brain shows increased oxidative damage, mitochondrial dysfunction, selective neuron loss, decreased hippocampal neurogenesis, and accumulation of beta-amyloid (Aβ) pathology with age. The aged canine is a natural model of Aβ accumulation, as the canine and human Aβ protein is 100% homologous and the APP sequences share 98% homology. Indeed, the canine brain accumulates senile plaques with age, and the accumulation of Aβ1-42 and Aβ1-40 progresses in a similar fashion to that occurring in the human brain. Furthermore, pharmacological and dietary interventions, and exercise have been shown to improve health and cognitive decline in the aged dogs, lending some predictive validity to the use of the model (Cotman and Head, 2008; Fahnestock et al., 2012; Milgram et al., 2005).
However, despite the significant advantages of higher-animal models, rodents in general, and rats and mice in particular, continue to remain the most commonly used animals as experimental models of aging and AD. A high birth rate, short reproductive and life cycle, and small size make them ideal laboratory animals. Furthermore, with the increased use of transgenic and knockout mouse models, it has become more common to use induced models to evaluate the etiology of the disease and to develop pharmaceutical treatments.
1.2. Induced models
These models rely on induction of AD-like pathology using experimental manipulations such as lesions (Lescaudron and Stein, 1999 Vale-Martinez et al., 2002), drugs (Buccafusco, 2009; Decker and McGaugh, 1991; Taffe et al., 1999), amyloid-β infusion and genetic alterations (for review see (Gotz and Ittner, 2008; Zahs and Ashe, 2010)). Drug treatments that produce a disruption or loss of acetylcholine brain transmission are examples of pharmacological disruption that models Alzheimer’s dementia. Such a deficit can be reproduced in animals by blocking cholinergic receptors pharmacologically with drugs such as scopolamine and mecamylamine or by using neurotoxic, electrolytic, or mechanical lesions of cholinergic brain subregions (Toledano et al., 2010). Lesion models have the advantage of producing more chronic deficits than the pharmacological models. Genetic manipulations include knockouts, knockdowns, and transgenic mice, and are extensively used to study pathological characteristics of AD, such as amyloid plaques and neurofibrillary tangles. Despite some drawbacks (reviewed in Zahs and Ashe, 2010), genetically altered species can be considered the most valid induced model based on similarities in construct (Zahs and Ashe, 2010).
Although the study of aging and AD depends on the use of animal models, the correspondence between the behavioral assays used in humans and animals to measure the key cognitive domains of the NIH Toolbox is not well established. In the following sections, we describe and evaluate tests that can be used to measure subdomains from the NIH Toolbox CFB (overview in Table 1). Such a test battery would facilitate study comparisons between different groups, make data pooling much more feasible, and improve the translational properties of preclinical research. Our goal here is to outline a battery of preclinical behavioral tests that may be used to assess 5 of the 7 cognitive domains comprising the NIH Toolbox CFB. Language and reading, which involve drawing inferences from written or printed text, are extremely difficult to reliably model in animals, and will not be addressed in this article.
Table 1.
Overview of animal tasks that parallel the NIH Toolbox cognitive function battery
| Domain | Task in NIH Toolbox CFB | Parallel animal task |
|---|---|---|
| Executive function | ||
| Cognitive flexibility | Dimensional card sort task | Attentional set-shifting task |
| Reversal learning task | ||
| Inhibitory control | Flanker task | Go/No-go tasks |
| Working memory | Single and multiple list sorting task | Discussed as a separate domain |
| Episodic memory | Sequential memory/learning task | What–where tasks |
| What–when tasks | ||
| What–where–when task | ||
| Working memory | Single and multiple list sorting task | DNMP/DNMS |
| Self-order tasks | ||
| n-Back task | ||
| Processing speed | Pattern comparison task | Pattern comparison task |
| Attention | Flanker task | 5-Choice serial reaction task |
| Visual attention task | ||
| Language | Picture vocabulary test | N/A |
Key: CFB, cognitive function battery; DNMP/DNMS, delayed non-match to position/delayed non-match to sample; N/A, not available; NIH, National Institutes of Health.
2. NIH Toolbox CFB and comparable preclinical tests
2.1. Executive function
Overall, the NIH Toolbox CFB considers executive function as the top-down cognitive modulation of goal-directed activity. Executive function involves several different components or subdomains, including set shifting, inhibitory response, and updating/working memory. In the NIH Toolbox CFB, the Dimensional Change Card Sort task is used as a measure of set shifting. This task is a measure that was adapted for adults from pediatric research (Zelazo, 2006). In this task, subjects must respond to pairs of stimuli by selecting the one that is the same shape as a third target stimulus or one that is the same color. The sorting contingencies shift, requiring the ability to inhibit previous response strategies and to try new ones. In accordance, we propose that the attentional set-shifting task be used as a measure of set-shifting in animals.
The NIH Toolbox CFB describes a flanker task to evaluate the inhibitory component of visual attention and executive function. The test presents a line of arrows (or fish, for children) in the center of the computer screen (Fan et al., 2002). The central stimulus points leftward or rightward, whereas the others point either in the same direction as the central stimuli (congruent trials) or in the opposite direction (incongruent trials). The task is to focus on the central arrow and indicate its direction, ignoring the “flankers.” Reaction time is slower on incongruent than on congruent trials, reflecting the contribution of an inhibitory response to the incongruent flankers. This task on the NIH Toolbox CFB yields a score for the executive component and can also yield a score reflecting responses to congruent conditions, reflecting in turn sustained attention without inhibitory responses. Here, we propose that Go/No-go paradigms can provide excellent tests to measure inhibitory response.
Working memory, which is both a component of executive function and a separate domain in the NIH Toolbox, will be discussed later here.
2.1.1. Attentional set shifting
Cognitive flexibility in animals can be measured by using an attentional set-shifting or intra-extra-dimensional shift task (Birrell and Brown, 2000; Dias et al.,1996). In this test, animals are trained to carry out a series of 7 discriminations (Fig. 1A), namely, simple discrimination (SD), compound discrimination (CD), reversal 1 (R1), intra-dimensional shift (IDS), reversal 2 (R2), extra-dimensional shift (EDS), and reversal 3 (R3). Animals are trained to select the target (associated with the positive reward) based on a single criterion. For instance, in rodent studies, the first stage is often an SD, in which the animals chose 1 food bowl over the other based on the shape of bowl. This is identical to the SD in the training session on the previous day, except that new bowls are used. The animal is expected to reach a predetermined criterion of “n” consecutive correct responses. For CD, a second dimension (odor) is introduced, but the correct and incorrect exemplars remain the same (shape of bowl). For the IDS, the relevant stimulus is still the shape of bowl, but a new shape is introduced. For the reversal phase, the exemplars and relevant dimensions remain the same, but the animals have to learn that the previously baited shape or odor is now incorrect and the other odor is now the correct one. For the ED shift, the previously irrelevant parameter (i.e., odor) is now relevant. It should be noted that new exemplars are used for the ID and ED shifts.
Fig. 1.
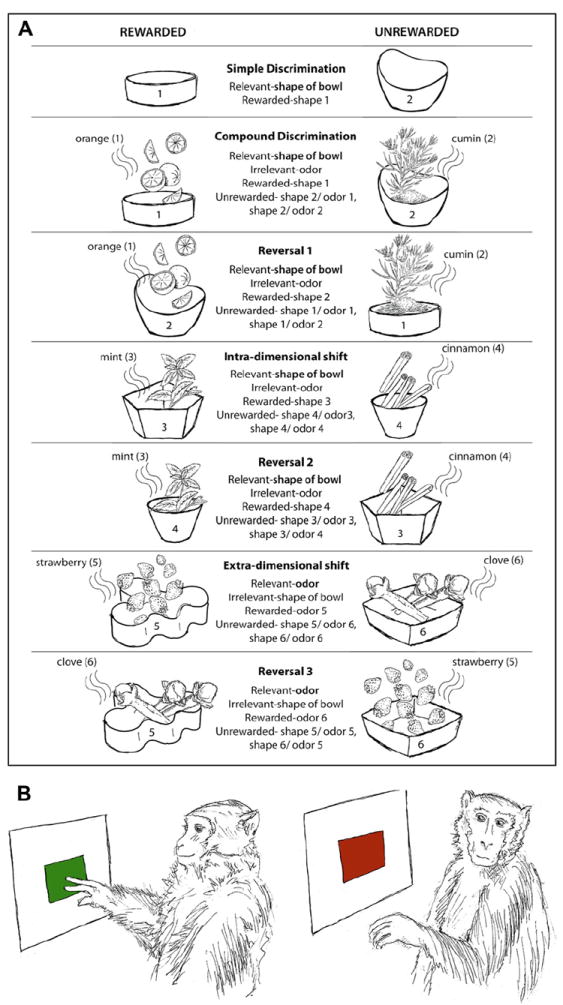
(A) Attentional set-shifting task. The image shows a common form of the attentional set-shifting task. Rats are trained to choose the appropriate bowl to dig for food reward based on defined discrimination rules that change at every successive stage of the task. Each bowl can vary in the shape of the bowl and the odor of the media. Intradimensional shift comprises a total stimulus change, but with the same measure (odor or shape) still being the relevant stimulus to attend to. During extradimensional shifting, the other dimension, which had so far been irrelevant, becomes the relevant measure. (B) Go/No-go task. Representative image showing subjects performing on go trials (in response to the color green on screen) and inhibiting response on no-go trials (in response to the color red on screen).
2.1.2. Task validity and effects of aging
Manipulations, which impair performance in the set shifting task, include lesions in the medial prefrontal cortex (PFC) in rats (Birrell and Brown, 2000; Dias et al., 1996) and dorsolateral PFC in monkeys (Dias et al., 1996). Aged rats are also impaired in task performance (Nicolle and Baxter, 2003). Pharmacologically, scopolamine selectively disrupts task performance during the ED shift but not in the ID phase (Chen et al., 2004). This dissociation is consistent with human studies using the Wisconsin Card Sorting Test(WCST) and is suggestive of some construct validity (Owen et al., 1991). Fewer studies have used mice to evaluate set-shifting deficits. Recently, Young et al. have used the set-shifting task in mice to show that aged mice may use a strategy of “speed to accuracy trade off” to maintain accurate performance in the task (Young et al., 2010). Specifically, these investigators reported that older mice were not specifically impaired in trials to criterion but were significantly slower in making correct responses. The only other study that used the attentional set-shifting task in a murine model used a transgenic APP precursor protein mutation model (TG2576). The authors reported an overall effect of aging, but there was no evidence of an ID/ED shift or performance difference between simple and reversal phases, which in turn questions the formation of attentional set in the mice (Zhuo et al., 2007). A key difference between the 2 studies was the use of normal aged versus transgenic mice. Collectively, these studies suggest that the attentional set-shifting task is a good model of set shifting in primates and rats, but that additional work is needed to adapt it to mice.
Reversal learning, which is often used as an independent test of executive function, is a part of the set-shifting task described previously. However, when used as an independent test, rodent models commonly use an operant chamber (Neese et al., 2010) or odor discrimination/reversal task (Schoenbaum et al., 2002a) to measure reversal learning. Briefly, the operant chamber method uses a 2-lever operant box to first train the animals on 1 lever and not the other, following which the active lever is switched, requiring the rat to reverse it response to the previously nonbaited lever. This is similar to the R1 phase of the attentional set-shifting task. However, a study by Roberts and Wallis (2000) in primates, showed that whereas set-shifting ability is impaired by lateral lesions of the PFC, reversal learning is impaired by orbital but not lateral lesions. This is consistent with the work of Schoenbaum et al. (Schoenbaum et al., 2003) in rodents. It must be noted that Schoenbaum have also reported that the orbitofrontal cortex is necessary for inhibiting responses only when the responses need to be altered to reflect changing relationships between cues and outcomes (Schoenbaum et al., 2002b). This suggests that a loss of inhibitory control can occur independently from other components of executive function. Although reversal learning can be used to test cognitive flexibility and inhibitory control, it is difficult to isolate these 2 components in the task. Thus, we recommend investigating set shifting and inhibitory control independently using the attentional set-shifting task and go/no-go paradigms, respectively.
2.1.3. Go/No-go paradigms
A go/no-go task requires subjects to perform a learned response on “go” trials (e.g., to push a lever in response to a color) but withholding responding on “no-go” trials (e.g., not to push lever on a different color) (Fig. 1B). The index of inhibitory control is the number of errors that a subject makes on no-go trials (Iversen and Mishkin, 1970, Sakagami and Tsutsui, 1999, Sakagami et al., 2001).
2.1.4. Task validity and effects of aging
The main function of the PFC is one of “executive control”; however, there is some controversy over whether subregions of PFC are functionally differentiated in executing this control, and whether there is localization of inhibitory functions to dorsolateral PFC (Diamond, 1990), orbital frontal cortex or right inferior frontal cortex (IFC) (Aron et al., 2004; Roberts and Wallis, 2000). Nonetheless most studies agree that the prefrontal regions of the brain are involved in inhibitory responses and in signaling for overriding an automatic response tendency. Neuroimaging studies using go/no-go tasks consistently point to involvement of a right-lateralized IFC region (Garavan et al., 2002; Menon et al., 2001; Nielson et al., 2002). This has also been demonstrated in nonhuman primate studies, lending further credibility to the model (Iversen and Mishkin, 1970). The differences in the specific subregions underlying control of different tasks listed here suggest that the ability to suppress response tendencies may be an intrinsic property of the prefrontal cortex, whereas the form of the “disinhibition” is more dependent on the nature of the whole operation (or task) being performed.
2.2. Episodic memory
Episodic memory refers to the ability to remember information about personal experiences along with the context in which they occurred (Dickerson and Eichenbaum, 2010; Tulving and Thomson, 1973; Tulving, 1983). This specific form of memory is the last to be acquired during childhood (Perner and Ruffman, 1995) and is among the first to be lost with progressive aging (Herlitz and Forsell, 1996). The NIH Toolbox CFB refers to episodic memory as the sum of cognitive processes involved in the acquisition, storage, and effortful recall of episodes or information within a context. The task incorporated into the NIH Toolbox CFB is the Picture Sequence Memory Test. It was developed for adults from the Imitation Based Assessment of Memory paradigm used to test learning and recall in infants and young children (Bauer, 1993). Single pictures depicting scenes with a common theme (e.g., playing in the park) are sequentially presented, after which the pictures are “gathered” on the computer screen to the center. The subject must recapitulate the sequence demonstrated by the examiner. There are 3 trials, and the sequence length varies depending on the age group being tested: 6 pictures for 3- to 4-year-olds; 9 pictures for 5- to 6-year-olds; 12 for 7- and 8-year-olds; 15 for subjects aged 9 to 60 years; and 9 pictures for subjects aged 65 to 85 years. Because of constraints on the total time allocated to the NIH Toolbox CFB, there is no delayed recall trial. However, there is ample evidence to support a very high correlation between performance on learning trials and subsequent recall of the same material after a delay.
As mentioned earlier, a defining feature of episodic memory is that the memory for individual events is intrinsically tied to the context in which they occurred. This operational definition is the basis of most animal models of episodic memory. In most paradigms, animals demonstrate episodic memory capacity by remembering specific events as well as the spatial and/or temporal context in which they occurred. Approaches developed to study episodic memory in animals range from the original model of memory for “what–where–when” (Clayton and Dickinson,1999) to more recent characterizations of the retrieval dynamics of episodic recollection using receiver-operating characteristics (ROC) (Fortin et al., 2004; Sauvage et al., 2008). These approaches are comprehensively reviewed elsewhere (Clayton et al., 2003; Crystal, 2009; Dere et al., 2006; Eichenbaum et al., 2012; Fouquet et al., 2010; Kesner and Hunsaker, 2010). Here, we consider approaches focusing on the spatial context, the temporal context, and the integration of the 2 contexts.
2.2.1. Memory for events in their spatial context (what–where)
The common cognitive demand of the following tasks is to remember where a specific event (usually the presentation of a specific stimulus) occurred in the environment (Fig. 2A). This capacity involves distinguishing similar events and/or spatial locations and is thought to depend on pattern separation processes (Gilbert et al., 1998; Yassa et al., 2011b). The “where” component can refer to a specific place in an environment, or to a specific location on a screen or complex visual scene.
Fig. 2.
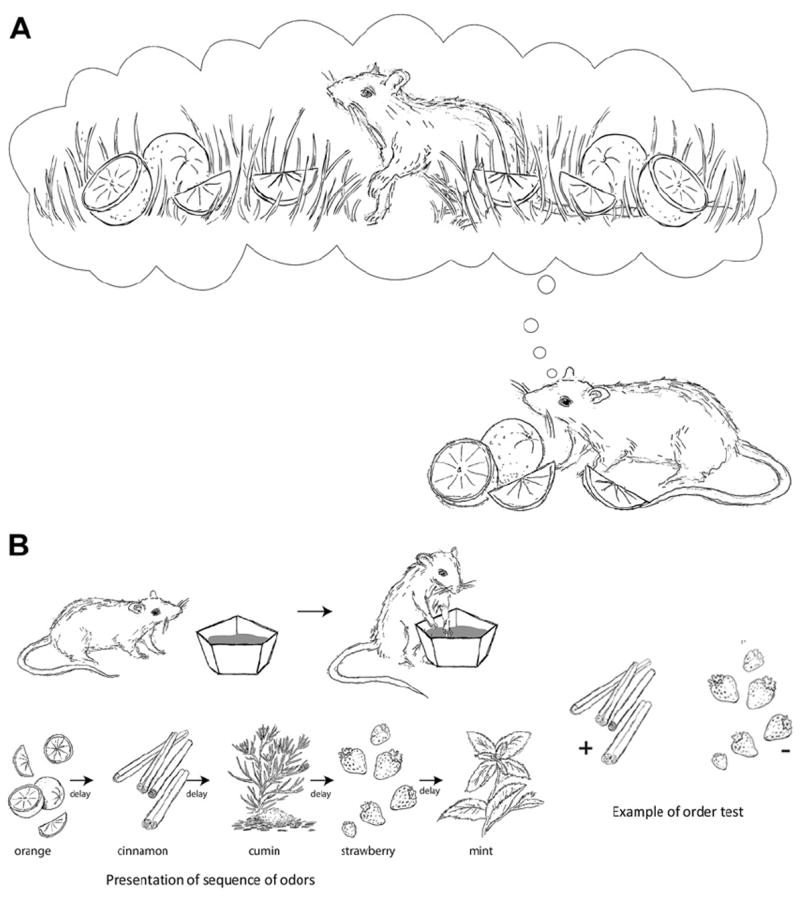
(A) One general approach for paradigms that aim to evaluate episodic memory is that animals demonstrate episodic memory capacity by remembering specific events as well as the spatial context in which they occurred. The image shows the principle underlying cognitive demands involved in spatial episodic memory tasks, which is to remember the association between presentation of a specific stimulus (oranges) and the specific environment (grass) in which it occurred. (B) Memory for events in their temporal context: In each trial, rats are presented with a series of odors, following which the animal is probed for its memory of the order of elements in the series. The stimulus (odor) associated with reward in the retention phase is the stimulus that was presented first in the sample phase.
This is in contrast to simple left/right position differences between stimuli as seen in delayed nonmatch or match to sample tasks. In rodents, a common approach is to use paired-associates paradigms in which animals must remember specific odor–place (Day et al., 2003, Rajji et al., 2006) or object–place associations (Gilbert and Kesner, 2003, 2004). Similar item-in-place paradigms have been developed using computer screens in nonhuman primates (Baxter et al., 2007; Gaffan, 1994), and have proved successful in detecting early symptoms of cognitive decline associated with AD dementia (Taffe et al., 2004). Overall, these approaches can also be adapted to evaluate episodic memory in other animals such as rodents, dogs, and nonhuman primates.
2.2.2. Task validity and effects of aging
The main advantages of this general approach are that the tasks are relatively simple to administer, use common apparati, and are learned relatively quickly. The latter is particularly true for tasks relying on spontaneous preference measures (Dix and Aggleton, 1999; Langston and Wood, 2010; Lee et al., 2005; Mumby et al., 2002a; Mumby et al., 2002b). Overall these paradigms have proved to be sensitive measures of hippocampal dysfunction (Kesner et al., 2008) and of episodic memory dysfunction in the preclinical stages of AD (Fowler et al., 2002; Lee et al., 2003; Swainson et al., 2001). In particular, changes in the CA3 and dentate gyrus of the hippocampus are implicated in decreased efficiency in pattern separation in the aging brain (Holden et al., 2012; Yassa et al., 2011a). This measure of episodic memory is a considerable improvement over item-based recognition tests (Dalla Barba, 1997; Lipinska and Backman, 1997; Tierney et al., 1996; Welsh et al., 1991), which rely more on recognition memory than episodic memory. Finally, it should be noted that spatial paradigms do not strongly parallel the NIH Toolbox CFB measure of episodic memory, which is a nonspatial sequence memory task.
2.2.3. Memory for events in their temporal context (what–when)
This approach focuses on the memory for “when” events occurred. Importantly, this approach is nonspatial, so the memory for the temporal context can be studied independently of the spatial context. One task of particular interest has focused on the capacity to remember trial-unique sequences of events (Fortin et al., 2002; Kesner et al., 2002). In this task, animals are presented with a series of randomly selected odors in the sample phase. A single-choice test is then used to probe for memory of the sequence where the animal is rewarded for selecting the odor that had appeared earlier in the series (Fig. 2B). The task also includes a control condition in which animals are tested for their memory (recognition) of the odors (but not the temporal relationship), a capacity that can be supported by recognition memory processes. Animals with damage to the hippocampus can remember the individual odors presented in each sequence, but not their temporal, sequence-specific relationship (Fortin et al., 2002; Kesner et al., 2002). Similar tasks have been used in monkeys (Kumaran and Maguire, 2006a; Naya and Suzuki, 2011; Petrides, 1995) and humans (Kumaran and Maguire, 2006a, 2006b) as well.
2.2.4. Task validity and effects of aging
The main advantage of this general approach is that it closely parallels the Picture Sequence Memory Test used to measure episodic memory in the Toolbox, lending much face validity to the model. The main disadvantage is that it requires extensive training to teach animals the rules of the task and to comprehensively assess sequence memory capacity. Note that other types of sequence memory tasks have been developed, including versions based on spontaneous preference (Hauser et al., 2009; Hoge and Kesner, 2007), which provide a simpler, but quicker, assay of the capacity to remember sequences of events, as reviewed by Kesner and Churchwell (Kesner and Churchwell, 2011). Furthermore, the hippocampus has been shown to be essential for successful execution of the sequence memory task (Fortin et al., 2002; Kesner et al., 2002) and for animals to retain the ability to disambiguate overlapping sequences of odors (Agster et al., 2002). However, to the best of our knowledge, no animal studies have attempted to directly use the sequence memory task mentioned above to evaluate episodic memory in aging, making it difficult to comment on the predictive validity of the task.
2.2.5. “What–where–when” paradigms
The third approach is centered on the original animal model of episodic memory, the memory for what–where–when in food-storing scrub jays (Clayton and Dickinson, 1999). Whereas the studies described earlier focused on either the spatial or the temporal context, this approach requires memory for both types of contexts.
The original model took advantage of the natural caching behavior of scrub jays, to demonstrate that they could remember specific episodic memories: they could remember “what” they stored (worms or peanuts), and well as “where” (the location in the cage) and “when” (4 hours or 124 hours ago) they did it. Since then, what–where-when memory has been demonstrated in rodents (Babb and Crystal, 2006a, b; Eacott and Norman, 2004; Ergorul and Eichenbaum, 2004; Kart-Teke et al., 2006) and primates (Hoffman et al., 2009).
2.2.6. Task validity and effects of aging
A positive aspect of this approach is that it has a very stringent criterion for the demonstration of episodic memory in animals. In that sense, this model could be considered better than the others. However, the stringent criterion is also a downside, as simultaneously probing for what, where, and when memory makes it difficult to isolate the nature of memory impairments or the critical neural substrates. For that reason, approaches focusing on either the spatial or temporal context appear to be better suited for investigating the effects of aging or AD. It should be noted that this approach also requires extensive training, although simpler versions using spontaneous preference tests have also been developed (Kart-Teke et al., 2006).
2.3. Working memory
As defined in the NIH Toolbox CFB, working memory is a step up from the conventional construct of short-term memory storage and clearly includes the concept of an “active computational work-space.” The task, based on one developed by Mungas et al. for English and Spanish speakers (Mungas et al., 2011), consists of sequential presentation of pictures of objects and their corresponding auditory words (e.g., “strawberry,” “apple”). Once the series, which varies in the number of items presented, is complete, the subject must report the objects in order of size. In 1 condition, all items from a sequence are from a single category (e.g., animals or fruit). In a second condition, items from 2 categories are presented in random sequence, and subjects are instructed to report the items in order of size from smallest to largest but also to list all items from 1 category first and then all items from the second. Therefore, for a comparable animal test paradigm it is essential to include pre-clinical tests that require short-term storage of information together with manipulation of information to achieve a defined goal. Unfortunately, current animal models of working memory do not fully capture both features. Here, we highlight delay-type tasks that are most suitable for examining the short-term memory storage aspect of working memory, and we identify promising approaches for investigating the capacity of animals to manipulate information in working memory.
2.3.1. Short-term memory storage
There is a large body of literature showing that primates and rodents can hold specific stimuli “in mind” for short periods of time (reviewed in Castner et al., 2004; Dudchenko et al., 2012). Typically, this capacity is investigated using delay-type paradigms, in which each trial is divided in 3 phases: (1) a sample phase, in which the animal is presented with the stimulus to remember; (2) a delay phase, during which the stimulus is not present; and (3) a choice phase in which the animal is rewarded for demonstrating its memory of the sample stimulus. Many variants of this general approach have been developed, including the use of different types of sample stimuli (e.g., pictures, objects, tones, levers, locations), reward contingencies for the choice phase (“matching” or “non-matching” to the sample), and apparatus (manual or automated). Overall, these different versions provide consistent findings (Dudchenko et al., 2012); however, before choosing a specific version, it is important to consider the following. First, it is critical that the set of sample stimuli be small and repeated across trials. This interference is important to ensure that animals cannot solve the task based on the degree of familiarity with particular stimuli (recognition memory), or by storing and subsequently retrieving the specific experience of being presented with the stimulus (episodic memory). Second, as discussed in Jeneson and Squire (2012), it is preferable not to test short-term memory in rodents using tasks requiring complex spatial processing (e.g., use specific arm entries as sample stimuli). Not only is the use of complex spatial cues not typical in human (Stern et al., 2001) and nonhuman primate studies (Carlson et al., 1997; Shamy et al., 2011), it increases the likelihood that performance will critically depend on the hippocampus (Olton and Papas, 1979; Olton et al., 1978), which is generally not the case for the conventional version of the task involving simpler stimuli (e.g., visual or auditory stimuli, position of levers or items on a screen). Third, although the use of either match or non-match contingencies lead to similar findings (Dudchenko et al., 2012), animals tend to learn the rules of the tasks more quickly if a non-match contingency is used (Mishkin and Delacour, 1975).
For these reasons, we recommend the use of the Delayed Non-Match to Position task (DNMP) (Bussey et al., 2003; Dunnett et al., 1988) to study the short-term memory storage component of working memory. This task has been used extensively in rodents, so considerable data are available on its critical neural substrate and pharmacology (reviewed by Dudchenko et al., 2012). Similar paradigms have been widely used in nonhuman primate studies as well (delayed-response task, Funahashi et al., 1989; Joseph and Barone, 1987). The DNMP task can be run manually or with an automated apparatus (with levers, or lights on touchscreen), the latter being more advantageous in terms of standardization and throughput. The Delayed Non-Match to Sample Task (DNMS) (Mishkin and Delacour, 1975; Rapp and Amaral, 1989) is a good alternative to the DNMP; it is essentially the same task, with the exception that sample stimuli are discrete cues (e.g., pictures, objects, tones) rather than positions. However, as mentioned earlier, it is critical that sample stimuli be repeated to avoid tapping into recognition memory processes.
2.3.2. Manipulation of information in working memory
In addition to the short-term storage of information, the NIH Toolbox definition of working memory includes a requirement for “on line” manipulation of that information. To our knowledge, the type of manipulation required by the Mungas et al. (2011) task has not been demonstrated in animals; however, simpler forms have been reported in nonhuman primates, such as the capacity to “monitor” information within working memory. For instance, monkeys can keep track of the specific sequence (Fig. 3) in which highly familiar stimuli were last presented (Petrides, 1991a; Warden and Miller, 2010), and can keep track of which stimuli from a similar set have already been rewarded on the current trials (Petrides, 1995). This capacity has been shown to depend on the integrity of the dorsolateral prefrontal cortex in monkeys as well as in humans (Petrides, 1991a, b, Petrides et al., 1993a, Petrides et al., 1993b, Petrides, 1995) Although rodents can remember trial-unique sequences of stimuli (Fortin et al., 2002; Kesner et al., 2002), their ability to do so using a set of repeated stimuli has not been well characterized.
Fig. 3.
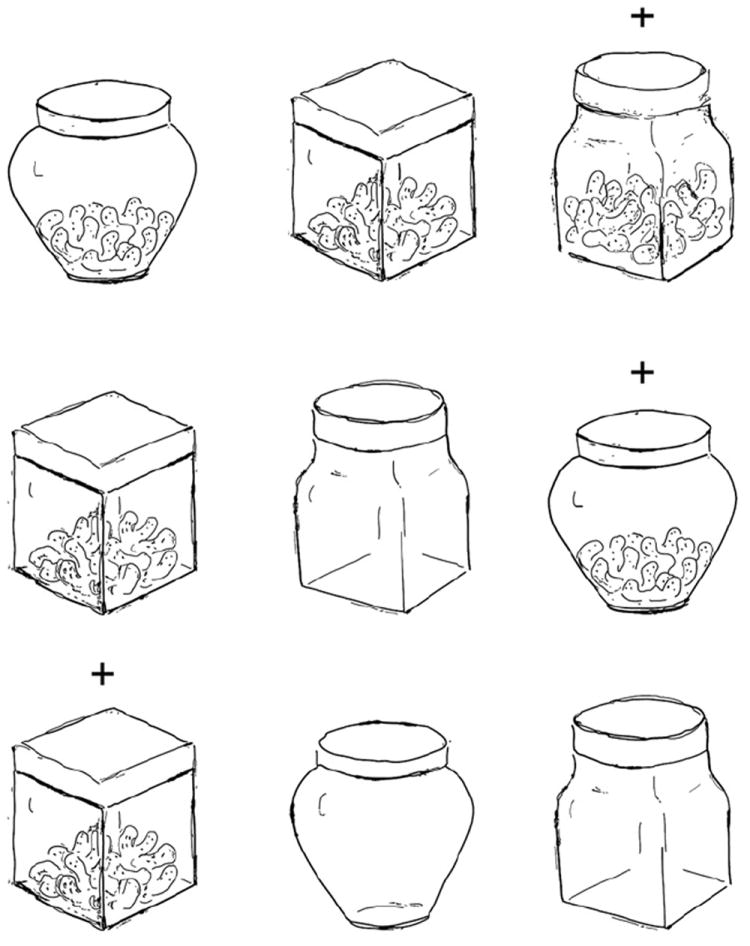
Representative image showing the self order task: In each trial, 3 containers, which differ in color and shape, are presented. Subjects keep track of the specific sequence in which containers are presented and rewarded in the current trials. The container that has already been rewarded in the last trial(s) is no longer rewarded in the next trial.
Other forms of monitoring of information in working memory include the capacity to perform “n-back” tasks (Barch et al., 1997; Cohen et al., 1997), which require subjects to indicate whether the current stimulus matches the one n steps earlier in the series. Older human subjects have been shown to perform as well as younger subjects at 1-Back version of this task but are significantly impaired at 2-Back and 3-Back versions (Mattay et al., 2006). The n-back task in humans is a continuous performance task in which every stimulus that is presented is used as a cue to guide the next current response. Interestingly, recent evidence suggests that rodents can perform similar tests that use sequential presentation of objects and tap into continuous recognition memory procedures (Kesner et al., 2001). In fact, 1-back and 2-back version of the human task have also been successfully used in rodents (Ko and Evenden, 2009). Note that continuous recognition paradigms offer another approach to test this capacity (Jackson-Smith et al., 1993; Kesner et al., 2001; Otto and Eichenbaum, 1992). Although it is clear that additional research is needed in this area, these findings support the idea that a more complete animal model of working memory will be available in the future.
2.4. Processing speed
Processing speed is defined in the NIH Toolbox CFB as “either the amount of time it takes to process a set amount of information or the amount of information that can be processed within a certain unit of time.” A pattern comparison task based on that designed by Salthouse is used to assess processing speed (Salthous and Babcok, 1991; Salthouse, 1991). Pairs of stimuli are presented, and the subject must simply indicate, as quickly as possible, whether they are the same or different. To our knowledge, this task has not yet been developed for animals. Here, we discuss existing paradigms in primates and rodents that could be adapted to measure this subdomain.
2.4.1. Pattern comparison task
Pattern comparison tasks using morphed stimuli have been used to study feature-ambiguous discriminations in primates (Bussey et al., 2003; Saksida et al., 2006). The basic idea is to train animals to discriminate between 2 very different pictures, and subsequently measure performance as the 2 pictures are made progressively more similar (by morphing them). Although previous studies have focused exclusively on accuracy (Bussey et al., 2003; Saksida et al., 2006), the design could be updated to measure reaction time as well. It is important to note that age-related decline in speed of motor functioning may be a confounding factor in this test. However, this can be addressed by taking baseline measures of response time for each subject. A similar pattern comparison task could be developed in rodents using touchscreen testing methods (Bartko et al., 2011; Bussey et al., 2008).
2.4.2. Task validity and effects of aging
Distraction has a strong effect on tests of processing speed particularly in older adults, with irrelevant information influencing estimates of processing speed by more than 15% (Lustig et al., 2006). The task has been replicated to an extent in a canine model of aging in which performance accuracy declined with increased similarity of the distracter (Milgram et al., 2002). The study did not report response time and focused on errors alone, but the task described would be an excellent measure of speed of processing and would parallel the NIH Toolbox task.
2.5. Attention
The NIH Toolbox CFB recognizes the large array of processes that fall under the rubric of attention (e.g., selective [visual] attention, sustained attention, and divided attentions) and focuses on sustained attention. However, attention also forms the basis for many conscious mental processes and overlaps with processes involved in executive function. The flanker task described in the NIH Toolbox CFB was designed to assess sustained attention but also response inhibition, a component of visual attention and executive function. It is based on a paradigm developed by Posner et al. (2002), and has been described previously.
We have examined 2 tasks that assess attention in animal models. The first task is a visual attention task, which has shown age-dependent performance effects in higher animal models. The second task is the 5-choice serial reaction test, which is extensively used in the literature to measure various aspects of attentional processes. Although the effects of aging on attention have been shown using this task, the other major selling point is the variety of cognitive measures that can be assessed using the single task. Both tasks are dependent on the frontal lobe and provide an excellent model to assess attention function in animals.
2.5.1. Five-choice serial reaction task
The 5-choice serial reaction (5-CSR) task is a rodent-based task designed to assess attention and several attentional processes. This task was originally developed (Carli et al., 1983) to assess attention-deficit/hyperactivity disorder (ADHD), and has been used to assess different aspects of attention in both humans and laboratory species. The test procedure consists of placing animals in the operant test box with a food delivery magazine on 1 side and a curved wall on the other. Five apertures are recessed in the curved wall on the same plane. The animal initiates a trial by a nose-poke into the magazine, which results in the onset of the intertrial interval (ITI; typically 5 seconds). Following the ITI, 1 of the 5 apertures is illuminated, and the animal is required simply to nose-poke in the lit aperture. A food reward is delivered for every correct nose poke. Errors result in a time-out phase (typically 5 seconds). The task typically lasts for 100 to 120 trials or 30 minutes, whichever is completed sooner. To manipulate the task load, it is also possible to use a “9-hole box.” This task provides a variety of response measures, including accuracy (proportion correct responses of total correct and incorrect responses, or percent correct), omissions (proportion of omitted trials, or percent omissions), anticipatory responses (premature responses, responses before stimulus presentation), perseverative responses (repeated responses at the response apertures), total trials completed, as well as several response latency indices. The 5-CSR is extensively used in the literature to assess attention deficits associated with neurodegenerative conditions (Harati et al., 2011; Muir et al., 1999; Romberg et al., 2011). Typically, selective attention is assessed by accuracy, whereas processing speed is assessed by measures of latency. Omissions measure sustained attention deficits (Young et al., 2009). This task has also been used to show that aging-induced deficits in attentional function is similar to that observed following lesions of the basalo-cortical cholinergic system (Muir et al., 1999). Furthermore, lesions of the hippocampus do not impair rat performance of the 5-CSR task (Kirkby and Higgins, 1998), suggesting that there are similarities between brain regions that mediate human and rodent attention function.
2.5.2. Task validity and effects of aging
The forebrain cholinergic system shows a marked degeneration with age. Therefore, it should be expected that aged subjects show impairments in tests of attention. In 1992, Moore et al. (1992) attempted to assess the effects of aging in old Fisher-344 rats using simple- and choice-reaction time tasks (SRTT and CRTT, respectively), and reported age-related decrease in vigilance (Moore et al., 1992). Soon after, Jones et al. also demonstrated impairments in the 5-CSR task using aged rats (Jones et al., 1995). Scopolamine has also been used to induce behavioral deficits in the 5-CSR task, and AChEIs including tacrine, donepezil, and physostigmine all reverse scopolamine-induced deficits in performance (Kirkby et al., 1996). Recently, a computer-automated touchscreen version of the 5-Choice Serial Reaction Time Task (5-CSRTT) has been used to test the 3xTgAD mouse, which carries human APPswe and tauP301L transgenes as well as a PS1M146V knock-in (Romberg et al., 2011). These transgenic mice overexpress altered human genes, and many develop age-dependent learning and memory deficits. The authors report that the 3xTgAD mice were less accurate in responding to short, spatially unpredictable stimuli, particularly when the attentional demand of the task was high. Increase in perseverative responses was also reported. Another study has used the 5-CSRTT to report a prominent increase in premature responding (impulsivity) in transgenic mice expressing FTDP-17 tau mutations. These mice demonstrate filamentous neuropathology similar to that observed in human tauopathy conditions (Lambourne et al., 2007). The 5-CSR can therefore be used to study behavioral specificity and to delineate specific behaviors relating to attention, executive function and speed of responding.
2.5.3. Visual attention task
Although the 5-CSR is an excellent measure of attention function, it is possible to use another task that aligns more closely with the flanker task described in the NIH Toolbox CFB. However, it is important to note that perception of targets in humans is influenced a number of parameters, including image contrast and orientation of distracters in target detection tasks (Chen and Tyler, 2008; Polat and Sagi, 1993; Williams and Hess, 1998), so developing an analog of the flanker task for other species is a significant challenge. The behavioral effects of flanking stimuli have mostly been studied in nonhuman primates (Li et al., 2006), although a recent study revealed that rats are also sensitive to the configuration of flanking elements of visual stimuli (Meier et al., 2011). More specifically, the authors report that rats are impaired in detecting collinear flankers, compared to configurations in which flankers differ from the target in orientation or angular position (Meier et al., 2011). Therefore, it would be an oversimplification to exactly imitate the flanker task in rodents, or in other laboratory species, if the correspondence in visual acuity were not fully characterized. However, a selective attention task can model the age-related attentional deficits by modifying the distracters used. Specifically the ability of young and old animals to respond to the target stimulus when it is presented along with 0, 1, 2, or 3 distracters is tested for the first sessions of the task. The difficulty of the task is further increased by modifying the distracter stimuli and using a distracter with greater physical similarity to the target for the later sessions of the task (Snigdha et al., 2012).
2.5.4. Task validity and effects of aging
In a recent study, we have shown that older dogs show decreased response accuracy coupled with increased response latency, and a greater disruption is seen when there is an increased similarity of distracter to the positive stimulus (Snigdha et al., 2012). This is consistent with studies in human subjects that reveal that challenging discrimination ability by using physically similar stimuli taxes attentional resources in older subjects (Scialfa et al., 1998) and in AD patients (Gainotti et al., 2001). These studies suggest that the visual attention task is a valid tool to predict age-related changes, as measured by decreased response accuracy coupled with increased response latency in animal models.
2.6. Language and reading
Language and reading involve the capacity that humans have for using a symbol system to categorize and communicate. In the NIH Toolbox CFB, there are 2 language measures, Oral Reading and Vocabulary Comprehension. Reading vocabulary, measured by oral pronunciation of single written words, is a surrogate for overall cognitive ability level but also for educational opportunity. Vocabulary Comprehension tests auditory comprehension of single words. Although some attempts have been made to measure communication in animals, this subdomain cannot be comprehensively investigated in animals and thus is not discussed in this article.
3. Conclusion
Animal experiments and preclinical testing have had an impact on our understanding of mechanisms of the aging process. However, their significance in predicting the effectiveness of treatment strategies in clinical trials has remained less than accurate. This is largely due to the translational gap between clinical and preclinical studies. This article attempts to draw attention to some of those gaps, in addition to identifying animal paradigms that closely parallel the tests included in the NIH Toolbox CFB. To parallel a human test battery, there remains a need to refine animal tests so that they are quick to administer. Although we have tried to choose tasks to fit this criterion, this is not always possible and is a limitation that needs to be addressed in the future. However, we believe that tests described in this review will provide a tool to define aspects of cognition that benefits from lifestyle intervention, such as exercise and cognitive enrichment, as well as other pharmacological interventions. A comprehensive and standardized tool that closely parallels human tests of cognition will advance our understanding of the cognitive, structural, and molecular features associated with successful aging and will help to improve outcome measures in research settings.
Footnotes
Disclosure statement
The authors declare no actual or potential conflicts of interest.
References
- Agster KL, Fortin NJ, Eichenbaum H. The hippocampus and disambiguation of overlapping sequences. J Neurosci. 2002;22:5760–5768. doi: 10.1523/JNEUROSCI.22-13-05760.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Babb SJ, Crystal JD. Discrimination of what, when, and where is not based on time of day. Learn Behav. 2006a;34:124–130. doi: 10.3758/bf03193188. [DOI] [PubMed] [Google Scholar]
- Babb SJ, Crystal JD. Episodic-like memory in the rat. Curr Biol CB. 2006b;16:1317–1321. doi: 10.1016/j.cub.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Nystrom LE, Forman SD, Noll DC, Cohen JD. Dissociating working memory from task difficulty in human prefrontal cortex. Neuropsychologia. 1997;35:1373–1380. doi: 10.1016/s0028-3932(97)00072-9. [DOI] [PubMed] [Google Scholar]
- Bartko SJ, Vendrell I, Saksida LM, Bussey TJ. A computer-automated touchscreen paired-associates learning (PAL) task for mice: impairments following administration of scopolamine or dicyclomine and improvements following donepezil. Psychopharmacology (Berl) 2011;214:537–548. doi: 10.1007/s00213-010-2050-1. [DOI] [PubMed] [Google Scholar]
- Bauer PJ. Memory for gender-consistent and gender-inconsistent event sequences by twenty-five-month-old children. Child Dev. 1993;64:285–297. [PubMed] [Google Scholar]
- Baxter MG, Gaffan D, Kyriazis DA, Mitchell AS. Orbital prefrontal cortex is required for object-in-place scene memory but not performance of a strategy implementation task. J Neurosci. 2007;27:11327–11333. doi: 10.1523/JNEUROSCI.3369-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccafusco JJ. The revival of scopolamine reversal for the assessment of cognition-enhancing drugs. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. Boca Raton, FL: CRC Press; 2009. [PubMed] [Google Scholar]
- Bussey TJ, Padain TL, Skillings EA, Winters BD, Morton AJ, Saksida LM. The touchscreen cognitive testing method for rodents: how to get the best out of your rat. Learn Mem. 2008;15:516–523. doi: 10.1101/lm.987808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, Murray EA. Impairments in visual discrimination after perirhinal cortex lesions: testing ‘declarative’ vs. ‘perceptual-mnemonic’ views of perirhinal cortex function. Eur J Neurosci. 2003;17:649–660. doi: 10.1046/j.1460-9568.2003.02475.x. [DOI] [PubMed] [Google Scholar]
- Carli M, Robbins TW, Evenden JL, Everitt BJ. Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Res. 1983;9:361–380. doi: 10.1016/0166-4328(83)90138-9. [DOI] [PubMed] [Google Scholar]
- Carlson S, Rama P, Tanila H, Linnankoski I, Mansikka H. Dissociation of mnemonic coding and other functional neuronal processing in the monkey prefrontal cortex. J Neurophysiol. 1997;77:761–774. doi: 10.1152/jn.1997.77.2.761. [DOI] [PubMed] [Google Scholar]
- Castner SA, Goldman-Rakic PS, Williams GV. Animal models of working memory: insights for targeting cognitive dysfunction in schizophrenia. Psychopharmacology (Berl) 2004;174:111–125. doi: 10.1007/s00213-003-1710-9. [DOI] [PubMed] [Google Scholar]
- Chen CC, Tyler CW. Excitatory and inhibitory interaction fields of flankers revealed by contrast-masking functions. J Vis. 2008;8:1–14. doi: 10.1167/8.4.10. [DOI] [PubMed] [Google Scholar]
- Chen KC, Baxter MG, Rodefer JS. Central blockade of muscarinic cholinergic receptors disrupts affective and attentional set-shifting. Eur J Neurosci. 2004;20:1081–1088. doi: 10.1111/j.1460-9568.2004.03548.x. [DOI] [PubMed] [Google Scholar]
- Clayton NS, Bussey TJ, Dickinson A. Can animals recall the past and plan for the future? Nature Rev Neurosci. 2003;4:685–691. doi: 10.1038/nrn1180. [DOI] [PubMed] [Google Scholar]
- Clayton NS, Dickinson A. Motivational control of caching behaviour in the scrub jay, Aphelocoma coerulescens. Anim Behav. 1999;57:435–444. doi: 10.1006/anbe.1998.0989. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Head E. The canine (dog) model of human aging and disease: dietary, environmental and immunotherapy approaches. J Alzheimers Dis. 2008;15:685–707. doi: 10.3233/jad-2008-15413. [DOI] [PubMed] [Google Scholar]
- Crystal JD. Elements of episodic-like memory in animal models. Behav Process. 2009;80:269–277. doi: 10.1016/j.beproc.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Dalla Barba G. Recognition memory and recollective experience in Alzheimer’s disease. Memory. 1997;5:657–672. doi: 10.1080/741941546. [DOI] [PubMed] [Google Scholar]
- Day M, Langston R, Morris RG. Glutamate-receptor-mediated encoding and retrieval of paired-associate learning. Nature. 2003;424:205–209. doi: 10.1038/nature01769. [DOI] [PubMed] [Google Scholar]
- Decker MW, McGaugh JL. The role of interactions between the cholinergic system and other neuromodulatory systems in learning and memory. Synapse. 1991;7:151–168. doi: 10.1002/syn.890070209. [DOI] [PubMed] [Google Scholar]
- Dere E, Kart-Teke E, Huston JP, De Souza Silva MA. The case for episodic memory in animals. Neurosci Biobehav Rev. 2006;30:1206–1224. doi: 10.1016/j.neubiorev.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Diamond A. Developmental time course in human infants and infant monkeys, and the neural bases of, inhibitory control in reaching. Ann N Y Acad Sci. 1990;608:637–669. doi: 10.1111/j.1749-6632.1990.tb48913.x. discussion 669–676. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav Neurosci. 1996;110:872–886. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Eichenbaum H. The episodic memory system: neurocircuitry and disorders. Neuropsychopharmacology. 2010;35:86–104. doi: 10.1038/npp.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix SL, Aggleton JP. Extending the spontaneous preference test of recognition: evidence of object-location and object-context recognition. Behav Brain Res. 1999;99:191–200. doi: 10.1016/s0166-4328(98)00079-5. [DOI] [PubMed] [Google Scholar]
- Dudchenko PA, Talpos J, Young J, Baxter MG. Animal models of working memory: a review of tasks that might be used in screening drug treatments for the memory impairments found in schizophrenia. Neurosci Biobehav Rev. 2012 doi: 10.1016/j.neubiorev.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Dunnett SB, Evenden JL, Iversen SD. Delay-dependent short-term memory deficits in aged rats. Psychopharmacology (Berl) 1988;96:174–180. doi: 10.1007/BF00177557. [DOI] [PubMed] [Google Scholar]
- Eacott MJ, Norman G. Integrated memory for object, place, and context in rats: a possible model of episodic-like memory? J Neurosci. 2004;24:1948–1953. doi: 10.1523/JNEUROSCI.2975-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Sauvage M, Fortin N, Komorowski R, Lipton P. Towards a functional organization of episodic memory in the medial temporal lobe. Neurosci Biobehav Rev. 2012;36:1597–1608. doi: 10.1016/j.neubiorev.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergorul C, Eichenbaum H. The hippocampus and memory for “what,” “where,” and “when”. Learn Mem. 2004;11:397–405. doi: 10.1101/lm.73304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahnestock M, Marchese M, Head E, Pop V, Michalski B, Milgram WN, Cotman CW. BDNF increases with behavioral enrichment and an antioxidant diet in the aged dog. Neurobiol Aging. 2012;33:546–554. doi: 10.1016/j.neurobiolaging.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. J Cogn Neurosci. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Fortin NJ, Agster KL, Eichenbaum HB. Critical role of the hippocampus in memory for sequences of events. Nature Neurosci. 2002;5:458–462. doi: 10.1038/nn834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin NJ, Wright SP, Eichenbaum H. Recollection-like memory retrieval in rats is dependent on the hippocampus. Nature. 2004;431:188–191. doi: 10.1038/nature02853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouquet C, Tobin C, Rondi-Reig L. A new approach for modeling episodic memory from rodents to humans: the temporal order memory. Behav Brain Res. 2010;215:172–179. doi: 10.1016/j.bbr.2010.05.054. [DOI] [PubMed] [Google Scholar]
- Fowler KS, Saling MM, Conway EL, Semple JM, Louis WJ. Paired associate performance in the early detection of DAT. J Int Neuropsychol Soc. 2002;8:58–71. [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey’s dorsolateral prefrontal cortex. J Neurophysiol. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- Gaffan D. Scene-specific memory for objects—a model of episodic memory impairment in monkeys with fornix transection. J Cogn Neurosci. 1994;6:305–320. doi: 10.1162/jocn.1994.6.4.305. [DOI] [PubMed] [Google Scholar]
- Gainotti G, Marra C, Villa G. A double dissociation between accuracy and time of execution on attentional tasks in Alzheimer’s disease and multi-infarct dementia. Brain. 2001;124:731–738. doi: 10.1093/brain/124.4.731. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RA, Stein EA. Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. Neuroimage. 2002;17:1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Gearing M, Tigges J, Mori H, Mirra SS. beta-Amyloid (A beta) deposition in the brains of aged orangutans. Neurobiol Aging. 1997;18:139–146. doi: 10.1016/s0197-4580(97)00012-2. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP. Localization of function within the dorsal hippocampus: the role of the CA3 subregion in paired-associate learning. Behav Neurosci. 2003;117:1385–1394. doi: 10.1037/0735-7044.117.6.1385. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP. Memory for objects and their locations: the role of the hippocampus in retention of object-place associations. Neurobiol Learn Mem. 2004;81:39–45. doi: 10.1016/s1074-7427(03)00069-8. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP, DeCoteau WE. Memory for spatial location: role of the hippocampus in mediating spatial pattern separation. J Neurosci. 1998;18:804–810. doi: 10.1523/JNEUROSCI.18-02-00804.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz J, Ittner LM. Animal models of Alzheimer’s disease and frontotemporal dementia. Nature Rev Neurosci. 2008;9:532–544. doi: 10.1038/nrn2420. [DOI] [PubMed] [Google Scholar]
- Harati H, Majchrzak M, Cosquer B, Galani R, Kelche C, Cassel JC, Barbelivien A. Attention and memory in aged rats: Impact of lifelong environmental enrichment. Neurobiol Aging. 2011;32:718–736. doi: 10.1016/j.neurobiolaging.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Hauser E, Tolentino JC, Pirogovsky E, Weston E, Gilbert PE. The effects of aging on memory for sequentially presented objects in rats. Behav Neurosci. 2009;123:1339–1345. doi: 10.1037/a0017681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head E, Torp R. Insights into Abeta and presenilin from a canine model of human brain aging. Neurobiol Dis. 2002;9:1–10. doi: 10.1006/nbdi.2002.0476. [DOI] [PubMed] [Google Scholar]
- Herlitz A, Forsell Y. Episodic memory deficit in elderly adults with suspected delusional disorder. Acta Psychiatr Scand. 1996;93:355–361. doi: 10.1111/j.1600-0447.1996.tb10660.x. [DOI] [PubMed] [Google Scholar]
- Herndon JG, Moss MB, Rosene DL, Killiany RJ. Patterns of cognitive decline in aged rhesus monkeys. Behav Brain Res. 1997;87:25–34. doi: 10.1016/s0166-4328(96)02256-5. [DOI] [PubMed] [Google Scholar]
- Hoffman ML, Beran MJ, Washburn DA. Memory for “what”, “where”, and “when” information in rhesus monkeys (Macaca mulatta) J Exp Psychol Anim B. 2009;35:143–152. doi: 10.1037/a0013295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge J, Kesner RP. Role of CA3 and CA1 subregions of the dorsal hippocampus on temporal processing of objects. Neurobiol Learn Mem. 2007;88:225–231. doi: 10.1016/j.nlm.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden HM, Hoebel C, Loftis K, Gilbert PE. Spatial pattern separation in cognitively normal young and older adults. Hippocampus. 2012;22:1826–1832. doi: 10.1002/hipo.22017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen SD, Mishkin M. Perseverative interference in monkeys following selective lesions of the inferior prefrontal convexity. Exp Brain Res. 1970;11:376–386. doi: 10.1007/BF00237911. [DOI] [PubMed] [Google Scholar]
- Jackson-Smith P, Kesner RP, Chiba AA. Continuous recognition of spatial and nonspatial stimuli in hippocampal-lesioned rats. Behavioral and neural biology. 1993;59:107–119. doi: 10.1016/0163-1047(93)90821-x. [DOI] [PubMed] [Google Scholar]
- Jeneson A, Squire LR. Working memory, long-term memory, and medial temporal lobe function. Learn Mem. 2012;19:15–25. doi: 10.1101/lm.024018.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DN, Barnes JC, Kirkby DL, Higgins GA. Age-associated impairments in a test of attention: evidence for involvement of cholinergic systems. J Neurosci. 1995;15:7282–7292. doi: 10.1523/JNEUROSCI.15-11-07282.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph JP, Barone P. Prefrontal unit activity during a delayed oculomotor task in the monkey. Exp Brain Res. 1987;67:460–468. doi: 10.1007/BF00247279. [DOI] [PubMed] [Google Scholar]
- Kart-Teke E, De Souza Silva MA, Huston JP, Dere E. Wistar rats show episodic-like memory for unique experiences. Neurobiol Learn Mem. 2006;85:173–182. doi: 10.1016/j.nlm.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Churchwell JC. An analysis of rat prefrontal cortex in mediating executive function. Neurobiol Learn Mem. 2011;96:417–431. doi: 10.1016/j.nlm.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Gilbert PE, Barua LA. The role of the hippocampus in memory for the temporal order of a sequence of odors. Behav Neurosci. 2002;116:286–290. doi: 10.1037//0735-7044.116.2.286. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Hunsaker MR. The temporal attributes of episodic memory. Behav Brain Res. 2010;215:299–309. doi: 10.1016/j.bbr.2009.12.029. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Hunsaker MR, Warthen MW. The CA3 subregion of the hippocampus is critical for episodic memory processing by means of relational encoding in rats. Behav Neurosci. 2008;122:1217–1225. doi: 10.1037/a0013592. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Ravindranathan A, Jackson P, Giles R, Chiba AA. A neural circuit analysis of visual recognition memory: role of perirhinal, medial, and lateral entorhinal cortex. Learn Mem. 2001;8:87–95. doi: 10.1101/lm.29401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkby DL, Higgins GA. Characterization of perforant path lesions in rodent models of memory and attention. Eur J Neurosci. 1998;10:823–838. doi: 10.1046/j.1460-9568.1998.00087.x. [DOI] [PubMed] [Google Scholar]
- Kirkby DL, Jones DN, Barnes JC, Higgins GA. Effects of anticholinesterase drugs tacrine and E2020, the 5-HT(3) antagonist ondansetron, and the H(3) antagonist thioperamide, in models of cognition and cholinergic function. Behav Pharmacol. 1996;7:513–525. [PubMed] [Google Scholar]
- Ko T, Evenden J. The effects of psychotomimetic and putative cognitive-enhancing drugs on the performance of a n-back working memory task in rats. Psychopharmacology (Berl) 2009;202:67–78. doi: 10.1007/s00213-008-1314-5. [DOI] [PubMed] [Google Scholar]
- Kumaran D, Maguire EA. The dynamics of hippocampal activation during encoding of overlapping sequences. Neuron. 2006a;49:617–629. doi: 10.1016/j.neuron.2005.12.024. [DOI] [PubMed] [Google Scholar]
- Kumaran D, Maguire EA. An unexpected sequence of events: mismatch detection in the human hippocampus. PloS Biol. 2006b;4:2372–2382. doi: 10.1371/journal.pbio.0040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambourne SL, Humby T, Isles AR, Emson PC, Spillantini MG, Wilkinson LS. Impairments in impulse control in mice transgenic for the human FTDP-17 tauV337M mutation are exacerbated by age. Hum Mol Genet. 2007;16:1708–1719. doi: 10.1093/hmg/ddm119. [DOI] [PubMed] [Google Scholar]
- Langston RF, Wood ER. Associative recognition and the hippocampus: differential effects of hippocampal lesions on object-place, object-context and object-place-context memory. Hippocampus. 2010;20:1139–1153. doi: 10.1002/hipo.20714. [DOI] [PubMed] [Google Scholar]
- Lee AC, Rahman S, Hodges JR, Sahakian BJ, Graham KS. Associative and recognition memory for novel objects in dementia: implications for diagnosis. Eur J Neurosci. 2003;18:1660–1670. doi: 10.1046/j.1460-9568.2003.02883.x. [DOI] [PubMed] [Google Scholar]
- Lee I, Hunsaker MR, Kesner RP. The role of hippocampal subregions in detecting spatial novelty. Behav Neurosci. 2005;119:145–153. doi: 10.1037/0735-7044.119.1.145. [DOI] [PubMed] [Google Scholar]
- Lescaudron L, Stein DG. Differences in memory impairment and response to GM1 ganglioside treatment following electrolytic or ibotenic acid lesions of the nucleus basalis magnocellularis. Restor Neurol Neurosci. 1999;15:25–37. [PubMed] [Google Scholar]
- Li W, Piech V, Gilbert CD. Contour saliency in primary visual cortex. Neuron. 2006;50:951–962. doi: 10.1016/j.neuron.2006.04.035. [DOI] [PubMed] [Google Scholar]
- Lipinska B, Backman L. Encoding-retrieval interactions in mild Alzheimer’s disease: the role of access to categorical information. Brain Cogn. 1997;34:274–286. doi: 10.1006/brcg.1997.0916. [DOI] [PubMed] [Google Scholar]
- Lustig C, Hasher L, Tonev ST. Distraction as a determinant of processing speed. Psychonom Bull Rev. 2006;13:619–625. doi: 10.3758/bf03193972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Pardo CA, Cork LC, Price DL. Synaptic pathology and glial responses to neuronal injury precede the formation of senile plaques and amyloid deposits in the aging cerebral cortex. Am J Pathol. 1994;145:1358–1381. [PMC free article] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Berman KF, Das S, Meyer-Lindenberg A, Goldberg TE, Callicott JH, Weinberger DR. Neuro-physiological correlates of age-related changes in working memory capacity. Neurosci Lett. 2006;392:32–37. doi: 10.1016/j.neulet.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Meier P, Flister E, Reinagel P. Collinear features impair visual detection by rats. J Vis. 2011;11:1–16. doi: 10.1167/11.3.22. [DOI] [PubMed] [Google Scholar]
- Menon V, Adleman NE, White CD, Glover GH, Reiss AL. Error-related brain activation during a Go/NoGo response inhibition task. Hum Brain Mapp. 2001;12:131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milgram NW, Head E, Zicker SC, Ikeda-Douglas CJ, Murphey H, Muggenburg B, Siwak C, Tapp D, Cotman CW. Learning ability in aged beagle dogs is preserved by behavioral enrichment and dietary fortification: a two-year longitudinal study. Neurobiol Aging. 2005;26:77–90. doi: 10.1016/j.neurobiolaging.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Milgram NW, Zicker SC, Head E, Muggenburg BA, Murphey H, Ikeda-Douglas CJ, Cotman CW. Dietary enrichment counteracts age-associated cognitive dysfunction in canines. Neurobiol Aging. 2002;23:737–745. doi: 10.1016/s0197-4580(02)00020-9. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Delacour J. An analysis of short-term visual memory in the monkey. J Exp Psychol Anim Behav Process. 1975;1:326–334. doi: 10.1037//0097-7403.1.4.326. [DOI] [PubMed] [Google Scholar]
- Moore H, Dudchenko P, Bruno JP, Sarter M. Toward modeling age-related changes of attentional abilities in rats: simple and choice reaction time tasks and vigilance. Neurobiol Aging. 1992;13:759–772. doi: 10.1016/0197-4580(92)90100-c. [DOI] [PubMed] [Google Scholar]
- Muir JL, Fischer W, Bjorklund A. Decline in visual attention and spatial memory in aged rats. Neurobiol Aging. 1999;20:605–615. doi: 10.1016/s0197-4580(99)00098-6. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn Mem. 2002a;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby DG, Glenn MJ, Nesbitt C, Kyriazis DA. Dissociation in retrograde memory for object discriminations and object recognition in rats with perirhinal cortex damage. Behav Brain Res. 2002b;132:215–226. doi: 10.1016/s0166-4328(01)00444-2. [DOI] [PubMed] [Google Scholar]
- Mungas D, Widaman KF, Reed BR, Tomaszewski Farias S. Measurement invariance of neuropsychological tests in diverse older persons. Neuropsychology. 2011;25:260–269. doi: 10.1037/a0021090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naya Y, Suzuki WA. Integrating what and when across the primate medial temporal lobe. Science. 2011;333:773–776. doi: 10.1126/science.1206773. [DOI] [PubMed] [Google Scholar]
- Neese SL, Wang VC, Doerge DR, Woodling KA, Andrade JE, Helferich WG, Korol DL, Schantz SL. Impact of dietary genistein and aging on executive function in rats. Neurotoxicol Teratol. 2010;32:200–211. doi: 10.1016/j.ntt.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolle MM, Baxter MG. Glutamate receptor binding in the frontal cortex and dorsal striatum of aged rats with impaired attentional set-shifting. Eur J Neurosci. 2003;18:3335–3342. doi: 10.1111/j.1460-9568.2003.03077.x. [DOI] [PubMed] [Google Scholar]
- Nielson KA, Langenecker SA, Garavan H. Differences in the functional neuroanatomy of inhibitory control across the adult life span. Psychol Aging. 2002;17:56–71. doi: 10.1037//0882-7974.17.1.56. [DOI] [PubMed] [Google Scholar]
- Olton DS, Papas BC. Spatial memory and hippocampal function. Neuropsychologia. 1979;17:669–682. doi: 10.1016/0028-3932(79)90042-3. [DOI] [PubMed] [Google Scholar]
- Olton DS, Walker JA, Gage FH. Hippocampal connections and spatial discrimination. Brain Res. 1978;139:295–308. doi: 10.1016/0006-8993(78)90930-7. [DOI] [PubMed] [Google Scholar]
- Otto T, Eichenbaum H. Complementary roles of the orbital prefrontal cortex and the perirhinal-entorhinal cortices in an odor-guided delayed-nonmatching-to-sample task. Behav Neurosci. 1992;106:762–775. doi: 10.1037//0735-7044.106.5.762. [DOI] [PubMed] [Google Scholar]
- Owen AM, Roberts AC, Polkey CE, Sahakian BJ, Robbins TW. Extradimensional versus intra-dimensional set shifting performance following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia. 1991;29:993–1006. doi: 10.1016/0028-3932(91)90063-e. [DOI] [PubMed] [Google Scholar]
- Perner J, Ruffman T. Episodic memory and autonoetic consciousness: developmental evidence and a theory of childhood amnesia. J Exp Child Psychol. 1995;59:516–548. doi: 10.1006/jecp.1995.1024. [DOI] [PubMed] [Google Scholar]
- Petrides M. Functional specialization within the dorsolateral frontal cortex for serial order memory. Proc Biol Sci R Soc. 1991a;246:299–306. doi: 10.1098/rspb.1991.0158. [DOI] [PubMed] [Google Scholar]
- Petrides M. Monitoring of selections of visual stimuli and the primate frontal cortex. Proc Biol Sci R Soc. 1991b;246:293–298. doi: 10.1098/rspb.1991.0157. [DOI] [PubMed] [Google Scholar]
- Petrides M. Impairments on nonspatial self-ordered and externally ordered working-memory tasks after lesions of the mid-dorsal part of the lateral frontal-cortex in the monkey. J Neurosci. 1995;15:359–375. doi: 10.1523/JNEUROSCI.15-01-00359.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Alivisatos B, Evans AC, Meyer E. Dissociation of human middorsolateral from posterior dorsolateral frontal cortex in memory processing. Proc Natl Acad Sci U S A. 1993a;90:873–877. doi: 10.1073/pnas.90.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Alivisatos B, Meyer E, Evans AC. Functional activation of the human frontal cortex during the performance of verbal working memory tasks. Proc Natl Acad Sci U S A. 1993b;90:878–882. doi: 10.1073/pnas.90.3.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polat U, Sagi D. Lateral interactions between spatial channels: suppression and facilitation revealed by lateral masking experiments. Vis Res. 1993;33:993–999. doi: 10.1016/0042-6989(93)90081-7. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK, Vizueta N, Levy KN, Evans DE, Thomas KM, Clarkin JF. Attentional mechanisms of borderline personality disorder. Proc Natl Acad Sci U S A. 2002;99:16366–16370. doi: 10.1073/pnas.252644699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DL, Martin LJ, Sisodia SS, Wagster MV, Koo EH, Walker LC, Koliatsos VE, Cork LC. Aged non-human primates: an animal model of age-associated neurodegenerative disease. Brain Pathol. 1991;1:287–296. doi: 10.1111/j.1750-3639.1991.tb00672.x. [DOI] [PubMed] [Google Scholar]
- Pugliese M, Geloso MC, Carrasco JL, Mascort J, Michetti F, Mahy N. Canine cognitive deficit correlates with diffuse plaque maturation and S100beta (-) astrocytosis but not with insulin cerebrospinal fluid level. Acta Neuropathol. 2006;111:519–528. doi: 10.1007/s00401-006-0052-1. [DOI] [PubMed] [Google Scholar]
- Rajji T, Chapman D, Eichenbaum H, Greene R. The role of CA3 hippocampal NMDA receptors in paired associate learning. J Neurosci. 2006;26:908–915. doi: 10.1523/JNEUROSCI.4194-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp PR, Amaral DG. Evidence for task-dependent memory dysfunction in the aged monkey. J Neurosci. 1989;9:3568–3576. doi: 10.1523/JNEUROSCI.09-10-03568.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AC, Wallis JD. Inhibitory control and affective processing in the prefrontal cortex: neuropsychological studies in the common marmoset. Cereb Cortex. 2000;10:252–262. doi: 10.1093/cercor/10.3.252. [DOI] [PubMed] [Google Scholar]
- Romberg C, Mattson MP, Mughal MR, Bussey TJ, Saksida LM. Impaired attention in the 3xTgAD mouse model of Alzheimer’s disease: rescue by donepezil (Aricept) J Neurosci. 2011;31:3500–3507. doi: 10.1523/JNEUROSCI.5242-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakagami M, Tsutsui K. The hierarchical organization of decision making in the primate prefrontal cortex. Neurosci Res. 1999;34:79–89. doi: 10.1016/s0168-0102(99)00038-3. [DOI] [PubMed] [Google Scholar]
- Sakagami M, Tsutsui K, Lauwereyns J, Koizumi M, Kobayashi S, Hikosaka O. A code for behavioral inhibition on the basis of color, but not motion, in ventrolateral prefrontal cortex of macaque monkey. J Neurosci. 2001;21:4801–4808. doi: 10.1523/JNEUROSCI.21-13-04801.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksida LM, Bussey TJ, Buckmaster CA, Murray EA. No effect of hippocampal lesions on perirhinal cortex-dependent feature-ambiguous visual discriminations. Hippocampus. 2006;16:421–430. doi: 10.1002/hipo.20170. [DOI] [PubMed] [Google Scholar]
- Salthous TA, Babcok RL. Decomposing adult age differences in working memory. Dev Psychol. 1991;27:763–776. [Google Scholar]
- Salthouse TA. Age and experience effects on the interpretation of orthographic drawings of three-dimensional objects. Psychol Aging. 1991;6:426–433. doi: 10.1037//0882-7974.6.3.426. [DOI] [PubMed] [Google Scholar]
- Sarasa M, Pesini P. Natural non-trasgenic animal models for research in Alzheimer’s disease. Curr Alzheimer Res. 2009;6:171–178. doi: 10.2174/156720509787602834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvage MM, Fortin NJ, Owens CB, Yonelinas AP, Eichenbaum H. Recognition memory: opposite effects of hippocampal damage on recollection and familiarity. Nat Neurosci. 2008;11:16–18. doi: 10.1038/nn2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Nugent S, Saddoris MP, Gallagher M. Teaching old rats new tricks: age-related impairments in olfactory reversal learning. Neurobiol Aging. 2002a;23:555–564. doi: 10.1016/s0197-4580(01)00343-8. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Nugent SL, Saddoris MP, Setlow B. Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odor discriminations. Neuroreport. 2002b;13:885–890. doi: 10.1097/00001756-200205070-00030. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Nugent SL, Saddoris MP, Gallagher M. Lesions of orbitofrontal cortex and basolateral amygdala complex disrupt acquisition of odor-guided discriminations and reversals. Learn Mem. 2003;10:129–140. doi: 10.1101/lm.55203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scialfa CT, Esau SP, Joffe KM. Age, target-distractor similarity, and visual search. Exp Aging Res. 1998;24:337–358. doi: 10.1080/036107398244184. [DOI] [PubMed] [Google Scholar]
- Shamy JL, Habeck C, Hof PR, Amaral DG, Fong SG, Buonocore MH, Stern Y, Barnes CA, Rapp PR. Volumetric correlates of spatiotemporal working and recognition memory impairment in aged rhesus monkeys. Cereb Cortex. 2011;21:1559–1573. doi: 10.1093/cercor/bhq210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DE, Rapp PR, McKay HM, Roberts JA, Tuszynski MH. Memory impairment in aged primates is associated with focal death of cortical neurons and atrophy of subcortical neurons. J Neurosci. 2004;24:4373–4381. doi: 10.1523/JNEUROSCI.4289-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snigdha S, Christie LA, De Rivera C, Araujo JA, Milgram NW, Cotman CW. Age and distraction are determinants of performance on a novel visual search task in aged beagle dogs. Age (Dordr) 2012;34:67–73. doi: 10.1007/s11357-011-9219-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CE, Sherman SJ, Kirchhoff BA, Hasselmo ME. Medial temporal and prefrontal contributions to working memory tasks with novel and familiar stimuli. Hippocampus. 2001;11:337–346. doi: 10.1002/hipo.1048. [DOI] [PubMed] [Google Scholar]
- Summers JB, Buccafusco JJ, Hill WD, Prendergast MA. Localization of ubiquitin in the plaques of five aged primates by dual-label fluorescent immunohistochemistry. Alzheimers Res. 1997;3:11–21. [Google Scholar]
- Swainson R, Hodges JR, Galton CJ, Semple J, Michael A, Dunn BD, Iddon JL, Robbins TW, Sahakian BJ. Early detection and differential diagnosis of Alzheimer’s disease and depression with neuropsychological tasks. Dement Geriatr Cogn Disord. 2001;12:265–280. doi: 10.1159/000051269. [DOI] [PubMed] [Google Scholar]
- Taffe MA, Weed MR, Gold LH. Scopolamine alters rhesus monkey performance on a novel neuropsychological test battery. Brain Res Cogn Brain Res. 1999;8:203–212. doi: 10.1016/s0926-6410(99)00021-x. [DOI] [PubMed] [Google Scholar]
- Taffe MA, Weed MR, Gutierrez T, Davis SA, Gold LH. Modeling a task that is sensitive to dementia of the Alzheimer’s type: individual differences in acquisition of a visuo-spatial paired-associate learning task in rhesus monkeys. Behav Brain Res. 2004;149:123–133. doi: 10.1016/s0166-4328(03)00214-6. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Buccafusco JJ, Jackson WJ. Scopolamine reversal of nicotine enhanced delayed matching-to-sample performance in monkeys. Pharmacol Biochem Behav. 1993;45:925–929. doi: 10.1016/0091-3057(93)90141-f. [DOI] [PubMed] [Google Scholar]
- Tierney MC, Szalai JP, Snow WG, Fisher RH, Nores A, Nadon G, Dunn E, St George-Hyslop PH. Prediction of probable Alzheimer’s disease in memory-impaired patients: a prospective longitudinal study. Neurology. 1996;46:661–665. doi: 10.1212/wnl.46.3.661. [DOI] [PubMed] [Google Scholar]
- Toledano A, Alvarez MI, Toledano-Diaz A. Diversity and variability of the effects of nicotine on different cortical regions of the brain—therapeutic and toxicological implications. Centr Nerv Syst Agents Medic Chem. 2010;10:180–206. doi: 10.2174/1871524911006030180. [DOI] [PubMed] [Google Scholar]
- Tulving E. Ecphoric processes in episodic memory. Philos Trans R Soc B. 1983;302:361–371. [Google Scholar]
- Tulving E, Thompson DM. Encoding specificity and retrieval processes in episodic memory. Psychol Rev. 1973;80:352–373. [Google Scholar]
- Vale-Martinez A, Baxter MG, Eichenbaum H. Selective lesions of basal forebrain cholinergic neurons produce anterograde and retrograde deficits in a social transmission of food preference task in rats. Eur J Neurosci. 2002;16:983–998. doi: 10.1046/j.1460-9568.2002.02153.x. [DOI] [PubMed] [Google Scholar]
- Voytko ML, Mach RH, Gage HD, Ehrenkaufer RL, Efange SM, Tobin JR. Cholinergic activity of aged rhesus monkeys revealed by positron emission tomography. Synapse. 2001;39:95–100. doi: 10.1002/1098-2396(20010101)39:1<95::AID-SYN12>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Warden MR, Miller EK. Task-dependent changes in short-term memory in the prefrontal cortex. J Neurosci. 2010;30:15801–15810. doi: 10.1523/JNEUROSCI.1569-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh K, Butters N, Hughes J, Mohs R, Heyman A. Detection of abnormal memory decline in mild cases of Alzheimer’s disease using CERAD neuropsychological measures. Arch Neurol. 1991;48:278–281. doi: 10.1001/archneur.1991.00530150046016. [DOI] [PubMed] [Google Scholar]
- Williams CB, Hess RF. Relationship between facilitation at threshold and suprathreshold contour integration. J Opt Soc Am A. 1998;15:2046–2051. doi: 10.1364/josaa.15.002046. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Lacy JW, Stark SM, Albert MS, Gallagher M, Stark CE. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2011a;21:968–979. doi: 10.1002/hipo.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Mattfeld AT, Stark SM, Stark CE. Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. Proc Natl Acad Sci U S A. 2011b;108:8873–8878. doi: 10.1073/pnas.1101567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Light GA, Marston HM, Sharp R, Geyer MA. The 5-choice continuous performance test: evidence for a translational test of vigilance for mice. PloS One. 2009;4:e4227. doi: 10.1371/journal.pone.0004227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Powell SB, Geyer MA, Jeste DV, Risbrough VB. The mouse attentional-set-shifting task: a method for assaying successful cognitive aging? Cogn Affect Behav Neurosci. 2010;10:243–251. doi: 10.3758/CABN.10.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahs KR, Ashe KH. ‘Too much good news’—are Alzheimer mouse models trying to tell us how to prevent, not cure, Alzheimer’s disease? Trends Neurosci. 2010;33:381–389. doi: 10.1016/j.tins.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Zelazo PD. The Dimensional Change Card Sort (DCCS): a method of assessing executive function in children. Nature Protoc. 2006;1:297–301. doi: 10.1038/nprot.2006.46. [DOI] [PubMed] [Google Scholar]
- Zhuo JM, Prescott SL, Murray ME, Zhang HY, Baxter MG, Nicolle MM. Early discrimination reversal learning impairment and preserved spatial learning in a longitudinal study of Tg2576 APPsw mice. Neurobiol Aging. 2007;28:1248–1257. doi: 10.1016/j.neurobiolaging.2006.05.034. [DOI] [PubMed] [Google Scholar]


