Abstract
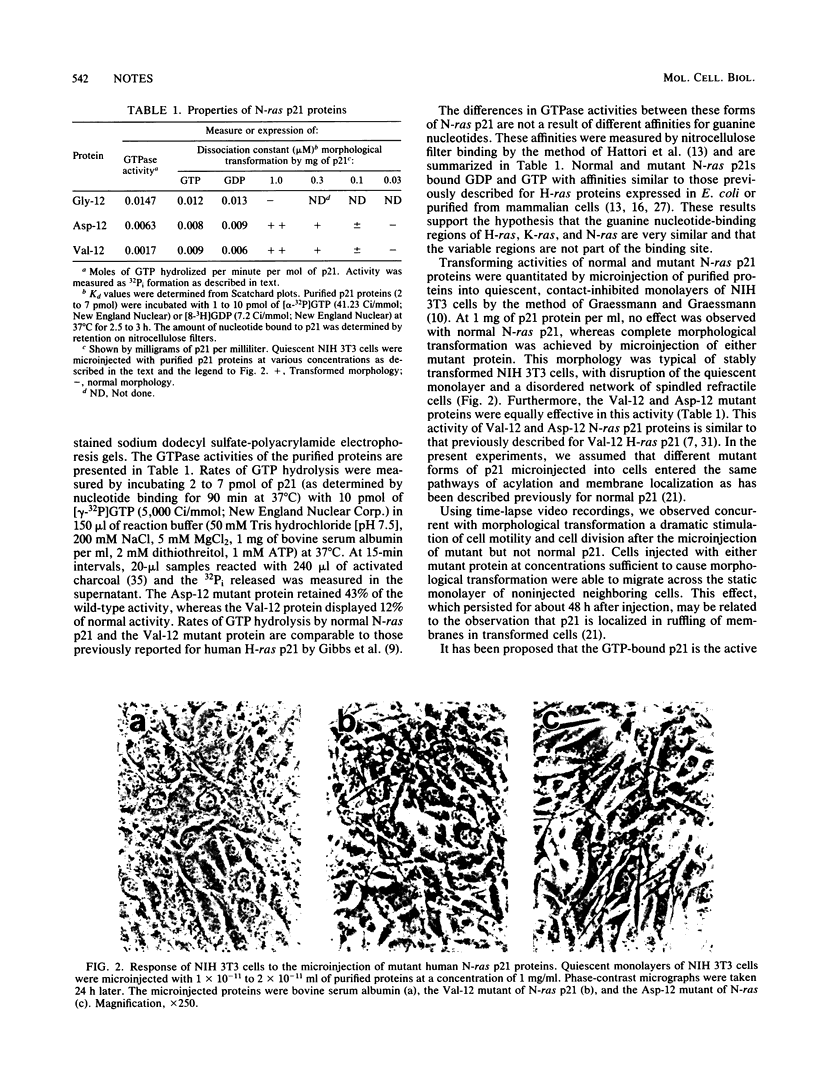
We characterized the normal (Gly-12) and two mutant (Asp-12 and Val-12) forms of human N-ras proteins produced by Escherichia coli. No significant differences were found between normal and mutant p21 proteins in their affinities for GTP or GDP. Examination of GTPase activities revealed significant differences between the mutant p21s: the Val-12 mutant retained 12% of wild-type GTPase activity, whereas the Asp-12 mutant retained 43%. Both mutant proteins, however, were equally potent in causing morphological transformation and increased cell motility after their microinjection into quiescent NIH 3T3 cells. This lack of correlation between transforming potency and GTPase activity or guanine nucleotide binding suggests that position 12 mutations affect other aspects of p21 function.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bos J. L., Toksoz D., Marshall C. J., Verlaan-de Vries M., Veeneman G. H., van der Eb A. J., van Boom J. H., Janssen J. W., Steenvoorden A. C. Amino-acid substitutions at codon 13 of the N-ras oncogene in human acute myeloid leukaemia. 1985 Jun 27-Jul 3Nature. 315(6022):726–730. doi: 10.1038/315726a0. [DOI] [PubMed] [Google Scholar]
- Capon D. J., Seeburg P. H., McGrath J. P., Hayflick J. S., Edman U., Levinson A. D., Goeddel D. V. Activation of Ki-ras2 gene in human colon and lung carcinomas by two different point mutations. Nature. 1983 Aug 11;304(5926):507–513. doi: 10.1038/304507a0. [DOI] [PubMed] [Google Scholar]
- Colby W. W., Hayflick J. S., Clark S. G., Levinson A. D. Biochemical characterization of polypeptides encoded by mutated human Ha-ras1 genes. Mol Cell Biol. 1986 Feb;6(2):730–734. doi: 10.1128/mcb.6.2.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der C. J., Finkel T., Cooper G. M. Biological and biochemical properties of human rasH genes mutated at codon 61. Cell. 1986 Jan 17;44(1):167–176. doi: 10.1016/0092-8674(86)90495-2. [DOI] [PubMed] [Google Scholar]
- Der C. J., Krontiris T. G., Cooper G. M. Transforming genes of human bladder and lung carcinoma cell lines are homologous to the ras genes of Harvey and Kirsten sarcoma viruses. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3637–3640. doi: 10.1073/pnas.79.11.3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano O., Aldrich T., Tamanoi F., Taparowsky E., Furth M., Wigler M. Analysis of the transforming potential of the human H-ras gene by random mutagenesis. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4008–4012. doi: 10.1073/pnas.81.13.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feramisco J. R., Gross M., Kamata T., Rosenberg M., Sweet R. W. Microinjection of the oncogene form of the human H-ras (T-24) protein results in rapid proliferation of quiescent cells. Cell. 1984 Aug;38(1):109–117. doi: 10.1016/0092-8674(84)90531-2. [DOI] [PubMed] [Google Scholar]
- Gambke C., Hall A., Moroni C. Activation of an N-ras gene in acute myeloblastic leukemia through somatic mutation in the first exon. Proc Natl Acad Sci U S A. 1985 Feb;82(3):879–882. doi: 10.1073/pnas.82.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J. B., Sigal I. S., Poe M., Scolnick E. M. Intrinsic GTPase activity distinguishes normal and oncogenic ras p21 molecules. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5704–5708. doi: 10.1073/pnas.81.18.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graessmann M., Graessmann A. Microinjection of tissue culture cells. Methods Enzymol. 1983;101:482–492. doi: 10.1016/0076-6879(83)01033-2. [DOI] [PubMed] [Google Scholar]
- Gross M., Sweet R. W., Sathe G., Yokoyama S., Fasano O., Goldfarb M., Wigler M., Rosenberg M. Purification and characterization of human H-ras proteins expressed in Escherichia coli. Mol Cell Biol. 1985 May;5(5):1015–1024. doi: 10.1128/mcb.5.5.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A., Brown R. Human N-ras: cDNA cloning and gene structure. Nucleic Acids Res. 1985 Jul 25;13(14):5255–5268. doi: 10.1093/nar/13.14.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori S., Ulsh L. S., Halliday K., Shih T. Y. Biochemical properties of a highly purified v-rasH p21 protein overproduced in Escherichia coli and inhibition of its activities by a monoclonal antibody. Mol Cell Biol. 1985 Jun;5(6):1449–1455. doi: 10.1128/mcb.5.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lacal J. C., Srivastava S. K., Anderson P. S., Aaronson S. A. Ras p21 proteins with high or low GTPase activity can efficiently transform NIH/3T3 cells. Cell. 1986 Feb 28;44(4):609–617. doi: 10.1016/0092-8674(86)90270-9. [DOI] [PubMed] [Google Scholar]
- Manne V., Bekesi E., Kung H. F. Ha-ras proteins exhibit GTPase activity: point mutations that activate Ha-ras gene products result in decreased GTPase activity. Proc Natl Acad Sci U S A. 1985 Jan;82(2):376–380. doi: 10.1073/pnas.82.2.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manne V., Yamazaki S., Kung H. F. Guanosine nucleotide binding by highly purified Ha-ras-encoded p21 protein produced in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6953–6957. doi: 10.1073/pnas.81.22.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark D. F., Lu S. D., Creasey A. A., Yamamoto R., Lin L. S. Site-specific mutagenesis of the human fibroblast interferon gene. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5662–5666. doi: 10.1073/pnas.81.18.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy M. S., Bargmann C. I., Weinberg R. A. Human colon carcinoma Ki-ras2 oncogene and its corresponding proto-oncogene. Mol Cell Biol. 1984 Aug;4(8):1577–1582. doi: 10.1128/mcb.4.8.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J. P., Capon D. J., Goeddel D. V., Levinson A. D. Comparative biochemical properties of normal and activated human ras p21 protein. Nature. 1984 Aug 23;310(5979):644–649. doi: 10.1038/310644a0. [DOI] [PubMed] [Google Scholar]
- Myrdal S. E., Auersperg N. p21ras. Heterogeneous localization in transformed cells. Exp Cell Res. 1985 Aug;159(2):441–450. doi: 10.1016/s0014-4827(85)80017-3. [DOI] [PubMed] [Google Scholar]
- Parada L. F., Tabin C. J., Shih C., Weinberg R. A. Human EJ bladder carcinoma oncogene is homologue of Harvey sarcoma virus ras gene. Nature. 1982 Jun 10;297(5866):474–478. doi: 10.1038/297474a0. [DOI] [PubMed] [Google Scholar]
- Poe M., Scolnick E. M., Stein R. B. Viral Harvey ras p21 expressed in Escherichia coli purifies as a binary one-to-one complex with GDP. J Biol Chem. 1985 Apr 10;260(7):3906–3909. [PubMed] [Google Scholar]
- Reddy E. P., Reynolds R. K., Santos E., Barbacid M. A point mutation is responsible for the acquisition of transforming properties by the T24 human bladder carcinoma oncogene. Nature. 1982 Nov 11;300(5888):149–152. doi: 10.1038/300149a0. [DOI] [PubMed] [Google Scholar]
- Santos E., Tronick S. R., Aaronson S. A., Pulciani S., Barbacid M. T24 human bladder carcinoma oncogene is an activated form of the normal human homologue of BALB- and Harvey-MSV transforming genes. Nature. 1982 Jul 22;298(5872):343–347. doi: 10.1038/298343a0. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Shih T. Y., Maryak J., Ellis R., Chang E., Lowy D. Guanine nucleotide binding activity of the src gene product of rat-derived murine sarcoma viruses. Ann N Y Acad Sci. 1980;354:398–409. doi: 10.1111/j.1749-6632.1980.tb27981.x. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Papageorge A. G., Stokes P. E., Weeks M. O., Scolnick E. M. Guanine nucleotide-binding and autophosphorylating activities associated with the p21src protein of Harvey murine sarcoma virus. Nature. 1980 Oct 23;287(5784):686–691. doi: 10.1038/287686a0. [DOI] [PubMed] [Google Scholar]
- Shimizu K., Birnbaum D., Ruley M. A., Fasano O., Suard Y., Edlund L., Taparowsky E., Goldfarb M., Wigler M. Structure of the Ki-ras gene of the human lung carcinoma cell line Calu-1. Nature. 1983 Aug 11;304(5926):497–500. doi: 10.1038/304497a0. [DOI] [PubMed] [Google Scholar]
- Shimizu K., Goldfarb M., Suard Y., Perucho M., Li Y., Kamata T., Feramisco J., Stavnezer E., Fogh J., Wigler M. H. Three human transforming genes are related to the viral ras oncogenes. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2112–2116. doi: 10.1073/pnas.80.8.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal I. S., Gibbs J. B., D'Alonzo J. S., Temeles G. L., Wolanski B. S., Socher S. H., Scolnick E. M. Mutant ras-encoded proteins with altered nucleotide binding exert dominant biological effects. Proc Natl Acad Sci U S A. 1986 Feb;83(4):952–956. doi: 10.1073/pnas.83.4.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey D. W., Kung H. F. Transformation of NIH 3T3 cells by microinjection of Ha-ras p21 protein. Nature. 1984 Aug 9;310(5977):508–511. doi: 10.1038/310508a0. [DOI] [PubMed] [Google Scholar]
- Sweet R. W., Yokoyama S., Kamata T., Feramisco J. R., Rosenberg M., Gross M. The product of ras is a GTPase and the T24 oncogenic mutant is deficient in this activity. Nature. 1984 Sep 20;311(5983):273–275. doi: 10.1038/311273a0. [DOI] [PubMed] [Google Scholar]
- Tabin C. J., Bradley S. M., Bargmann C. I., Weinberg R. A., Papageorge A. G., Scolnick E. M., Dhar R., Lowy D. R., Chang E. H. Mechanism of activation of a human oncogene. Nature. 1982 Nov 11;300(5888):143–149. doi: 10.1038/300143a0. [DOI] [PubMed] [Google Scholar]
- Tjian R., Robbins A., Clark R. Catalytic properties of the SV40 large T antigen. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):103–111. doi: 10.1101/sqb.1980.044.01.012. [DOI] [PubMed] [Google Scholar]
- Walter M., Clark S. G., Levinson A. D. The oncogenic activation of human p21ras by a novel mechanism. Science. 1986 Aug 8;233(4764):649–652. doi: 10.1126/science.3487832. [DOI] [PubMed] [Google Scholar]
- Yuasa Y., Srivastava S. K., Dunn C. Y., Rhim J. S., Reddy E. P., Aaronson S. A. Acquisition of transforming properties by alternative point mutations within c-bas/has human proto-oncogene. Nature. 1983 Jun 30;303(5920):775–779. doi: 10.1038/303775a0. [DOI] [PubMed] [Google Scholar]