Abstract
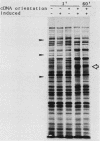
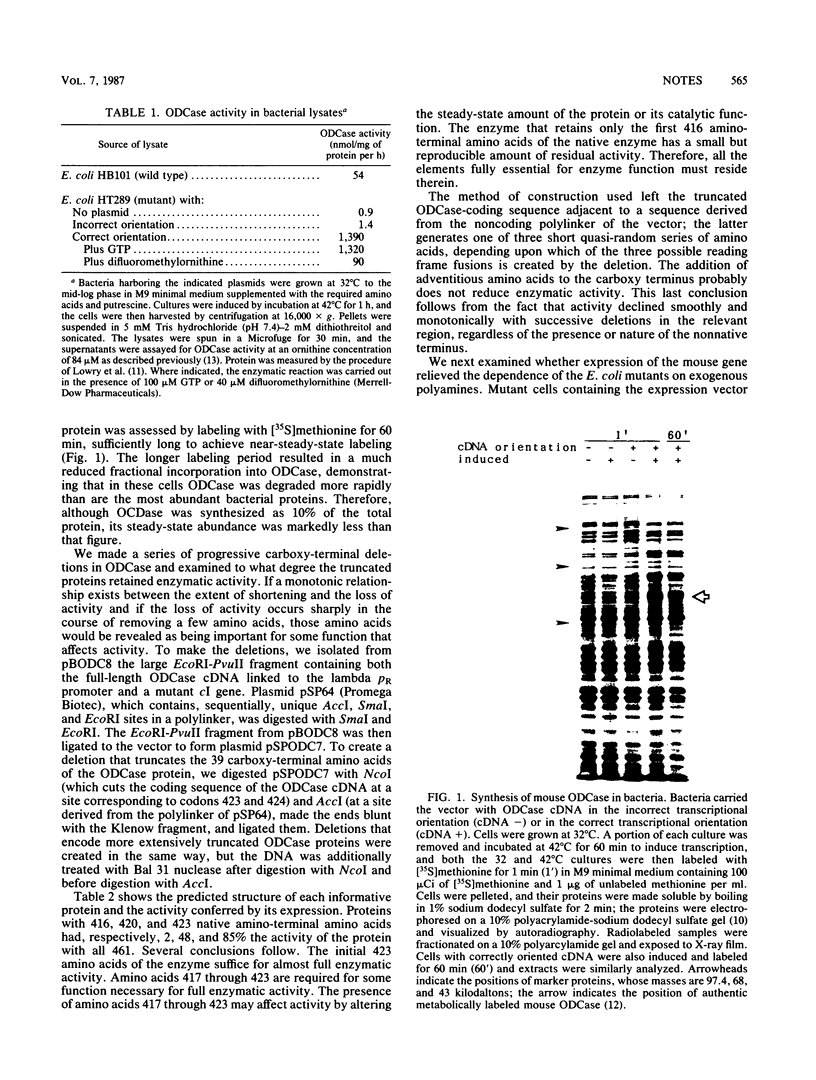
Mouse ornithine decarboxylase (ODCase) cDNA was expressed at a high level in an Escherichia coli mutant deficient in polyamine biosynthesis. The expression of mouse ornithine decarboxylase relieved the dependence of the mutant on an exogenous source of polyamines, presumably by providing putrescine, the product of the enzyme. The effect on the enzymatic activity of deletions that removed carboxy-terminal amino acids of the protein was determined.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Applebaum D. M., Dunlap J. C., Morris D. R. Comparison of the biosynthetic and biodegradative ornithine decarboxylases of Escherichia coli. Biochemistry. 1977 Apr 19;16(8):1580–1584. doi: 10.1021/bi00627a008. [DOI] [PubMed] [Google Scholar]
- Boucek R. J., Jr, Lembach K. J. Purification by affinity chromatography and preliminary characterization of ornithine decarboxylase from simian virus 40-transformed 3T3 mouse fibroblasts. Arch Biochem Biophys. 1977 Dec;184(2):408–415. doi: 10.1016/0003-9861(77)90368-x. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Nunberg J. H., Kaufman R. J., Erlich H. A., Schimke R. T., Cohen S. N. Phenotypic expression in E. coli of a DNA sequence coding for mouse dihydrofolate reductase. Nature. 1978 Oct 19;275(5681):617–624. doi: 10.1038/275617a0. [DOI] [PubMed] [Google Scholar]
- DasSarma S., Tischer E., Goodman H. M. Plant glutamine synthetase complements a glnA mutation in Escherichia coli. Science. 1986 Jun 6;232(4755):1242–1244. doi: 10.1126/science.2871626. [DOI] [PubMed] [Google Scholar]
- Fonzi W. A., Sypherd P. S. Expression of the gene for ornithine decarboxylase of Saccharomyces cerevisiae in Escherichia coli. Mol Cell Biol. 1985 Jan;5(1):161–166. doi: 10.1128/mcb.5.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta M., Coffino P. Mouse ornithine decarboxylase. Complete amino acid sequence deduced from cDNA. J Biol Chem. 1985 Mar 10;260(5):2941–2944. [PubMed] [Google Scholar]
- Hafner E. W., Tabor C. W., Tabor H. Mutants of Escherichia coli that do not contain 1,4-diaminobutane (putrescine) or spermidine. J Biol Chem. 1979 Dec 25;254(24):12419–12426. [PubMed] [Google Scholar]
- Kahana C., Nathans D. Nucleotide sequence of murine ornithine decarboxylase mRNA. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1673–1677. doi: 10.1073/pnas.82.6.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallio A., McCann P. P., Bey P. DL-a-Monofluoromethylputrescine is a potent irreversible inhibitor of Escherichia coli ornithine decarboxylase. Biochem J. 1982 Jun 15;204(3):771–775. doi: 10.1042/bj2040771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McConlogue L., Coffino P. A mouse lymphoma cell mutant whose major protein product is ornithine decarboxylase. J Biol Chem. 1983 Oct 25;258(20):12083–12086. [PubMed] [Google Scholar]
- McConlogue L., Coffino P. Ornithine decarboxylase in difluoromethylornithine-resistant mouse lymphoma cells. Two-dimensional gel analysis of synthesis and turnover. J Biol Chem. 1983 Jul 10;258(13):8384–8388. [PubMed] [Google Scholar]
- McConlogue L., Gupta M., Wu L., Coffino P. Molecular cloning and expression of the mouse ornithine decarboxylase gene. Proc Natl Acad Sci U S A. 1984 Jan;81(2):540–544. doi: 10.1073/pnas.81.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., McCann P. P. Polyamine metabolism and function. Am J Physiol. 1982 Nov;243(5):C212–C221. doi: 10.1152/ajpcell.1982.243.5.C212. [DOI] [PubMed] [Google Scholar]
- Queen C. A vector that uses phage signals for efficient synthesis of proteins in Escherichia coli. J Mol Appl Genet. 1983;2(1):1–10. [PubMed] [Google Scholar]
- Russell D. H., Snyder S. H. Amine synthesis in regenerating rat liver: effect of hypophysectomy and growth hormone on ornithine decarboxylase. Endocrinology. 1969 Feb;84(2):223–228. doi: 10.1210/endo-84-2-223. [DOI] [PubMed] [Google Scholar]
- Seely J. E., Pösö H., Pegg A. E. Measurement of the number of ornithine decarboxylase molecules in rat and mouse tissues under various physiological conditions by binding of radiolabelled alpha-difluoromethylornithine. Biochem J. 1982 Aug 15;206(2):311–318. doi: 10.1042/bj2060311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely J. E., Pösö H., Pegg A. E. Purification of ornithine decarboxylase from kidneys of androgen-treated mice. Biochemistry. 1982 Jul 6;21(14):3394–3399. doi: 10.1021/bi00257a023. [DOI] [PubMed] [Google Scholar]
- Struhl K., Cameron J. R., Davis R. W. Functional genetic expression of eukaryotic DNA in Escherichia coli. Proc Natl Acad Sci U S A. 1976 May;73(5):1471–1475. doi: 10.1073/pnas.73.5.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines in microorganisms. Microbiol Rev. 1985 Mar;49(1):81–99. doi: 10.1128/mr.49.1.81-99.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor H., Tabor C. W., Cohn M. S., Hafner E. W. Streptomycin resistance (rpsL) produces an absolute requirement for polyamines for growth of an Escherichia coli strain unable to synthesize putrescine and spermidine [delta(speA-speB) delta specC]. J Bacteriol. 1981 Aug;147(2):702–704. doi: 10.1128/jb.147.2.702-704.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor H., Tabor C. W. Polyamine requirement for efficient translation of amber codons in vivo. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7087–7091. doi: 10.1073/pnas.79.23.7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S. R., Goddard J. M., Martin D. W., Jr Human purine nucleoside phosphorylase cDNA sequence and genomic clone characterization. Nucleic Acids Res. 1984 Jul 25;12(14):5779–5787. doi: 10.1093/nar/12.14.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung C. Y., Ingolia D. E., Roth D. B., Shoemaker C., Al-Ubaidi M. R., Yen J. Y., Ching C., Bobonis C., Kaufman R. J., Kellems R. E. Identification of functional murine adenosine deaminase cDNA clones by complementation in Escherichia coli. J Biol Chem. 1985 Aug 25;260(18):10299–10307. [PubMed] [Google Scholar]