Abstract
For women with breast cancer who undergo mastectomy, immediate breast reconstruction (IR) offers a cosmetic and psychological advantage. We evaluated the association between demographic, hospital, surgeon and insurance factors and receipt of IR. We conducted a retrospective hospital-based analysis with the Perspective database. Women who underwent a mastectomy for invasive breast cancer (IBC) and ductal carcinoma in situ (DCIS) from 2000 to 2010 were included. Logistic regression analysis was used to determine factors predictive of IR. Analyses were stratified by age (<50 vs. ≥50) and IBC versus DCIS. Of the 108,992 women with IBC who underwent mastectomy, 30,859 (28.3 %) underwent IR, as compared to 6,501 (44.2 %) of the 14,710 women with DCIS who underwent mastectomy underwent IR. In a multivariable model for IBC, increasing age, black race, being married, rural location, and increased comorbidities were associated with decreased IR. Odds ratios (OR) of IR increased with commercial insurance (OR 3.38) and Medicare (OR 1.66) insurance (vs. self-pay), high surgeon-volume (OR 1.19), high hospital-volume (OR 2.24), and large hospital size (OR 1.20). The results were identical for DCIS, and by age category. The absolute difference between the proportion of patients who received IR with commercial insurance compared to other insurance, increased over time. Immediate in-hospital complication rates were higher for flap reconstruction compared to implant or no reconstruction (15.2, 4.0, and 6.1 %, respectively, P <.0001). IR has increased significantly over time; however, modifiable factors such as insurance status, hospital size, hospital location, and physician volume strongly predict IR. Public policy should ensure that access to reconstructive surgery is universally available.
Keywords: Breast reconstruction, Insurance, Hospital volume
Introduction
In 1983, the results of the only randomized trial comparing immediate breast reconstruction to delayed reconstruction in 64 women undergoing a mastectomy for breast cancer was published [1]. The study was conducted to determine if morbidity, cosmetic outcomes, and psychological benefits differed between these groups. The study concluded that women who underwent immediate breast reconstruction had less psychological morbidity at 3 months following mastectomy [1]. As then a number of cross-sectional and prospective studies of breast reconstruction have been reported, most of which suggest an improvement in psychological health, self-esteem, sexuality, and body image [2-5], without compromising local recurrence rates [6, 7]. This is particularly true for young women with breast cancer. Based on this evidence, in October 1998 the Women’s Health and Cancer Rights Act (WHCRA) was signed mandating that group health plans, health insurance companies, and HMOs that cover mastectomy, must also cover the costs of reconstruction.
Despite the benefits, surprisingly few patients undergo immediate breast reconstruction. Studies of women diagnosed in the mid-1990s found rates of immediate post-mastectomy reconstruction ranging from 3 to 8 % [8]. While subsequent studies in early 2000 reported rates increasing to 25 % [9], these rates are variable and dependent on the composition of the cohort being analyzed. The proportion of patients undergoing immediate reconstruction is higher when patients are treated in specialized centers with dedicated surgical oncologists and available reconstructive surgeons [10]. With regard to delayed reconstruction, in the trial by Dean et al. [1], only 6 of the 31 women randomized to delayed reconstruction underwent reconstructive surgery within a year after mastectomy. Recent population-based studies report that only 3 % of women undergo delayed reconstruction after mastectomy [11].
Several studies analyzing data from the 1990s have evaluated factors that influence receipt of post-mastectomy reconstruction in women with invasive cancer only [11-14]. African-American race and Asian ethnicity have consistently been associated with a reduced likelihood of undergoing reconstruction [8, 11, 12]. Younger age, higher education levels, and income increase the likelihood of reconstruction [8, 11]. The goal of this study was to evaluate the association of demographic, hospital, physician, and insurance factors with receipt of immediate breast reconstruction and complications in the decade following the signing of the WHCRA, and to evaluate the immediate complications associated with IR compared to mastectomy alone in women with invasive breast cancer (IBC) and ductal carcinoma in situ (DCIS).
Patients and methods
Data source
We utilized the Perspective database (Premier, Charlotte, North Carolina), a voluntary, fee-supported database originally developed to measure resource utilization and quality of care. Perspective contains a representative sample of more than 600 acute-care hospitals throughout the United States that contribute data on inpatient hospital admissions [15]. Each participating institution submits electronic updates on a quarterly basis. The data are audited regularly to ensure quality and integrity. In addition to patient demographics, disease characteristics, and procedures, the database collects information on all billed services. The Perspective database has been utilized in a number of outcomes studies [16, 17]. In 2006, perspective recorded approximately 5.5 million hospital discharges, representing approximately 15 % of hospitalizations nationwide [15, 17].
Cohort selection and surgical procedures
Our analysis included women between the ages of 18 and 90 who underwent a simple, radical, or skin sparing mastectomy (ICD-9: 85.33–85.36, 85.40–85.48) for IBC (ICD-9: 174.0–174.9) or DCIS (ICD-9: 233.0) between January 2000 and March 2010. Women who undergo mastectomy with DCIS do not undergo radiation and, therefore, were chosen as a comparison to avoid treatment and stage-related confounders. Patients were categorized based on the receipt of reconstructive surgery: flap (ICD-9: 85.7, 85.70–85.76, 85.79, 85.82–85.85, 85.87, 86.60, 86.70, 86.72, 86.74–86.75) or implant (ICD-9: 85.33, 85.35, 85.53, 85.54, 85.6, 85.89, 85.93–85.96)/tissue expander (ICD-9: 85.95). Anyone who had ICD-9 codes for both a flap and an implant procedure was categorized as a flap. Performance of lymphadenectomy and laterality was noted for each patient.
Clinical and demographic characteristics
Demographic data analyzed included age, race (white, black, other), marital status (married, single, unknown), and insurance status (Medicare, Medicaid, commercial, self-pay, and unknown). The hospitals at which patients were treated were characterized by location (urban, rural), region of the country (northeast, mid-west, west, south), size (<400 beds, 400–600 beds, and >600 beds) and teaching status (teaching, non-teaching). Risk adjustment for comorbid conditions was performed using the Charlson comorbidity index [18]. The ICD-9 coding to define the Charlson index as reported by Deyo et al. [19] was utilized.
Procedure volume
For each surgeon and hospital, we determined the total number of mastectomies performed during the study period. As not all physicians and hospitals contributed data for the entire study period, we calculated annualized procedure volumes. The annualized procedure volume was estimated by dividing the total number of subjects of a given surgeon or hospital who underwent a mastectomy by the number of years a given surgeon or hospital contributed at least one mastectomy to the database. The distribution of volumes was then inspected visually and cut-points selected to create three approximately equal tertiles of surgeon volume (low <5.1, intermediate 5.1–13.4, and high ≥13.5) and hospital volume: (low <32.7, intermediate 32.7–67.4, and high >67.4) [20, 21].
Outcomes and costs
The primary outcome of the study was receipt of immediate post-mastectomy reconstruction. Secondary outcomes included a stratified analysis by age group (<50; ≥50). This cutoff was chosen so that we could specifically look at receipt of IR in young women. In addition, individual complications, rates of transfusion, prolonged length of stay (mean), and perioperative mortality were compared. Perioperative morbidity was classified into the following categories: (1) perioperative complications (abscess, wound complication, operative injury) and (2) medical complications (venous thromboembolism, myocardial infarction, cardiopulmonary arrest, acute renal failure, respiratory failure, cerebrovascular accident, bacteremia/sepsis, shock, and pneumonia). A composite score of any morbidity was determined based on the occurrence of one or more of the above complications in a patient.
The Perspective database includes an itemized, data-stamped log of all items that are billed to a patient including drugs, laboratory and radiologic tests, and therapeutic services during the hospitalization. Within the Perspective database, approximately three quarters of hospitals submit direct cost data taken from internal accounting systems. The remaining institutions provide estimates based on Medicare cost to charge ratios [15, 17, 22]. Patients with a hospital cost of greater than or less than 3 standard deviations above or below the mean were excluded and all costs were reported in 2010 US dollars [23]. Cost data from perspectives have been utilized in a number of outcomes studies [15, 17, 22].
Statistical analysis
Frequency distributions between categorical variables were compared using χ2 tests, while continuous variables were compared with one-way ANOVA. The association between the outcomes of interest and reconstruction was assessed using multivariable logistic regression models that included patient, surgeon, and hospital characteristics. Results are reported with odds ratios (OR) and 95 % confidence intervals. Analyses were performed, stratified by age category and by invasive vs. DCIS. Interactions between key variables and year of mastectomy were evaluated. All analyses were performed with SAS version 9.2 (SAS Institute Inc, Cary, North Carolina). All statistical tests were two-sided.
Results
We identified 108,992 women with IBC and 14,710 women with DCIS who underwent mastectomy, and of these, 30,859 (28.3 %) of women with IBC and 6,501 (44.2 %) with DCIS underwent immediate breast reconstruction. The prevalence rate of immediate breast reconstruction was 2.5-fold higher among women <50 years (51.3 %) than those ≥50 years (20.5 %). The clinical and demographic characteristics of the sub-cohorts are displayed in Table 1.
Table 1.
Clinical and demographic characteristics of the cohort stratified by invasive cancer and DCIS
| Invasive cancer mastectomy
|
Invasive cancer reconstruction
|
Ductal carcinoma in situ mastectomy
|
Ductal carcinoma in situ reconstruction
|
|||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | %a | Total | % | N | %a | |
| Reconstruction | 108,992 | 30,859 | 28.3 | 14,710 | 6,501 | 44.2 | ||
| Age at surgery | ||||||||
| <40 | 6,539 | 6.0 | 3,521 | 53.8 | 761 | 5.2 | 580 | 76.2 |
| 40–49 | 19,980 | 18.3 | 10,437 | 52.2 | 3,313 | 22.5 | 2,358 | 71.2 |
| 50–59 | 25,235 | 23.1 | 9,836 | 39.0 | 3,895 | 26.5 | 2,258 | 58.0 |
| 60–69 | 23,501 | 21.6 | 5,280 | 22.5 | 3,162 | 21.5 | 1,029 | 32.5 |
| ≥70 | 33,737 | 31.0 | 1,785 | 5.8 | 3,579 | 24.3 | 276 | 7.7 |
| Race | ||||||||
| White | 75,850 | 69.6 | 18,599 | 24.5 | 10,357 | 70.4 | 4,657 | 45.0 |
| Black | 11,547 | 10.6 | 2,009 | 17.4 | 1,478 | 10.1 | 537 | 36.3 |
| Other | 21,595 | 19.8 | 4,949 | 22.9 | 2,875 | 19.5 | 1,307 | 45.5 |
| Year of diagnosis | ||||||||
| 2000–2002 | 23,761 | 21.8 | 5,469 | 17.7 | 3,604 | 24.5 | 1,407 | 39.0 |
| 2003–2004 | 21,516 | 19.7 | 5,813 | 27.0 | 2,721 | 18.5 | 1,197 | 44.0 |
| 2005–2006 | 21,270 | 19.5 | 5,700 | 26.8 | 2,867 | 19.5 | 1,239 | 42.2 |
| 2007–2008 | 24,594 | 22.6 | 7,503 | 30.5 | 3,179 | 21.6 | 1,468 | 46.2 |
| 2009–2010 | 17,851 | 16.4 | 6,374 | 35.7 | 2,339 | 15.9 | 1,190 | 50.9 |
| Marital status | ||||||||
| Married | 54,092 | 49.6 | 19,046 | 35.2 | 8,243 | 56.0 | 4,222 | 51.2 |
| Single | 13,460 | 12.3 | 3,750 | 27.9 | 1,628 | 11.1 | 731 | 44.9 |
| Unknown | 41,440 | 38.1 | 8,063 | 19.5 | 4,839 | 32.9 | 1,548 | 32.0 |
| Insurance status | ||||||||
| Commercial | 54,126 | 49.7 | 24,780 | 45.8 | 8,640 | 58.7 | 5,440 | 63.0 |
| Medicare | 44,164 | 40.5 | 3,828 | 8.7 | 4,938 | 33.6 | 665 | 13.5 |
| Medicaid | 6,265 | 5.7 | 1,194 | 19.1 | 604 | 4.1 | 185 | 30.6 |
| Self-pay | 2,216 | 2.0 | 359 | 16.2 | 202 | 1.4 | 40 | 19.8 |
| Unknown | 2,210 | 2.0 | 698 | 31.4 | 326 | 2.2 | 132 | 40.1 |
| Mastectomy | ||||||||
| Simple | 105,187 | 96.5 | 28,704 | 27.3 | 13,905 | 94.5 | 5,913 | 42.5 |
| Skin sparing | 1,955 | 1.8 | 1,526 | 78.6 | 151 | 1.0 | 514 | 78.6 |
| Radical | 1,850 | 1.7 | 629 | 34.0 | 654 | 4.4 | 74 | 50.0 |
| Laterality | ||||||||
| Unilateral | 95,055 | 87.2 | 22,240 | 23.4 | 11,483 | 78.1 | 4,159 | 36.2 |
| Bilateral | 13,937 | 12.8 | 8,619 | 61.8 | 3,227 | 21.9 | 2,342 | 72.6 |
| Lymphadenectomy | ||||||||
| No | 16,732 | 15.4 | 6,406 | 38.3 | 5,802 | 39.4 | 2,542 | 43.8 |
| Yes | 92,260 | 84.6 | 24,453 | 26.5 | 8,908 | 60.6 | 3,959 | 44.4 |
| Hospital location | ||||||||
| Urban | 97,346 | 89.3 | 29,637 | 30.4 | 13,194 | 89.7 | 6,153 | 46.6 |
| Rural | 11,646 | 10.7 | 1,222 | 10.5 | 1,516 | 10.3 | 348 | 23.0 |
| Hospital type | ||||||||
| Teaching | 45,011 | 41.3 | 14,778 | 32.8 | 6,077 | 41.3 | 3,016 | 49.6 |
| Non-teaching | 53,981 | 58.7 | 16,081 | 25.1 | 8,633 | 58.7 | 3,485 | 40.4 |
| Hospital size (beds) | ||||||||
| <400 | 52,379 | 48.1 | 11,804 | 22.5 | 7,011 | 47.7 | 2,233 | 31.8 |
| 400–600 | 32,594 | 29.9 | 10,359 | 31.8 | 4,236 | 28.8 | 1,727 | 40.8 |
| >600 | 24,019 | 22.0 | 8,696 | 36.2 | 3,463 | 23.5 | 1,389 | 40.1 |
| Hospital region | ||||||||
| Mid-west | 21,050 | 19.3 | 5,582 | 26.5 | 2,932 | 10.5 | 1,351 | 46.1 |
| Northeast | 14,939 | 13.7 | 6,033 | 40.4 | 1,546 | 10.5 | 801 | 51.8 |
| South | 53,580 | 49.2 | 13,933 | 26.0 | 7,410 | 50.4 | 3,123 | 42.1 |
| West | 19,423 | 17.8 | 5,311 | 27.3 | 2,822 | 19.2 | 1,226 | 43.4 |
| Charlson comorbidity | ||||||||
| 1 | 31,531 | 28.9 | 11,903 | 37.8 | 7,891 | 53.6 | 4,057 | 51.4 |
| 2 | 33,036 | 30.3 | 10,271 | 31.1 | 3,824 | 26.0 | 1,694 | 44.3 |
| ≥3 | 44,425 | 40.8 | 8,685 | 19.6 | 2,995 | 20.4 | 750 | 25.0 |
| Surgeon volume | ||||||||
| Low | 36,531 | 33.5 | 7,883 | 21.6 | 4,763 | 32.4 | 1,765 | 37.1 |
| Intermediate | 36,173 | 33.2 | 9,004 | 24.9 | 5,005 | 34.0 | 2,171 | 43.4 |
| High | 36,288 | 33.3 | 13,972 | 38.5 | 4,942 | 33.6 | 2,565 | 51.9 |
| Hospital volume | ||||||||
| Low | 36,379 | 33.4 | 5,993 | 16.5 | 4,932 | 33.5 | 1,624 | 32.9 |
| Intermediate | 36,076 | 33.1 | 9,881 | 27.4 | 4,889 | 33.2 | 2,204 | 45.1 |
| High | 36,537 | 33.5 | 14,985 | 41.0 | 4,889 | 33.2 | 2,673 | 54.7 |
| Type of reconstruction | ||||||||
| Implant | 19,038 | 61.7 | 4,230 | 65.0 | ||||
| Flap | 11,821 | 38.3 | 2,271 | 35.0 | ||||
All Univariate χ2 analysis significant to p < 0.001
Percent of patients in category who underwent immediate reconstruction
For women with IBC, the prevalence rate of immediate reconstruction increased with time from 17.7 % in 2000–2002 to 35.7 % in 2009–2010. While rates increased over time across all insurance types, the increase was striking for women with commercial insurance (40.1–57.8 %: Fig. 1a). The rates of reconstruction also increased dramatically for women under the age of 50 (46.4–63.9 %: Fig. 1c); however, the rates in women >70 years increased but remained low (4.8–9.3 %). Throughout the decade black women were less likely to undergo reconstruction (Fig. 1b). For women <50 with commercial insurance, the rate of reconstruction for women with IBC in 2009–2010 was 67.0 %, and for women with DCIS it was 78.9 %.
Fig. 1.
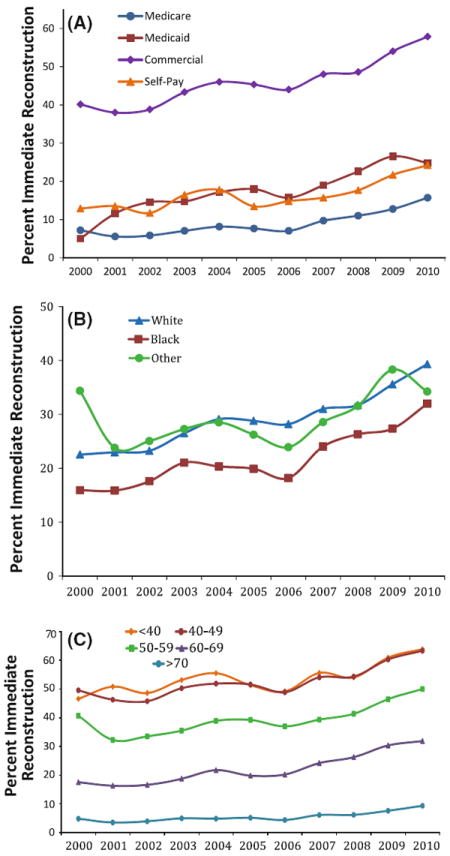
Annual rates of immediate breast cancer reconstruction following mastectomy by a type of insurance, b race, and c age category
All the clinical and demographic characteristics evaluated were associated with immediate reconstruction in women with IBC and DCIS (Table 1). In a multivariable model including only women with IBC (Table 2), women over age 59 were less likely than women age between 50 and 59 to have immediate reconstruction; women <50 were more likely to have IR. Immediate reconstruction was significantly less likely for women of black race (OR 0.68; 95 %CI 0.64–0.72), single marital status (OR 0.76, 95 %CI 0.72–0.80), rural hospital location (OR 0.46; 95 %CI 0.43–0.49), and >2 co-morbid conditions (OR 0.55 95 %CI 0.53–0.57). Odds of reconstruction increased with commercial (OR 3.38; 95 %CI 2.99–3.82) and Medicare (OR 1.66; 95 %CI 1.46–1.90) insurance (compared to self-pay), bilateral mastectomies (OR 2.77; 95 %CI 2.65–2.90), non-teaching hospital (OR 1.21; 95 %CI 1.16–1.25), high surgeon volume (OR 1.19; 95 %CI 1.15–1.25), high hospital volume (OR 2.24; 95 %CI 2.12–2.35), and large hospital size (OR 1.20; 95 %CI 1.14–1.26). Similar associations were seen in women with DCIS (Table 2). Table 3 shows the multivariable models stratified by age. The associations between younger and older women are similar; however, the association between commercial insurance and reconstruction was stronger for women <50 versus ≥50 years (OR 3.71 vs. 3.08, respectively). The absolute difference between the proportion of patients who received IR with commercial insurance versus the other types of insurances increased over time (interaction; p = 0.02) (Fig. 1a).
Table 2.
Multivariable analysis of predictors of immediate breast reconstruction in women with invasive cancer and DCIS who underwent mastectomy
| Invasive cancer OR (95 % CI) | DCIS OR (95 % CI) | |
|---|---|---|
| Age at surgery | ||
| <40 | 1.86 (1.75–1.98)* | 2.39 (1.97–2.90)* |
| 40–49 | 1.66 (1.59–1.73)* | 1.71 (1.53–1.90)* |
| 50–59 | Referent | Referent |
| 60–69 | 0.59 (0.56–0.62)* | 0.48 (0.43–0.54)* |
| ≥70 | 0.17 (0.16–0.19)* | 0.14 (0.12–0.17)* |
| Race | ||
| White | Referent | Referent |
| Black | 0.68 (0.64–0.72)* | 0.69 (0.61–0.80)* |
| Other | 0.89 (0.86–0.93)* | 0.87 (0.79–0.96)* |
| Year of diagnosis | ||
| 2000–2002 | Referent | Referent |
| 2003–2004 | 1.21 (1.15–1.28)* | 1.27 (1.12–1.44)* |
| 2005–2006 | 1.12 (1.06–1.18)* | 1.11 (0.99–1.26) |
| 2007–2008 | 1.29 (1.22–1.35)* | 1.24 (1.09–1.40)* |
| 2009–2010 | 1.68 (1.59–1.77)* | 1.47 (1.29–1.69)* |
| Marital status | ||
| Married | Referent | Referent |
| Single | 0.76 (0.72–0.80)* | 0.72 (0.63–0.82)* |
| Insurance status | ||
| Self–pay | Referent | Referent |
| Commercial | 3.38 (2.99–3.82)* | 3.76 (2.65–5.35)* |
| Medicare | 1.66 (1.46–1.90)* | 1.72 (1.19–2.49)* |
| Medicaid | 1.21 (1.05–1.39)* | 1.19 (0.80–1.77) |
| Unknown | 2.15 (1.84–2.51)* | 2.84 (1.86–4.35)* |
| Mastectomy | ||
| Simple | Referent | Referent |
| Skin sparing | 4.33 (3.83–4.91)* | 2.47 (1.98–3.08)* |
| Radical | 1.64 (1.46–1.85)* | 1.33 (0.89–1.98) |
| Laterality | ||
| Unilateral | Referent | Referent |
| Bilateral | 2.77 (2.65–2.90)* | 2.51 (2.26–2.77)* |
| Lymphadenectomy | ||
| No | Referent | Referent |
| Yes | 0.69 (0.66–0.72)* | 0.88 (0.81–0.96)* |
| Hospital location | ||
| Urban | Referent | Referent |
| Rural | 0.46 (0.43–0.49)* | 0.52 (0.45–0.61)* |
| Hospital type | ||
| Teaching | Referent | Referent |
| Non-teaching | 1.21 (1.16–1.25)* | 0.92 (0.84–1.02) |
| Hospital size (beds) | ||
| <400 | Referent | Referent |
| 400–600 | 1.13 (1.08–1.17)* | 1.30 (1.17–1.44)* |
| >600 | 1.20 (1.14–1.26)* | 1.18 (1.05–1.34)* |
| Hospital region | ||
| Mid-west | Referent | Referent |
| Northeast | 1.74(1.64–1.83)* | 1.31 (1.11–1.53)* |
| South | 0.93 (0.89–0.97)* | 0.95 (0.85–1.06) |
| West | 0.82 (0.78–0.87)* | 0.79 (0.69–0.91)* |
| Charlson comorbidity | ||
| 1 | Referent | Referent |
| 2 | 0.77 (0.74–0.80)* | 1.02 (0.92–1.12) |
| ≥3 | 0.55 (0.53–0.57)* | 0.59 (0.53–0.66)* |
| Surgeon volume | ||
| Low | Referent | Referent |
| Intermediate | 0.95 (0.91–0.99)* | 1.06 (0.95–1.17)* |
| High | 1.19 (1.15–1.25)* | 1.15 (1.03–1.28)* |
| Hospital volume | ||
| Low | Referent | Referent |
| Intermediate | 1.51 (1.44–1.58)* | 1.45 (1.30–1.61)* |
| High | 2.24 (2.12–2.35)* | 1.71 (1.51–1.94)* |
p < 0.01
Table 3.
Multivariable analysis of predictors of immediate breast reconstruction in women with invasive breast cancer <50 years and ≥50 years of age
| Women <50 years OR (95 % CI) | Women ≥50 years OR (95 % CI) | |
|---|---|---|
| Sample size | 26,519 | 82,473 |
| 13,958 (51.3 %) | 16,901 (20.5 %) | |
| Age at surgery | ||
| <40 | Referent | – |
| 40–49 | 0.89 (0.84–0.95)* | – |
| 50–59 | – | Referent |
| 60–69 | – | 0.58 (0.55–0.61)* |
| ≥70 | – | 0.17 (0.16–0.19)* |
| Race | ||
| White | Referent | Referent |
| Black | 0.70 (0.64–0.76)* | 0.66 (0.61–0.71)* |
| Other | 0.87 (0.81–0.93)* | 0.91 (0.87–0.96)* |
| Year of diagnosis | ||
| 2000–2002 | Referent | Referent |
| 2003–2004 | 1.17 (1.08–1.28)* | 1.24 (1.16–1.32)* |
| 2005–2006 | 1.06 (0.92–1.09)* | 1.20 (1.12–1.28)* |
| 2007–2008 | 1.14 (1.05–1.23)* | 1.38 (1.30–1.47)* |
| 2009–2010 | 1.52 (1.39–1.67)* | 1.77 (1.66–1.89)* |
| Marital status | ||
| Married | Referent | Referent |
| Single | 0.82 (0.76–0.89)* | 0.72 (0.68–0.77)* |
| Insurance status | ||
| Self-pay | Referent | Referent |
| Commercial | 3.71 (3.12–4.42)* | 3.08 (2.59–3.66)* |
| Medicare | 1.31 (1.02–1.68)* | 1.57 (1.31–1.87)* |
| Medicaid | 1.42 (1.17–1.73)* | 0.99 (0.80–1.23) |
| Unknown | 2.59 (2.05–3.27)* | 1.84 (1.49–2.28) |
| Mastectomy | ||
| Simple | Referent | Referent |
| Skin sparing | 2.81 (2.33–3.39)* | 5.70 (4.85–6.69)* |
| Radical | 1.38 (1.14–1.69)* | 1.79 (1.55–2.06)* |
| Laterality | ||
| Unilateral | Referent | Referent |
| Bilateral | 3.05 (2.83–3.29)* | 2.64 (2.49–2.79)* |
| Lymphadenectomy | ||
| No | Referent | Referent |
| Yes | 0.70 (0.64–0.75)* | 0.69 (0.65–0.73)* |
| Hospital location | ||
| Urban | Referent | Referent |
| Rural | 0.49 (0.43–0.55)* | 0.44 (0.41–0.49)* |
| Hospital type | ||
| Teaching | Referent | Referent |
| Non-teaching | 1.13 (1.06–1.21)* | 1.25 (1.19–1.31)* |
| Hospital size (beds) | ||
| <400 | Referent | Referent |
| 400–600 | 1.07 (1.00–1.16)* | 1.16 (1.10–1.22)* |
| >600 | 1.24 (1.14–1.35)* | 1.18 (1.11–1.25)* |
| Hospital region | ||
| Mid-west | Referent | Referent |
| Northeast | 1.77 (1.60–1.95)* | 1.73 (1.62–1.86)* |
| South | 0.82 (0.76–0.88)* | 1.00 (0.94–1.05) |
| West | 0.68 (0.62–0.75)* | 0.91 (0.85–0.98)* |
| Charlson comorbidity | ||
| 1 | Referent | Referent |
| 2 | 0.73 (0.69–0.78)* | 0.79(0.75–0.83)* |
| ≥3 | 0.53 (0.50–0.57)* | 0.56 (0.53–0.59)* |
| Surgeon volume | ||
| Low | Referent | Referent |
| Intermediate | 0.94 (0.88–1.01) | 0.95 (0.91–1.00) |
| High | 1.10 (1.03–1.18)* | 1.25 (1.18–1.31)* |
| Hospital volume | ||
| Low | Referent | Referent |
| Intermediate | 1.54 (1.43–1.66)* | 1.48 (1.40–1.57)* |
| High | 2.36 (2.16–2.57)* | 2.17 (2.04–2.32)* |
p < 0.01
As seen in Table 4, rates of any immediate in-hospital complication were low, but highest for women undergoing flap reconstruction (6.1 % mastectomy alone vs. 4.0 % with implant reconstruction and 15.2 % with flap reconstruction) (p < 0.0001). The difference was largely driven by blood transfusion rates, which were 2.1, 0.8, and 8.4 %, respectively. Mean length of stay decreased over time (Fig. 2), and was highest for women with a flap reconstruction (3.4 days) as compared to implant (1.8 days) and mastectomy alone (1.7 days).
Table 4.
Morbidity and mortality associated with breast reconstruction
| No reconstruction
|
Implant reconstruction
|
Flap reconstruction
|
p value | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Any complication | 4770 | 6.1 | 769 | 4.0 | 1799 | 15.2 | <0.0001 |
| Perioperative complication (any) | 826 | 1.1 | 159 | 0.8 | 297 | 2.5 | <0.0001 |
| Abscess | 507 | 0.7 | 74 | 0.4 | 171 | 1.4 | <0.0001 |
| Wound complication | 200 | 0.3 | 47 | 0.3 | 92 | 0.8 | <0.0001 |
| Operative Injury | 144 | 0.2 | 40 | 0.2 | 40 | 0.3 | 0.003 |
| Medical complication (any) | 1722 | 2.2 | 319 | 1.7 | 600 | 5.1 | <0.0001 |
| Venous thromboembolism | 234 | 0.3 | 33 | 0.2 | 67 | 0.6 | <0.0001 |
| Respiratory failure | 681 | 0.9 | 214 | 1.1 | 434 | 3.7 | <0.0001 |
| Renal failure | 275 | 0.4 | 14 | 0.1 | 38 | 0.3 | <0.0001 |
| Pneumonia | 205 | 0.3 | 17 | 0.1 | 37 | 0.3 | <0.0001 |
| Myocardial Infarction | 86 | 0.1 | 2 | 0.01 | 6 | 0.05 | <0.001 |
| Cardiopulmonary arrest | 55 | 0.1 | 3 | 0.02 | 4 | 0.03 | 0.01 |
| Shock | 413 | 0.5 | 62 | 0.3 | 73 | 0.6 | 0.0003 |
| Stroke | 114 | 0.2 | 3 | 0.02 | 7 | 0.06 | <0.0001 |
| Bacteremia/sepsis | 107 | 0.1 | 4 | 0.02 | 22 | 0.2 | <0.0001 |
| Process measures | |||||||
| Transfusion | 1594 | 2.1 | 159 | 0.8 | 988 | 8.4 | <0.0001 |
| Length of stay >2 days | 10,936 | 14.0 | 3,709 | 19.5 | 8,206 | 69.4 | <0.0001 |
| Perioperative death | 84 | 0.1 | 4 | 0.02 | 5 | 0.04 | 0.0003 |
Fig. 2.

Annual a length of stay and b initial hospital costs of mastectomy with and without immediate reconstruction. Costs are adjusted to 2010 dollars
The mean hospital costs over the 10-year period for women undergoing mastectomy alone was $5,724, whereas the mean costs for women undergoing mastectomy and flap reconstruction was $15,866. For those who underwent implants, the mean charges were $11,602. The hospital costs associated with mastectomy and either type of reconstruction increased at a greater pace over time than the cost of mastectomy alone (Fig. 2).
Discussion
Despite the reported benefits, our findings suggest that the rate of immediate reconstruction following a mastectomy for breast cancer remains low for women with both IBC (28 %) and DCIS (44 %) for the decade following the signing of the Women’s Health and Cancer Rights Act. Reassuringly, however, the rates have increased significantly over time, specifically in young women and women with commercial insurance. The likelihood of receiving this procedure still appears to be strongly influenced by modifiable factors, such as insurance status and physician and hospital characteristics. These findings are particularly important given the recent increase in the number of women undergoing mastectomy, and the increasing cost of immediate reconstruction over time [24, 25]. While flap reconstruction is associated with higher hospital costs and an increased mean length of stay, mean length of stay has decreased over time. In addition, while the acute complication rates and transfusion rates are higher for flap reconstruction, complications rates were similar for mastectomy alone and mastectomy with implant reconstruction, which may be related to patient selection factors
To date, there has been only one small randomized trial comparing immediate reconstruction to delayed reconstruction [1], which was limited by reporting bias, lack of inclusion of patient reported outcomes, and a limited number of patients for whom delayed reconstruction was performed [26]. Despite this, guidelines suggest that immediate reconstruction is safe, well accepted by patients and should be offered to all women because of its cosmetic and psychosocial benefits [6]. Furthermore, over the past 30 years, advances in breast reconstructive techniques have expanded the choices for women undergoing mastectomy. Options include autologous tissue flaps and implants with tissue expanders. Each reconstructive technique has its own advantages and limitations; however, it is clear that the cosmetic outcome with each has improved over time, and is superior to delayed reconstruction. Some debate exists, however, on the best timing for women undergoing post-mastectomy radiation therapy [27]. In addition, there are fewer surgical procedures and hospital admissions with immediate compared to delayed reconstruction, resulting in lower health care costs [28].
We found that commercial insurance had a strong influence on receipt of post-mastectomy reconstruction, which may have been influenced by the establishment of the WHCRA. Women with commercial insurance had a three-fold higher likelihood of undergoing immediate reconstruction compared to those without insurance. This is consistent with a study from California in which decreased reconstruction rates were observed in patients with Medical public insurance [11]. We also found that the influence of insurance status on receipt of reconstruction has increased over time as have the hospital costs of reconstructive surgery with mastectomy, which increased at a steeper rate than the cost of mastectomy alone. Treatment costs influence receipt of care. In a population-based study, evaluating guideline-based cancer therapy, adherence to guidelines was particularly low for patients with Medicaid or Medicare only, compared to commercial insurance [29]. Furthermore, the risks of high out-of-pocket financial burdens are significantly greater for patients with cancer compared with other chronically ill and well patients, and these high burdens may affect treatment choice and deter patients from getting care [30]. The association between socioeconomic status and a patient’s receipt of guideline-based cancer care as well as survival outcome is consistent [31-34], further supporting the influence of high cancer care costs on the administration of optimal cancer care. It is unclear why hospital costs of reconstruction have increased so sharply given the overall decrease in length of stay over time.
We were surprised to see that the rate of immediate reconstruction in women under age 50 was only 51 % given that the distress and changes in body image and sexuality may be more pronounced in younger women [35]. Young adults with cancer may be a particularly vulnerable group [36-38]. Patients in this age group have the lowest rates of health insurance coverage, frequent delays in diagnosis, and the lowest accrual to clinical trials [37]. Against this background, young adults with cancer have unique challenges—medically, psychosocially, and economically—that are now beginning to be appreciated and addressed, which may result in improved treatment quality [38]. Reassuringly, by 2009–2010 rates of reconstruction were highest amongst young women with DCIS who had commercial insurance at 79 %.
We have identified a number of other modifiable factors associated with breast reconstruction. The hospital setting in which patients received care and the volume of the surgeon who performed the mastectomy also had a strong impact on the allocation of care. Women treated at large facilities (more beds) with higher procedure rates and at non-teaching hospitals were more likely to undergo reconstruction while those treated at rural hospitals were more than 50 % less likely to undergo immediate reconstruction. We also found regional variation in the use of IR, which has been found in other studies, and may represent reimbursement policy, education, and access [39]. Patients treated by physicians with high volume are more likely to undergo immediate reconstruction compared to surgeons with low volume. A recent report from a survey mailed to plastic surgeons found that perceived financial constraints by third-party payers was inversely associated with the volume of breast reconstructive surgeries [40]. In other settings, hospital and surgeon characteristics have also been associated with the quality of care a patient receives [21, 41, 42]. These factors are modifiable, and educational interventions should be assessed to improve access to optimal care [43].
Like other studies [11-13], we found that black women were significantly less likely than white women to undergo reconstruction. This was true for both younger and older women. In a study by Alderman et al. [13], minority women were significantly less likely to see a plastic surgeon prior to surgery and to desire more information about reconstruction. Other investigators have also reported that lack of knowledge and a greater perception of barriers to the procedure were more common among African-American patients [14]. Prior research has shown that the quality of the hospital as measured by the number of patients that receive guideline care, and the hospital volume explain some of the reported racial disparities in the receipt of definitive breast cancer therapy [44].
We recognize several important limitations in our study. While the Perspective database contains a very large sample of women from throughout the US, the data have a relatively higher proportion of patients treated at small to mid-size, non-teaching, urban facilities. As such, our findings may not be generalizable to the entire US healthcare system. Perspective lacks data on tumor characteristics such as histology, grade, and stage, all of which are known to influence reconstruction rates, and are likely the reason for lower rates of IR in women with invasive cancer compared to women with DCIS. Post-mastectomy radiation may have decreased the likelihood of women with invasive cancer undergoing IR; however, the predictors of IR were identical in women with DCIS, where post-mastectomy radiation is not performed. We also note that the complications captured are only during the initial hospitalization and underestimate total complications, as many occur after discharge. The cost data were obtained from the initial hospitalization, but does not include costs associated with implant insertion that may occur at a later timepoint, or does it differentiate more specifics related to types of flap reconstruction. While the duration of recovery and risks of complications are significant concerns, many patients report that they ultimately follow their surgeon’s recommendations [40]. Even with patient reports, differentiating the influence of the surgeon and setting from patient choice can be difficult. Finally, there is no information on delayed reconstruction; however, studies suggest that the rates are only about 5 % [11].
While using immediate post-mastectomy reconstruction has increased over the past 10 years, the overall rates still remain somewhat low, with 28 % of all women, and 51 % of women <50 years receiving immediate reconstruction. Insurance status is one of the strongest predictors of immediate reconstruction, and its influence in the likelihood of undergoing reconstruction has increased over time. Furthermore, because these factors are modifiable, interventions could be assessed to improve the quality of care in hospitals with a low volume of procedures, increase access for women who live in rural areas and among surgeons with few cancer procedures. In New York state, one such intervention was a law passed in 2010 (A10094B/S6993-B) that states that “every hospital performing breast surgical procedures must provide information in writing to patients prior to any breast cancer surgery, explaining the reconstructive options and the advantages and disadvantages of each.” It will be important to determine if interventions such as this influence reconstruction rates.
Acknowledgments
Dr. Hershman is the recipient of a Grant from the National Cancer Institute (NCI R01 CA134964). Ms. Richards is the recipient of a T32 fellowship (NCI CA09529).
Footnotes
Conflict of interest The authors have no potential conflicts of interest to report.
Contributor Information
D. L. Hershman, Email: dlh23@columbia.edu, Department of Medicine, Columbia University College of Physicians and Surgeons, Columbia University Medical Center, 161 Fort Washington Avenue, 10-1068, New York, NY 10032, USA; Department of Epidemiology, Columbia University Mailman School of Public Health, New York, NY, USA; Herbert Irving Comprehensive Cancer Center, Columbia University College of Physicians and Surgeons, New York, NY, USA.
C. A. Richards, Department of Epidemiology, Columbia University Mailman School of Public Health, New York, NY, USA
K. Kalinsky, Department of Medicine, Columbia University College of Physicians and Surgeons, Columbia University Medical Center, 161 Fort Washington Avenue, 10-1068, New York, NY 10032, USA Herbert Irving Comprehensive Cancer Center, Columbia University College of Physicians and Surgeons, New York, NY, USA.
E. T. Wilde, Department of Health Policy and Management, Columbia University Mailman School of Public Health, New York, NY, USA
Y. S. Lu, Department of Obstetrics and Gynecology, Columbia University College of Physicians and Surgeons, New York, NY, USA
J. A. Ascherman, Division of Plastic Surgery, Columbia University College of Physicians and Surgeons, New York, NY, USA
A. I. Neugut, Department of Medicine, Columbia University College of Physicians and Surgeons, Columbia University Medical Center, 161 Fort Washington Avenue, 10-1068, New York, NY 10032, USA Department of Epidemiology, Columbia University Mailman School of Public Health, New York, NY, USA; Herbert Irving Comprehensive Cancer Center, Columbia University College of Physicians and Surgeons, New York, NY, USA.
J. D. Wright, Herbert Irving Comprehensive Cancer Center, Columbia University College of Physicians and Surgeons, New York, NY, USA Department of Obstetrics and Gynecology, Columbia University College of Physicians and Surgeons, New York, NY, USA.
References
- 1.Dean C, Chetty U, Forrest AP. Effects of immediate breast reconstruction on psychosocial morbidity after mastectomy. Lancet. 1983;1(8322):459–462. doi: 10.1016/s0140-6736(83)91452-6. [DOI] [PubMed] [Google Scholar]
- 2.Wilkins EG, Cederna PS, Lowery JC, Davis JA, Kim HM, Roth RS, Goldfarb S, Izenberg PH, Houin HP, Shaheen KW. Prospective analysis of psychosocial outcomes in breast reconstruction: one-year postoperative results from the Michigan Breast Reconstruction Outcome Study. Plast Reconstr Surg. 2000;106(5):1014–1025. doi: 10.1097/00006534-200010000-00010. discussion 1026–1017. [DOI] [PubMed] [Google Scholar]
- 3.Alderman AK, Wilkins EG, Lowery JC, Kim M, Davis JA. Determinants of patient satisfaction in postmastectomy breast reconstruction. Plast Reconstr Surg. 2000;106(4):769–776. doi: 10.1097/00006534-200009040-00003. [DOI] [PubMed] [Google Scholar]
- 4.Atisha D, Alderman AK, Lowery JC, Kuhn LE, Davis J, Wilkins EG. Prospective analysis of long-term psychosocial outcomes in breast reconstruction: two-year postoperative results from the Michigan Breast Reconstruction Outcomes Study. Ann Surg. 2008;247(6):1019–1028. doi: 10.1097/SLA.0b013e3181728a5c. [DOI] [PubMed] [Google Scholar]
- 5.Al-Ghazal SK, Sully L, Fallowfield L, Blamey RW. The psychological impact of immediate rather than delayed breast reconstruction. Eur J Surg Oncol. 2000;26(1):17–19. doi: 10.1053/ejso.1999.0733. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez-Frias AM, Aguilar J, Sanchez JA, Merck B, Pinero A, Calpena R. Immediate reconstruction after mastectomy for breast cancer: which factors affect its course and final outcome? J Am Coll Surg. 2009;208(1):126–133. doi: 10.1016/j.jamcollsurg.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Petit JY, Gentilini O, Rotmensz N, Rey P, Rietjens M, Garusi C, Botteri E, De Lorenzi F, Martella S, Bosco R, et al. Oncological results of immediate breast reconstruction: long term follow-up of a large series at a single institution. Breast Cancer Res Treat. 2008;112(3):545–549. doi: 10.1007/s10549-008-9891-x. [DOI] [PubMed] [Google Scholar]
- 8.Morrow M, Scott SK, Menck HR, Mustoe TA, Winchester DP. Factors influencing the use of breast reconstruction post-mastectomy: a National Cancer Database study. J Am Coll Surg. 2001;192(1):1–8. doi: 10.1016/s1072-7515(00)00747-x. [DOI] [PubMed] [Google Scholar]
- 9.Reuben BC, Manwaring J, Neumayer LA. Recent trends and predictors in immediate breast reconstruction after mastectomy in the United States. Am J Surg. 2009;198(2):237–243. doi: 10.1016/j.amjsurg.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 10.Christian CK, Niland J, Edge SB, Ottesen RA, Hughes ME, Theriault R, Wilson J, Hergrueter CA, Weeks JC. A multi-institutional analysis of the socioeconomic determinants of breast reconstruction: a study of the National Comprehensive Cancer Network. Ann Surg. 2006;243(2):241–249. doi: 10.1097/01.sla.0000197738.63512.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kruper L, Holt A, Xu XX, Duan L, Henderson K, Bernstein L, Ellenhorn J. Disparities in reconstruction rates after mastectomy: patterns of care and factors associated with the use of breast reconstruction in Southern California. Ann Surg Oncol. 2011;18:2158–2165. doi: 10.1245/s10434-011-1580-z. [DOI] [PubMed] [Google Scholar]
- 12.Tseng JF, Kronowitz SJ, Sun CC, Perry AC, Hunt KK, Babiera GV, Newman LA, Singletary SE, Mirza NQ, Ames FC, et al. The effect of ethnicity on immediate reconstruction rates after mastectomy for breast cancer. Cancer. 2004;101(7):1514–1523. doi: 10.1002/cncr.20529. [DOI] [PubMed] [Google Scholar]
- 13.Alderman AK, Hawley ST, Janz NK, Mujahid MS, Morrow M, Hamilton AS, Graff JJ, Katz SJ. Racial and ethnic disparities in the use of postmastectomy breast reconstruction: results from a population-based study. J Clin Oncol. 2009;27(32):5325–5330. doi: 10.1200/JCO.2009.22.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrow M, Mujahid M, Lantz PM, Janz NK, Fagerlin A, Schwartz K, Liu L, Deapen D, Salem B, Lakhani I, et al. Correlates of breast reconstruction: results from a population-based study. Cancer. 2005;104(11):2340–2346. doi: 10.1002/cncr.21444. [DOI] [PubMed] [Google Scholar]
- 15.Lagu T, Rothberg MB, Nathanson BH, Pekow PS, Steingrub JS, Lindenauer PK. The relationship between hospital spending and mortality in patients with sepsis. Arch Intern Med. 2011;171(4):292–299. doi: 10.1001/archinternmed.2011.12. [DOI] [PubMed] [Google Scholar]
- 16.Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353(4):349–361. doi: 10.1056/NEJMoa041895. [DOI] [PubMed] [Google Scholar]
- 17.Lindenauer PK, Pekow PS, Lahti MC, Lee Y, Benjamin EM, Rothberg MB. Association of corticosteroid dose and route of administration with risk of treatment failure in acute exacerbation of chronic obstructive pulmonary disease. JAMA. 2010;303(23):2359–2367. doi: 10.1001/jama.2010.796. [DOI] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 20.Hollenbeck BK, Wei Y, Birkmeyer JD. Volume, process of care, and operative mortality for cystectomy for bladder cancer. Urology. 2007;69(5):871–875. doi: 10.1016/j.urology.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birkmeyer JD, Sun Y, Wong SL, Stukel TA. Hospital volume and late survival after cancer surgery. Ann Surg. 2007;245(5):777–783. doi: 10.1097/01.sla.0000252402.33814.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rothberg MB, Pekow PS, Lahti M, Brody O, Skiest DJ, Lindenauer PK. Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA. 2010;303(20):2035–2042. doi: 10.1001/jama.2010.672. [DOI] [PubMed] [Google Scholar]
- 23.US Department of Labor Bureau of Labor Statistics Consumer Price Index. [September 1, 2011];2011 ftp://ftp.bls.gov/pub/special.requests/cpi/cpiai.txt.
- 24.Tuttle TM, Jarosek S, Habermann EB, Arrington A, Abraham A, Morris TJ, Virnig BA. Increasing rates of contralateral prophylactic mastectomy among patients with ductal carcinoma in situ. J Clin Oncol. 2009;27(9):1362–1367. doi: 10.1200/JCO.2008.20.1681. [DOI] [PubMed] [Google Scholar]
- 25.Tuttle TM, Habermann EB, Grund EH, Morris TJ, Virnig BA. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol. 2007;25(33):5203–5209. doi: 10.1200/JCO.2007.12.3141. [DOI] [PubMed] [Google Scholar]
- 26.D’Souza N, Darmanin G, Fedorowicz Z. Immediate versus delayed reconstruction following surgery for breast cancer. Cochrane Database Syst Rev. 2011;7 doi: 10.1002/14651858.CD008674.pub2. CD008674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marin-Gutzke M, Sanchez-Olaso A. Reconstructive surgery in young women with breast cancer. Breast Cancer Res Treat. 2010;123(Suppl 1):67–74. doi: 10.1007/s10549-010-1127-1. [DOI] [PubMed] [Google Scholar]
- 28.Neyt MJ, Blondeel PN, Morrison CM, Albrecht JA. Comparing the cost of delayed and immediate autologous breast reconstruction in Belgium. Br J Plast Surg. 2005;58(4):493–497. doi: 10.1016/j.bjps.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Harlan LC, Greene AL, Clegg LX, Mooney M, Stevens JL, Brown ML. Insurance status and the use of guideline therapy in the treatment of selected cancers. J Clin Oncol. 2005;23(36):9079–9088. doi: 10.1200/JCO.2004.00.1297. [DOI] [PubMed] [Google Scholar]
- 30.Bernard DS, Farr SL, Fang Z. National estimates of out-of-pocket health care expenditure burdens among nonelderly adults with cancer: 2001 to 2008. J Clin Oncol. 2010;29(20):2821–2826. doi: 10.1200/JCO.2010.33.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du XL, Fang S, Vernon SW, El-Serag H, Shih YT, Davila J, Rasmus ML. Racial disparities and socioeconomic status in association with survival in a large population-based cohort of elderly patients with colon cancer. Cancer. 2007;110(3):660–669. doi: 10.1002/cncr.22826. [DOI] [PubMed] [Google Scholar]
- 32.Sprague BL, Trentham-Dietz A, Gangnon RE, Ramchandani R, Hampton JM, Robert SA, Remington PL, Newcomb PA. Socioeconomic status and survival after an invasive breast cancer diagnosis. Cancer. 2011;117(7):1542–1551. doi: 10.1002/cncr.25589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McBride RB, Lebwohl B, Hershman DL, Neugut AI. Impact of socioeconomic status on extent of lymph node dissection for colon cancer. Cancer Epidemiol Biomarkers Prev. 2010;19(3):738–745. doi: 10.1158/1055-9965.EPI-09-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mackillop WJ, Zhang-Salomons J, Groome PA, Paszat L, Holowaty E. Socioeconomic status and cancer survival in Ontario. J Clin Oncol. 1997;15(4):1680–1689. doi: 10.1200/JCO.1997.15.4.1680. [DOI] [PubMed] [Google Scholar]
- 35.Fobair P, Stewart SL, Chang S, D’Onofrio C, Banks PJ, Bloom JR. Body image and sexual problems in young women with breast cancer. Psychooncology. 2006;15(7):579–594. doi: 10.1002/pon.991. [DOI] [PubMed] [Google Scholar]
- 36.Zebrack B, Bleyer A, Albritton K, Medearis S, Tang J. Assessing the health care needs of adolescent and young adult cancer patients and survivors. Cancer. 2006;107(12):2915–2923. doi: 10.1002/cncr.22338. [DOI] [PubMed] [Google Scholar]
- 37.Bleyer A, Barr R. Cancer in young adults 20 to 39 years of age: overview. Semin Oncol. 2009;36(3):194–206. doi: 10.1053/j.seminoncol.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Bleyer A. Young adult oncology: the patients and their survival challenges. CA Cancer J Clin. 2007;57(4):242–255. doi: 10.3322/canjclin.57.4.242. [DOI] [PubMed] [Google Scholar]
- 39.Agarwal S, Pappas L, Neumayer L, Agarwal J. An analysis of immediate postmastectomy breast reconstruction frequency using the surveillance, epidemiology, and end results database. Breast J. 2011;17(4):352–358. doi: 10.1111/j.1524-4741.2011.01105.x. [DOI] [PubMed] [Google Scholar]
- 40.Alderman AK, Atisha D, Streu R, Salem B, Gay A, Abrahamse P, Hawley ST. Patterns and correlates of postmastectomy breast reconstruction by US plastic surgeons: results from a national survey. Plast Reconstr Surg. 2011;127(5):1796–1803. doi: 10.1097/PRS.0b013e31820cf183. [DOI] [PubMed] [Google Scholar]
- 41.Schrag D, Cramer LD, Bach PB, Cohen AM, Warren JL, Begg CB. Influence of hospital procedure volume on outcomes following surgery for colon cancer. JAMA. 2000;284(23):3028–3035. doi: 10.1001/jama.284.23.3028. [DOI] [PubMed] [Google Scholar]
- 42.Liu JH, Zingmond DS, McGory ML, SooHoo NF, Ettner SL, Brook RH, Ko CY. Disparities in the utilization of high-volume hospitals for complex surgery. JAMA. 2006;296(16):1973–1980. doi: 10.1001/jama.296.16.1973. [DOI] [PubMed] [Google Scholar]
- 43.Bilimoria KY, Bentrem DJ, Feinglass JM, Stewart AK, Winchester DP, Talamonti MS, Ko CY. Directing surgical quality improvement initiatives: comparison of perioperative mortality and long-term survival for cancer surgery. J Clin Oncol. 2008;26(28):4626–4633. doi: 10.1200/JCO.2007.15.6356. [DOI] [PubMed] [Google Scholar]
- 44.Keating NL, Kouri E, He Y, Weeks JC, Winer EP. Racial differences in definitive breast cancer therapy in older women: are they explained by the hospitals where patients undergo surgery? Med Care. 2009;47(7):765–773. doi: 10.1097/MLR.0b013e31819e1fe7. [DOI] [PubMed] [Google Scholar]


