Abstract
Regulatory T (Treg) cells are considered to inhibit the development of both type 1 (Th1) and type 2 helper T (Th2) cells. However, it is recently reported that there are reduced numbers of Treg cells in patients with allergic diseases as compared with individuals who have high levels of serum immunoglobulin E and blood eosinophils but are asymptomatic. Therefore, Treg cells may suppress the onset of allergic disease by down-regulating other types of immune cells besides Th1 and Th2 cells. The newly discovered interleukin 17-producing helper T cells that are responsible for autoimmune inflammatory diseases may counteract Treg cells even in allergic diseases. The Th2 cells that are capable of producing of high levels of tumor necrosis factor-α may also be involved in inflammation in allergic diseases. In this review, we further discuss the role of Th1, Th2, interleukin 17-producing helper T cells, and Treg cells in allergic diseases by using the balancing square model and the factors differentiating between patients with clinical manifestations of allergic symptomatic and atopic individuals who are sensitized but asymptomatic.
Keywords: helper T cells, regulatory T cells, interleukin 17, mast cells, thymic stromal lymphopoietin
Th1/Th2 paradigm theorizing for allergic diseases is on the rise
There is an inverse correlation between the levels of endotoxin in house dust and the incidence of atopic sensitization and hay fever [1]. The major role of endotoxin is considered to be the stimulation of macrophages/antigen-presenting cells to produce interleukin 12 (IL-12), which triggers the development of antigen-specific type 1 helper T (Th1) cells and inhibits the development of type 2 helper T (Th2) cells. As such, the hygiene hypothesis associated with an increasing prevalence of allergic diseases has been theorized by the Th1/Th2 paradigm [2] since a long time.
Most epidemiological studies supporting the hygiene hypothesis also indicate that the preventive effect on allergy of an "unhygienic" environment surrounded by many microbial components is limited to early childhood [1]. According to the classic Th1/Th2 paradigm theory, this could be typically speculated as shown in Figure 1. A Th2-dominant immune system develops in an individual when the immune system is exposed to allergens without prior exposure to microbial components such as endotoxin early in life. On the other hand, the development of allergen (antigen)-specific Th1 cells is triggered by simultaneous exposure to the antigen and microbial components. After childhood, the proportion of Th1/Th2 cells is not drastically altered by microbial exposure because of a decrease in the number of naive helper T cells that can react with common allergens.
Figure 1.
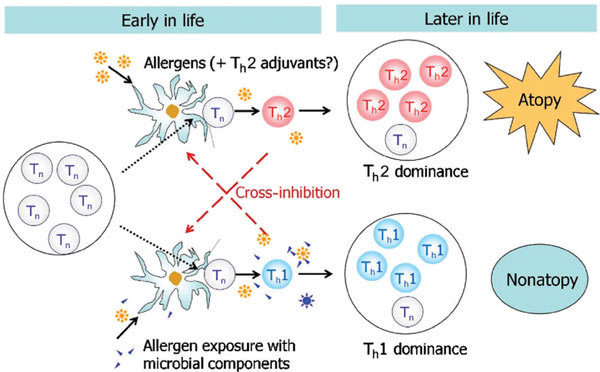
Environment surrounded by microbial components is effective only during early childhood. Only Tn's are present during infancy. The Th2-dominant immune system will develop when the immune system is exposed to allergens without microbial components such as endotoxin. Allergen-associated Th2 adjuvants such as prostaglandins may enhance Th2 development. On the other hand, antigen/allergen-specific Th1 cells can develop by simultaneous exposure to antigen/allergen and microbial components. After childhood, microbial exposure cannot drastically alter the proportion of Th1/Th2 balance because the proportion of Tn is decreased. Tn indicates naive helper T cells.
The incidence of Th1-mediated autoimmune diseases is known to have increased in the last half century in parallel with the increase of Th2-mediated allergic diseases [3]. The classic Th1/Th2 paradigm cannot be used to explain this point.
Roles of various regulatory T-cell populations
Several subsets of CD4+ cells are able to prevent immune responses against self-antigens or allergens. These cells are called regulatory T (Treg) cells. Because Treg cells inhibit both Th1- and Th2-cell development in vitro, increases in the incidence of Th1 diseases and Th2-mediated diseases are now thought to be related to an insufficient development of Treg cells. However, there is no evidence yet whether the "hygienic" environment with exposure to less microbial components during early life affects the development of Treg cells.
Among the several subsets, naturally occurring Treg (nTreg) cells have been well investigated. These cells originate in the thymus, express the repertoire of CD4+CD25+ (mouse) or CD4+CD25high (human) and the transcription factor forkhead box protein P3 (Foxp3), and have a major role in modulating the activity of self-reactive cells by inducing the destruction of autoreactive T cells mainly via cell-to-cell contact-dependent mechanisms [4]. Therefore, nTreg cells are considered to mainly have a preventive role in autoimmune diseases.
In humans, a population of CD4+CD25high T cells with regulatory function very similar to nTreg cells but derived from peripheral memory CD4+CD25- T cells has recently been described [5]. They are called adaptive Treg (aTreg) cells [6] or inducible Treg (iTreg) cells. One of iTreg-cell types is the IL-10-producing type 1 Treg (Tr1) cells, whose suppressive function has been well documented in allergy and autoimmunity [7]. The term "iTreg" is often used for the IL-10-producing Tr1, whereas the term aTreg is often used as CD4+CD25high T cells derived from peripheral memory CD4+CD25- T cells. Therefore, in this review, we have used the term aTreg as Foxp3+ CD4+CD25high T cells as shown in the recent review article written by Bacchetta et al [8]. Nevertheless, all subsets of Treg cells require a cytokine, transforming growth factor-β (TGF-β), for their development. The IL-10 is also produced not only by Tr1 cells, but also by various cell types including regulatory dendritic cells that can induce aTreg. [9] As such, these 2 cytokines play an important role in immune regulation.
Forkhead box protein P3 mutant mice develop an intense multiorgan inflammatory response, including allergic airway inflammation, striking hyperimmunoglobulinemia E, eosinophilia, and dysregulated Th1 and Th2 cytokine production [10,11]. In humans, genetic defects in Foxp3 cause immune dysregulation, polyendocrinopathy, and enteropathy, X-linked (IPEX) syndrome [12]. Most IPEX patients have food allergy and atopic dermatitis-related symptoms immediately after birth. It is thus suggested that Foxp3+ Treg cells play an important role in regulating common allergic disorders and IPEX. The number of Foxp3+ Treg cells is decreased in skin lesions in patients with atopic dermatitis and in patients with psoriasis [13].
Although Foxp3 is transiently expressed by antigen-activated helper T cells, [14] only persistent and high-level Foxp3 expression is related to the immunosuppressive functions.
IL-17-producing helper T cells that triggered the major revision for Th1/Th2 theory
A major role for the cytokine IL-17 has recently been described in various murine models of immune-mediated tissue injury, organ-specific autoimmunity, allergic disorders, and microbial infections. Interestingly, interferon-γ (IFN-γ) derived from Th1 cells often prevents the IL-17-mediated inflammation in mice with experimental autoimmune diseases [15,16]. A T-cell subpopulation that exclusively produces IL-17 (Th17) is now credited for causing and sustaining tissue damage. Human and mouse Th17 cells may require different sets of cytokines for their development. Thus, the identification of the Th17 cells triggered a shift in the immunologists' perspectives regarding the basis of tissue damage or auto-immune diseases, where for more than 20 years the role of Th1 cells was considered paramount [16].
However, it has been recently revealed that the expression of IL-17 in human CD4+ T cells may be completely different from that in mice [17]. Unlike mouse, human IL-6 and IL-21 do not induce IL-17 expression in either naive or effector T cells. The TGF-β inhibits human Th17-cell development but promotes mouse Th17-cell development when costimulated with IL-6 [17]. It should also be noted that in human adult peripheral blood, a large proportion of helper T cells can produce both IFN-γ and IL-4. The proportion of Th17 cells in peripheral blood CD4+ cells are consistently less than 1% in the peripheral blood of healthy individuals, and slightly higher among CD4+ T cells derived from patients with Crohn's disease [18]. Autologous Treg-cell clones suppress Th1 or Th2 cells, but not Th17 cells [18]. Nevertheless, IL-6 inhibits the development of both human and mouse Treg cells [19].
The key cytokine of Th17 cells, IL-17, is known to induce the production of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), IL-1β, IL-6, and proinflammatory chemokines CXCL1, 2, and 8 by acting on various cell types [20]. In humans, sputum IL-17A messenger RNA levels are significantly elevated in patients with asthma as compared with healthy controls [21]. Endogenous IL-17 contributes to the development of allergen-induced airway hyperresponsiveness, and there is also evidence that IL-17 stimulates the release of several cytokines with known capacity for airway remodeling from cells normally residing in the airways [22].
With the discovery of Th17 and Treg populations, the balancing square model is now needed to explain the pathogenesis of various immunological diseases. It also enables us to explain the epidemiological data demonstrating an increase in allergic diseases (Figure 2). According to this model, we speculate that substantial amounts of plant antigens, parasites, molds, viruses, and bacteria are required for balancing the total immune system (Figure 2). Autoimmune diseases are now considered to be initiated by an up-regulation of Th17 cells and a defect in nTreg cell function, whereas peritumor tissues are strikingly infiltrated with Foxp3+ nTreg cells implying that these cells impinge upon immunemediated rejection of the tumor [23].
Figure 2.
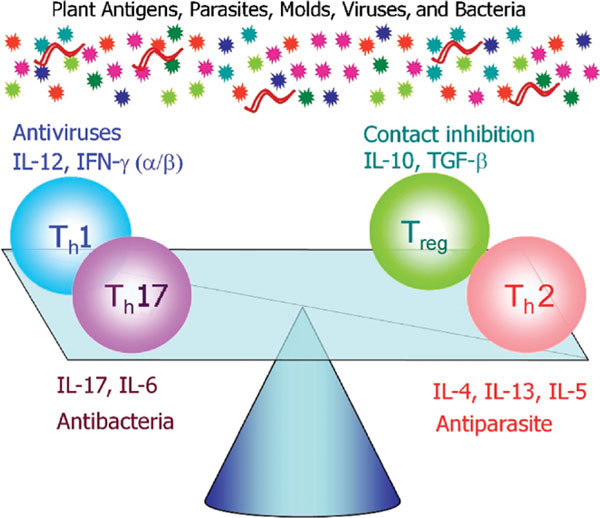
The balancing square model. The 4 T-cell types (Th1, Th2, Th17, and Treg) antagonize each other. The Th1-promoting cytokine, IL-12, is inhibitory to Th2-cell development, whereas the Th2-promoting cytokine, IL-4, blocks Th1-cell development. The Th1-derived IFN-γ blocks Th17-cell development. The Treg inhibits the development of both Th1 and Th2 cells by direct contact.
Role of aTreg cells in the onset of allergic diseases
Allergic diseases are caused by uncontrolled Th2-based immune responses to environmental antigens. It has been demonstrated that healthy nonatopic subjects have detectable IL-10-producing allergen-specific Tr1-like Treg cells, whereas the proportion of these Treg cells are very low in symptomatic allergic patients [24].
Several studies suggest a possible defect and impaired function of nTreg cells in the pathogenesis of immune responses toward allergens. However, like studies on auto-immune diseases, it is often difficult to distinguish the overlapping phenotypic characteristics between Treg cells and activated helper T cells [8].
We recently demonstrated that symptomatic atopic patients had a lower Foxp3+CD4+ ratio than asymptomatic controls having similar levels of serum IFN-γ, total immunoglobulin E (IgE), and eosinophils. These results suggest that circulating Foxp3+CD4+ cells regulate unknown factor(s) affecting the onset of allergic diseases, which are unrelated to these Th1/Th2 markers. Measurement of Foxp3+CD4+ cells has the potential to aid in evaluating the presence of active inflammation, which cannot be evaluated by known Th1- and Th2-related markers in patients with allergic diseases [25].
One of the many reasonable explanations for this observation is considered as follows, that is, down-regulation of Foxp3+ aTreg cells is often related to up-regulation of Th17 cells. In mice, IL-17 activates mast cells to release a proinflammatory cytokine, TNF-α, and thus can cause neutrophilic inflammation [26]. In humans, Th17 cells may enhance allergic inflammation by stimulating the tissue resident cells to release TNF-α, and are proven to evoke marked inflammation and airway remodeling. Using the balancing square model, the immunological features of symptomatic allergic diseases may be illustrated as in Figure 3A. Among Th2-cell subtypes, TNF-α-rich inflammatory Th2 (iTh2) cells may be developed by stimulation of dendritic cells with Th2 adjuvants associated with allergens or thymic stromal lymphopoietin (TSLP) often found in inflammatory tissues in allergic diseases [27]. Thus, iTh2 and Th17 cells can be both up-regulated in symptomatic allergic patients, where mast cells are also activated. On the other hand, in asymptomatic controls having similar high levels of IgE and eosinophils, both Th2 and aTreg cells may be up-regulated. Because of Th17 cells, the levels of IFN-γ may be kept at considerably high levels. However, these patients are often infected with viruses at the site of inflammatory tissues because of down-regulation of classic Th1 cells [28] capable of producing antiviral cytokine IFN-α.
Figure 3.
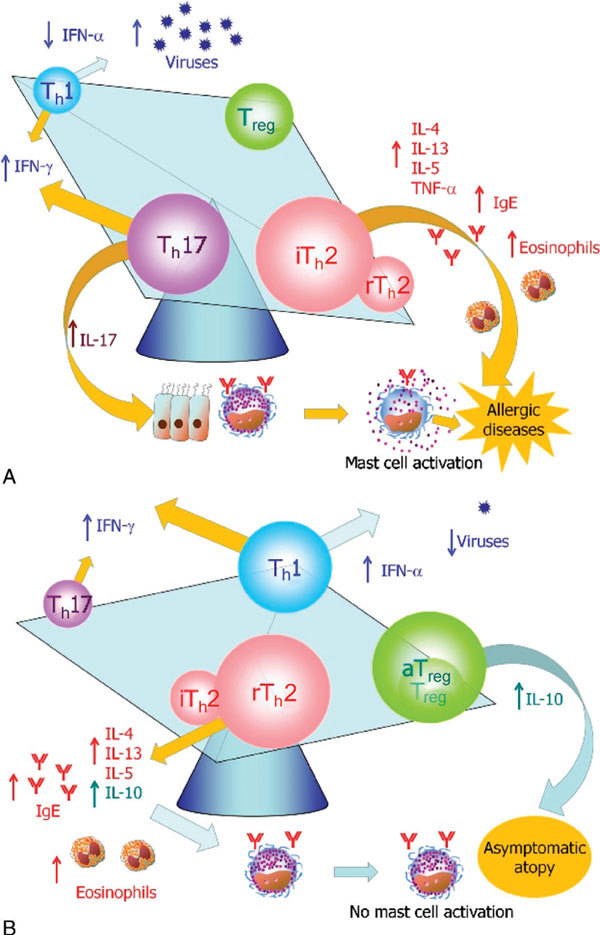
Onset of allergic diseases may be determined by the ratio of proinflammatory T-cell subsets (Th17 and iTh2) versus Treg subsets. A, In patients with chronic allergic diseases, proinflammatory T-cell subsets, that is, Th17 cells and Th2 cells that are capable of producing high levels of TNF-α (iTh2 cells) are upregulated. B, In asymptomatic atopic individuals, Th2 cells that are capable of producing IL-10 (rTh2 cells) may be up-regulated, and Th17 cells may be inactivated.
Among Th2 subsets, IL-10-producing Treg cells may be up-regulated in asymptomatic atopic individuals. Mast cells are therefore not activated, despite the presence of high levels of IgE antibodies and constant exposure to common allergens as shown in Figure 3B. Nevertheless, it will be necessary to determine why some people do not have marked mast cell activation even though they are sensitized to multiple allergens.
The nTreg cells can suppress not only T cells, but also natural killer cells, dendritic cell maturation, and antibody production by B cells [29]. Recently, mast cells and nTreg-derived IL-9 are found indispensable in nTreg-mediated peripheral tolerance to allograft transplantation in a mouse model [30]. Pollen immunotherapy that is known to induce allergen-specific aTreg or Tr1 cells inhibits seasonal increases in IL-9 protein expression and c-Kit+ mast cell infiltration in the nasal mucosa during the pollen season [31]. It is of particular interest to investigate a further relationship between aTreg and mast cells in future studies.
Conclusion
An atopic predisposition is acquired via up-regulation of Th2 cells compared with IFN-γ-producing helper T cells (Th1 cells and Th17 cells) specific for each allergen as shown in Figure 1. Numerous epidemiological studies indicate that microbial components affect the balance between these T-cell types [1]. It should be noted, however, that most of the people who acquired an atopic predisposition in a hygienic environment are still asymptomatic or having very mild symptoms [25]. At present, we have no answer as to why some further develop the clinical manifestations of allergic disease, whereas others remain asymptomatic.
We have reported that active atopic patients had a lower Foxp3+CD4+ ratio than asymptomatic controls having similar levels of serum IFN-γ, total IgE, and eosinophils, [25] suggesting that the development of clinical manifestations of allergic diseases may be determined by the ratio of proinflammatory T-cell subsets (Th17 and iTh2) versus Treg subsets. Increased ratio of proinflammatory cytokines (IL-17, TSLP, and IL-6) versus regulatory cytokines (IL-10 and TGF-β) in severe allergic diseases [8,19,20,27] would activate further the balance shift and form a positive feedback loop in chronic inflammation (Figure 4). Nevertheless, we will have to identify the factors influencing the balance shift of proinflammatory and regulatory T cell population.
Figure 4.
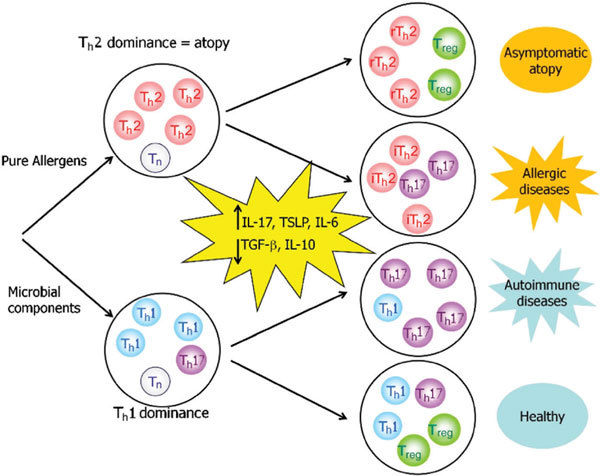
A model for development of allergic and autoimmune diseases in the absence of microbial components. Increased ratio of proinflammatory cytokines (IL-17, TSLP, and IL-6) versus regulatory cytokines (IL-10 and TGF-β) may play a key role in determining inflammatory allergic/autoimmune diseases versus asymptomatic individuals.
Notes
Supported by the National Institute of Biomedical Innovation Grant ID 05-24.
The authors state that they have no financial relationship with any commercial entity that has an interest in the subject of this article.
Acknowledgements
The authors thank Dr. Kimishige Ishizaka for valuable discussion via e-mail.
References
- Braun-Fahrländer C, Riedler J, Herz U. et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002;1:869–877. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- Mosmann TR, Cherwinski H, Bond MW. et al. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;1:2348–2357. [PubMed] [Google Scholar]
- Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;1:911–920. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Ono M, Setoguchi R. et al. Foxp3+CD25+CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;1:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- Walker MR, Carson BD, Nepom GT. et al. De novo generation of antigen-specific CD4+CD25+ regulatory T cells from human CD4+CD25- cells. Proc Natl Acad Sci USA. 2005;1:4103–4108. doi: 10.1073/pnas.0407691102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;1:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- Roncarolo MG, Gregori S, Battaglia M. et al. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;1:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- Bacchetta R, Gambineri E, Roncarolo MG. Role of regulatory T cells and Foxp3 in human diseases. J Allergy Clin Immunol. 2007;1:227–235. doi: 10.1016/j.jaci.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Svensson M, Maroof A, Ato M, Kaye PM. Stromal cells direct local differentiation of regulatory dendritic cells. Immunity. 2004;1:805–816. doi: 10.1016/j.immuni.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Schubert LA, Jeffery E, Zhang Y. et al. Scurfin (Foxp3) acts as a repressor of transcription and regulates T cell activation. J Biol Chem. 2001;1:37672–37679. doi: 10.1074/jbc.M104521200. [DOI] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;1:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- Wildin RS, Smyk-Pearson S, Filipovich AH. Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, × linked (IPEX) syndrome. J Med Genet. 2002;1:537–545. doi: 10.1136/jmg.39.8.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen J, Akdis M, Traidl-Hoffmann C. et al. Absence of T-regulatory cell expression and function in atopic dermatitis skin. J Allergy Clin Immunol. 2006;1:176–183. doi: 10.1016/j.jaci.2005.10.040. [DOI] [PubMed] [Google Scholar]
- Allan SE, Crome SQ, Crellin NK. et al. Activation-induced Foxp3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;1:345–354. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- Nakae S, Komiyama Y, Nambu A. et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;1:375–387. doi: 10.1016/S1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- Steinman L. A brief history of TH17, the first major revision in the TH1/TH2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;1:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- Evans HG, Suddason T, Jackson I, Taams LS, Lord GM. Optimal induction of T helper 17 cells in humans requires T cell receptor ligation in the context of Toll-like receptor-activated monocytes. Proc Natl Acad Sci USA. 2007;1:17034–17039. doi: 10.1073/pnas.0708426104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziato F, Cosmi L, Santarlasci V. et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;1:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnani S. Regulation of the T cell response. Clin Exp Allergy. 2006;1:1357–1366. doi: 10.1111/j.1365-2222.2006.02606.x. [DOI] [PubMed] [Google Scholar]
- Schmidt-Weber CB, Akdis M, Akdis CA. TH17 cells in the big picture of immunology. J Allergy Clin Immunol. 2007;1:247–254. doi: 10.1016/j.jaci.2007.06.039. [DOI] [PubMed] [Google Scholar]
- Bullens DM, Truyen E, Coteur L, Dilissen E, Hellings PW, Dupont LJ, Ceuppens JL. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res. 2006;1:135. doi: 10.1186/1465-9921-7-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden A. Interleukin-17 and airway remodelling. Pulm Pharmacol Ther. 2006;1:47–50. doi: 10.1016/j.pupt.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Betts G, Twohig J, Van den Broek M. et al. The impact of regulatory T cells on carcinogen-induced sarcogenesis. Br J Cancer. 2007;1:1849–1854. doi: 10.1038/sj.bjc.6603824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akdis M, Verhagen J, Taylor A. et al. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J Exp Med. 2004;1:1567–1575. doi: 10.1084/jem.20032058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orihara K, Narita M, Tobe T. et al. Circulating Foxp3+CD4+ cell numbers in atopic patients and healthy control subjects. J Allergy Clin Immunol. 2007;1:960–962. doi: 10.1016/j.jaci.2007.05.036. [DOI] [PubMed] [Google Scholar]
- Nakae S, Suto H, Berry GJ. et al. Mast cell-derived TNF can promote Th17 cell-dependent neutrophil recruitment in ovalbumin-challenged OTII mice. Blood. 2007;1:3640–3648. doi: 10.1182/blood-2006-09-046128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ. Thymic stromal lymphopoietin and OX40 ligand pathway in the initiation of dendritic cell-mediated allergic inflammation. J Allergy Clin Immunol. 2007;1:238–244. doi: 10.1016/j.jaci.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Gern JE, Vrtis R, Grindle KA, Swenson C, Busse WW. Relationship of upper and lower airway cytokines to outcome of experimental rhinovirus infection. Am J Respir Crit Care Med. 2000;1:2226–2231. doi: 10.1164/ajrccm.162.6.2003019. [DOI] [PubMed] [Google Scholar]
- Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol Med. 2007;1:108–116. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Lu LF, Lind EF, Gondek DC. et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;1:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- Nouri-Aria KT, Pilette C, Jacobson MR. et al. IL-9 and c-Kit+ mast cells in allergic rhinitis during seasonal allergen exposure: effect of immunotherapy. J Allergy Clin Immunol. 2005;1:73–79. doi: 10.1016/j.jaci.2005.03.011. [DOI] [PubMed] [Google Scholar]


