Abstract
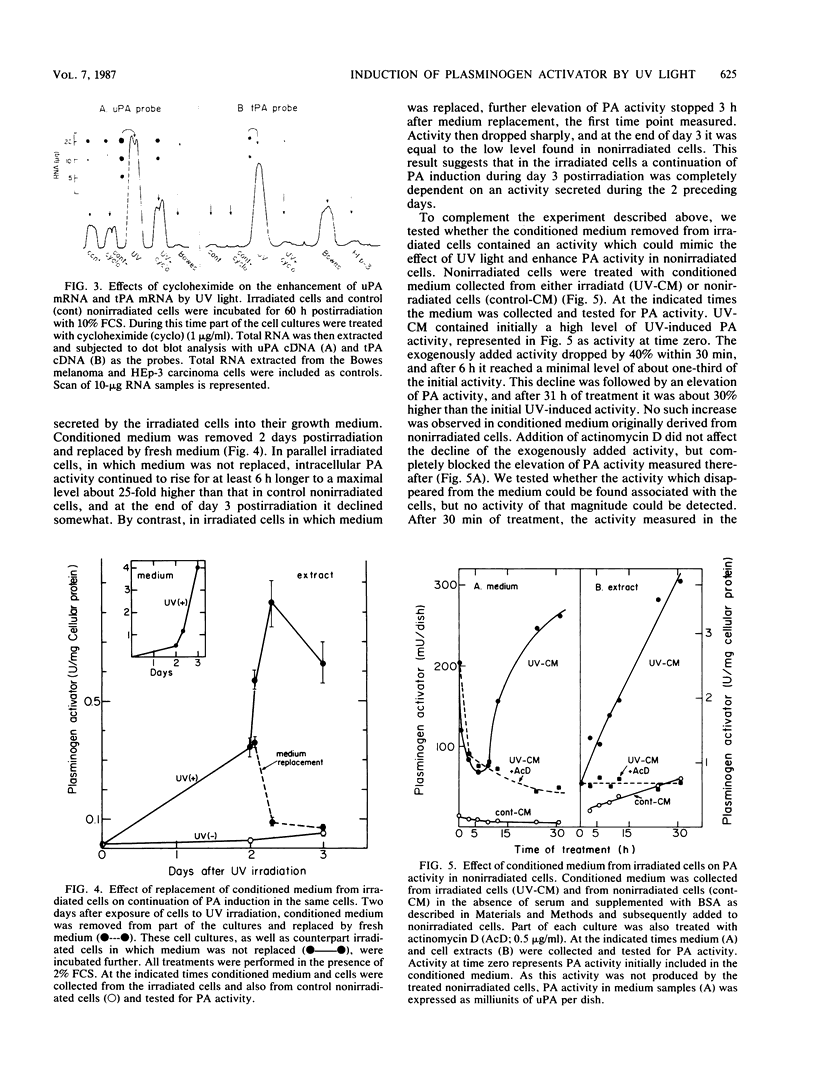
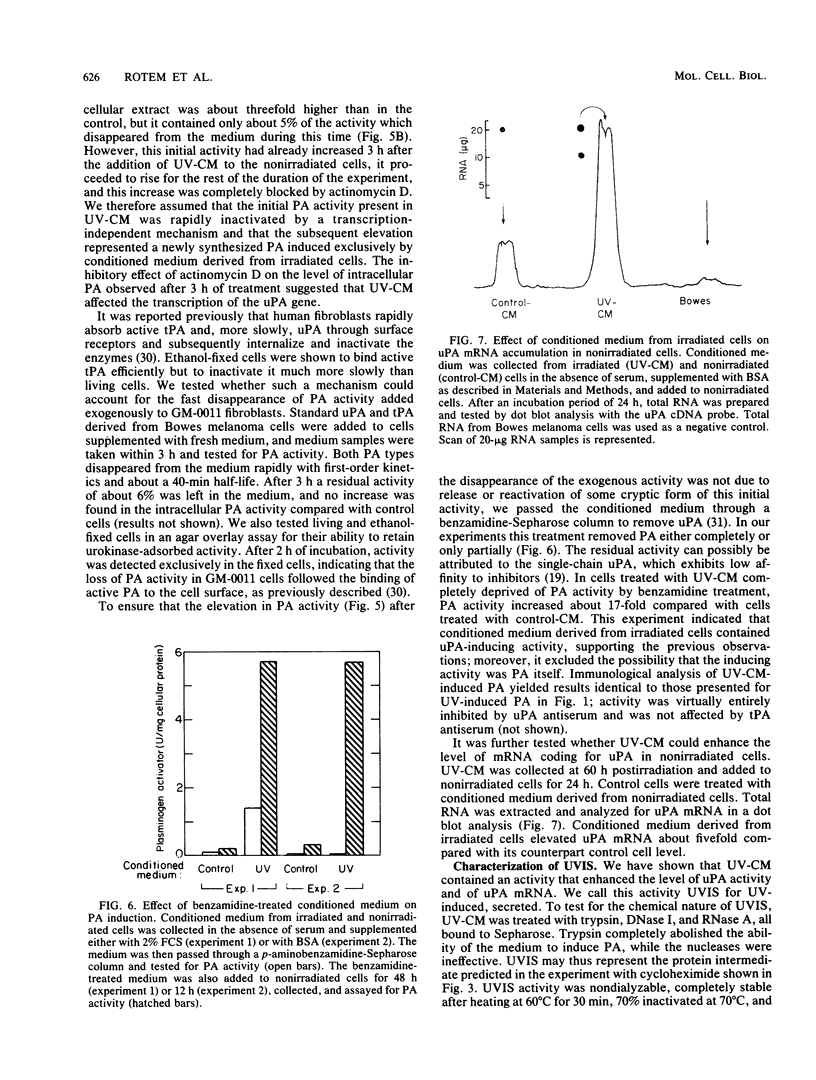
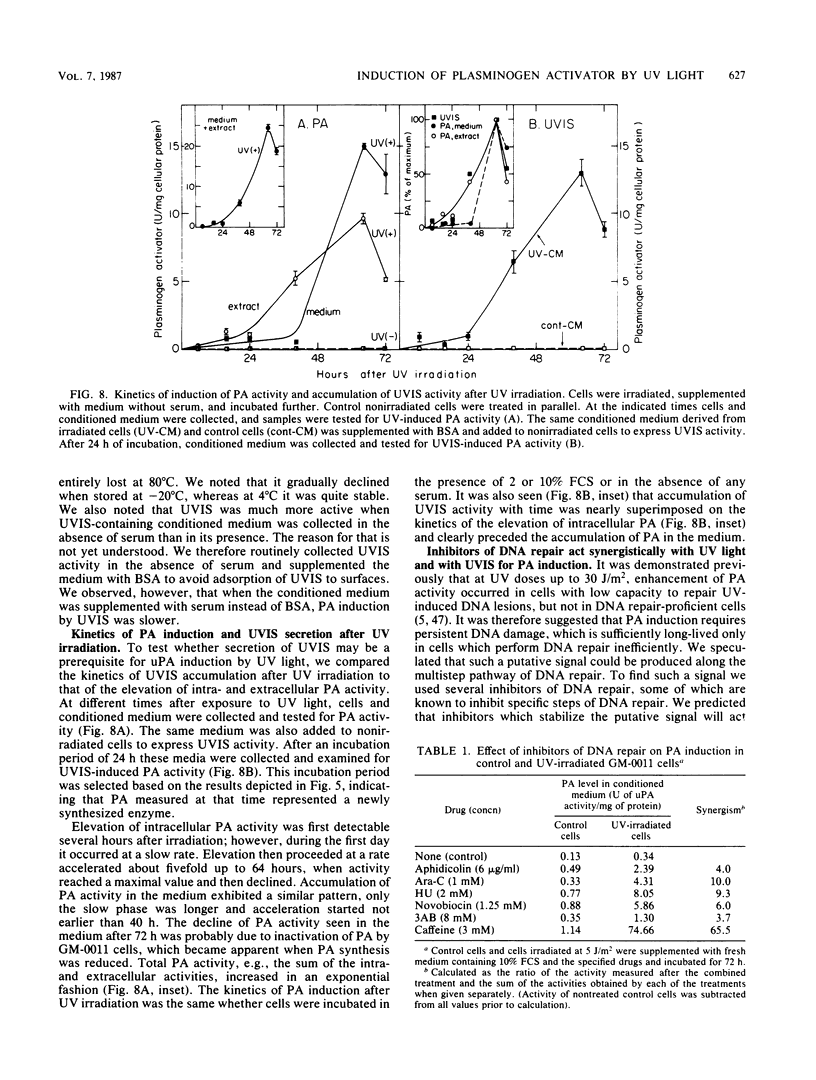
Plasminogen activator was previously shown to be induced by UV light in human cells with low capacity to repair UV-induced DNA lesions. We now show that in human fetal fibroblasts UV light enhanced the two mRNA species coding for the urokinase-type plasminogen activator (uPA) and the tissue-type plasminogen activator, but immunological analysis revealed exclusively uPA activity. Several independent and complementary experiments indicated that induction of uPA was mediated, apparently entirely, through a UV-induced, secreted protein (UVIS) in the growth medium of irradiated cells. First, elevation of uPA mRNA after irradiation was severely blocked by cycloheximide. Second, replacement of conditioned medium in irradiated cells while the rate of plasminogen activator induction was maximal rapidly and completely stopped any further increase in uPA activity. Third, addition of the same removed conditioned medium to nonirradiated cells mimicked UV light in enhancing the level of uPA activity as well as that of uPA mRNA. Fourth, UVIS activity was completely lost by treating the conditioned medium with trypsin but not with nucleases. Kinetic measurements indicated that the accumulation of UVIS rather than the induction of uPA by UVIS conferred the rate-limiting step in the overall process of uPA induction. Both UV light and UVIS acted synergistically with inhibitors of DNA repair for uPA induction. Based on these results, a model is proposed implicating relaxation of DNA torsional stress of an as yet undefined DNA sequence(s) in the induction of UVIS, which is then responsible for activation of the uPA gene.
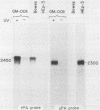
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Rahmsdorf H. J., Pöting A., Lücke-Huhle C., Herrlich P. 12-O-Tetradecanoylphorbol-13-acetate (TPA)-induced gene sequences in human primary diploid fibroblasts and their expression in SV40-transformed fibroblasts. J Cell Biochem. 1985;29(4):351–360. doi: 10.1002/jcb.240290408. [DOI] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ishai R., Sharon R., Rothman M., Miskin R. DNA repair and induction of plasminogen activator in human fetal cells treated with ultraviolet light. Carcinogenesis. 1984 Mar;5(3):357–362. doi: 10.1093/carcin/5.3.357. [DOI] [PubMed] [Google Scholar]
- Bockstahler L. E. Induction and enhanced reactivation of mammalian viruses by light. Prog Nucleic Acid Res Mol Biol. 1981;26:303–313. doi: 10.1016/s0079-6603(08)60413-4. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buhl S. N., Regan J. D. Effect of caffeine on postreplication repair in human cells. Biophys J. 1974 Jul;14(7):519–527. doi: 10.1016/S0006-3495(74)85932-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camiolo S. M., Markus G., Englander L. S., Siuta M. R., Hobika G. H., Kohga S. Plasminogen activator content and secretion in explants of neoplastic and benign human prostate tissues. Cancer Res. 1984 Jan;44(1):311–318. [PubMed] [Google Scholar]
- Camiolo S. M., Markus G., Evers J. L., Hobika G. H., DePasquale J. L., Beckley S., Grimaldi J. P. Plasminogen activator content of neoplastic and benign human prostate tissues; fibrin augmentation of an activator activity. Int J Cancer. 1981 Feb 15;27(2):191–198. doi: 10.1002/ijc.2910270211. [DOI] [PubMed] [Google Scholar]
- Chen B. D., Lin H. S. Colony-stimulating factor (CSF-1): its enhancement of plasminogen activator production and inhibition of cell growth in a mouse macrophage cell line. J Immunol. 1984 Jun;132(6):2955–2960. [PubMed] [Google Scholar]
- Christman J. K., Silagi S., Newcomb E. W., Silverstein S. C., Acs G. Correlated suppression by 5-bromodeoxyuridine of tumorigenicity and plasminogen activator in mouse melanoma cells. Proc Natl Acad Sci U S A. 1975 Jan;72(1):47–50. doi: 10.1073/pnas.72.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins A. R., Squires S., Johnson R. T. Inhibitors of repair DNA synthesis. Nucleic Acids Res. 1982 Feb 25;10(4):1203–1213. doi: 10.1093/nar/10.4.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins A., Johnson R. Novobiocin; an inhibitor of the repair of UV-induced but not X-ray-induced damage in mammalian cells. Nucleic Acids Res. 1979 Nov 10;7(5):1311–1320. doi: 10.1093/nar/7.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corasanti J. G., Celik C., Camiolo S. M., Mittelman A., Evers J. L., Barbasch A., Hobika G. H., Markus G. Plasminogen activator content of human colon tumors and normal mucosae: separation of enzymes and partial purification. J Natl Cancer Inst. 1980 Aug;65(2):345–351. [PubMed] [Google Scholar]
- Creissen D., Shall S. Regulation of DNA ligase activity by poly(ADP-ribose). Nature. 1982 Mar 18;296(5854):271–272. doi: 10.1038/296271a0. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio S. M., Setlow R. B. Defective and enhanced postreplication repair in classical and variant xeroderma pigmentosum cells treated with N-acetoxy-2-acetylaminofluorene. Cancer Res. 1978 Apr;38(4):1147–1153. [PubMed] [Google Scholar]
- D'Ambrosio S. M., Setlow R. B. Enhancement of postreplication repair in Chinese hamster cells. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2396–2400. doi: 10.1073/pnas.73.7.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danø K., Andreasen P. A., Grøndahl-Hansen J., Kristensen P., Nielsen L. S., Skriver L. Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res. 1985;44:139–266. doi: 10.1016/s0065-230x(08)60028-7. [DOI] [PubMed] [Google Scholar]
- Degen J. L., Estensen R. D., Nagamine Y., Reich E. Induction and desensitization of plasminogen activator gene expression by tumor promoters. J Biol Chem. 1985 Oct 15;260(23):12426–12433. [PubMed] [Google Scholar]
- Deutsch D. G., Mertz E. T. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970 Dec 4;170(3962):1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- Dunn W. C., Regan J. D. Inhibition of DNA excision repair in human cells by arabinofuranosyl cytosine: effect on normal and xeroderma pigmentosum cells. Mol Pharmacol. 1979 Mar;15(2):367–374. [PubMed] [Google Scholar]
- Durkacz B. W., Omidiji O., Gray D. A., Shall S. (ADP-ribose)n participates in DNA excision repair. Nature. 1980 Feb 7;283(5747):593–596. doi: 10.1038/283593a0. [DOI] [PubMed] [Google Scholar]
- Gellert M., O'Dea M. H., Itoh T., Tomizawa J. Novobiocin and coumermycin inhibit DNA supercoiling catalyzed by DNA gyrase. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4474–4478. doi: 10.1073/pnas.73.12.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glikin G. C., Ruberti I., Worcel A. Chromatin assembly in Xenopus oocytes: in vitro studies. Cell. 1984 May;37(1):33–41. doi: 10.1016/0092-8674(84)90298-8. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Whitmore G. F. The effect of-beta-D-arabinofuranosylcytosine on growth, viability, and DNA synthesis of mouse L-cells. Cancer Res. 1970 Nov;30(11):2627–2635. [PubMed] [Google Scholar]
- Gross J. L., Krupp M. N., Rifkin D. B., Lane M. D. Down-regulation of epidermal growth factor receptor correlates with plasminogen activator activity in human A431 epidermoid carcinoma cells. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2276–2280. doi: 10.1073/pnas.80.8.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S., Udvardy A., Schedl P. Novobiocin blocks the Drosophila heat shock response. J Mol Biol. 1985 May 5;183(1):13–29. doi: 10.1016/0022-2836(85)90277-3. [DOI] [PubMed] [Google Scholar]
- Helland D., Nes I. F., Kleppe K. Mammalian DNA-repair endonuclease acts only on supercoiled DNA. FEBS Lett. 1982 Jun 1;142(1):121–124. doi: 10.1016/0014-5793(82)80233-0. [DOI] [PubMed] [Google Scholar]
- Hoal E. G., Wilson E. L., Dowdle E. B. The regulation of tissue plasminogen activator activity by human fibroblasts. Cell. 1983 Aug;34(1):273–279. doi: 10.1016/0092-8674(83)90158-7. [DOI] [PubMed] [Google Scholar]
- Holmberg L., Bladh B., Astedt B. Purification of urokinase by affinity chromatography. Biochim Biophys Acta. 1976 Aug 12;445(1):215–222. doi: 10.1016/0005-2744(76)90174-1. [DOI] [PubMed] [Google Scholar]
- Huarte J., Belin D., Vassalli J. D. Plasminogen activator in mouse and rat oocytes: induction during meiotic maturation. Cell. 1985 Dec;43(2 Pt 1):551–558. doi: 10.1016/0092-8674(85)90184-9. [DOI] [PubMed] [Google Scholar]
- Huberman E., Fogel M. Activation of carcinogenic polycyclic hydrocarbons in polyoma-virus-transformed cells as a prerequisite for polyoma virus induction. Int J Cancer. 1975 Jan 15;15(1):91–98. doi: 10.1002/ijc.2910150111. [DOI] [PubMed] [Google Scholar]
- Ikegami S., Taguchi T., Ohashi M., Oguro M., Nagano H., Mano Y. Aphidicolin prevents mitotic cell division by interfering with the activity of DNA polymerase-alpha. Nature. 1978 Oct 5;275(5679):458–460. doi: 10.1038/275458a0. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakunaga T. Neoplastic transformation of human diploid fibroblast cells by chemical carcinogens. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1334–1338. doi: 10.1073/pnas.75.3.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann A. R. Effect of caffeine on DNA synthesis in mammalian cells. Biophys J. 1972 Oct;12(10):1316–1325. doi: 10.1016/S0006-3495(72)86165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M. W., Beach L. R., Palmiter R. D. Ultraviolet radiation-induced metallothionein-I gene activation is associated with extensive DNA demethylation. Cell. 1983 Nov;35(1):207–214. doi: 10.1016/0092-8674(83)90223-4. [DOI] [PubMed] [Google Scholar]
- Little J. W. Autodigestion of lexA and phage lambda repressors. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1375–1379. doi: 10.1073/pnas.81.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Maher V. M., Rowan L. A., Silinskas K. C., Kateley S. A., McCormick J. J. Frequency of UV-induced neoplastic transformation of diploid human fibroblasts is higher in xeroderma pigmentosum cells than in normal cells. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2613–2617. doi: 10.1073/pnas.79.8.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak T. W., Rutledge G., Sutherland D. J. Androgen-dependent fibrinolytic activity in a murine mammary carcinoma (Shionogi SC-115 cells) in vitro. Cell. 1976 Feb;7(2):223–226. doi: 10.1016/0092-8674(76)90021-0. [DOI] [PubMed] [Google Scholar]
- Maltzman W., Czyzyk L. UV irradiation stimulates levels of p53 cellular tumor antigen in nontransformed mouse cells. Mol Cell Biol. 1984 Sep;4(9):1689–1694. doi: 10.1128/mcb.4.9.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattern M. R., Scudiero D. A. Dependence of mammalian DNA synthesis on DNA supercoiling. III. Characterization of the inhibition of replicative and repair-type DNA synthesis by novobiocin and nalidixic acid. Biochim Biophys Acta. 1981 Apr 27;653(2):248–258. doi: 10.1016/0005-2787(81)90160-x. [DOI] [PubMed] [Google Scholar]
- Mezzina M., Nocentini S., Sarasin A. DNA ligase activity in carcinogen-treated human fibroblasts. Biochimie. 1982 Aug-Sep;64(8-9):743–748. doi: 10.1016/s0300-9084(82)80122-3. [DOI] [PubMed] [Google Scholar]
- Miskin R., Ben-Ishai R. Induction of plasminogen activator by UV light in normal and xeroderma pigmentosum fibroblasts. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6236–6240. doi: 10.1073/pnas.78.10.6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskin R., Reich E. Plasminogen activator: induction of synthesis by DNA damage. Cell. 1980 Jan;19(1):217–224. doi: 10.1016/0092-8674(80)90403-1. [DOI] [PubMed] [Google Scholar]
- Ny T., Elgh F., Lund B. The structure of the human tissue-type plasminogen activator gene: correlation of intron and exon structures to functional and structural domains. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5355–5359. doi: 10.1073/pnas.81.17.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossowski L., Biegel D., Reich E. Mammary plasminogen activator: correlation with involution, hormonal modulation and comparison between normal and neoplastic tissue. Cell. 1979 Apr;16(4):929–940. doi: 10.1016/0092-8674(79)90108-9. [DOI] [PubMed] [Google Scholar]
- Ossowski L., Reich E. Antibodies to plasminogen activator inhibit human tumor metastasis. Cell. 1983 Dec;35(3 Pt 2):611–619. doi: 10.1016/0092-8674(83)90093-4. [DOI] [PubMed] [Google Scholar]
- Ossowski L., Unkeless J. C., Tobia A., Quigley J. P., Rifkin D. B., Reich E. An enzymatic function associated with transformation of fibroblasts by oncogenic viruses. II. Mammalian fibroblast cultures transformed by DNA and RNA tumor viruses. J Exp Med. 1973 Jan 1;137(1):112–126. doi: 10.1084/jem.137.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter R. B. Effect of caffeine on DNA synthesis in irradiated and unirradiated mammalian cells. J Mol Biol. 1980 Nov 5;143(3):289–301. doi: 10.1016/0022-2836(80)90191-6. [DOI] [PubMed] [Google Scholar]
- Sarasin A. R., Hanawalt P. C. Carcinogens enhance survival of UV-irradiated simian virus 40 in treated monkey kidney cells: induction of a recovery pathway? Proc Natl Acad Sci U S A. 1978 Jan;75(1):346–350. doi: 10.1073/pnas.75.1.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarasin A., Bourre F., Benoit A. Error-prone replication of ultraviolet-irradiated simian virus 40 in carcinogen-treated monkey kidney cells. Biochimie. 1982 Aug-Sep;64(8-9):815–821. doi: 10.1016/s0300-9084(82)80135-1. [DOI] [PubMed] [Google Scholar]
- Schorpp M., Mallick U., Rahmsdorf H. J., Herrlich P. UV-induced extracellular factor from human fibroblasts communicates the UV response to nonirradiated cells. Cell. 1984 Jul;37(3):861–868. doi: 10.1016/0092-8674(84)90421-5. [DOI] [PubMed] [Google Scholar]
- Setlow R. B. Repair deficient human disorders and cancer. Nature. 1978 Feb 23;271(5647):713–717. doi: 10.1038/271713a0. [DOI] [PubMed] [Google Scholar]
- Sisskin E. E., Weinstein I. B., Evans C. H., Dipaolo J. A. Plasminogen activator synthesis accompanying chemical carcinogen-induced in vitro transformation of Syrian hamster and guinea-pig fetal cells. Int J Cancer. 1980 Sep 15;26(3):331–335. doi: 10.1002/ijc.2910260312. [DOI] [PubMed] [Google Scholar]
- Snyder R. D., Regan J. D. Aphidicolin inhibits repair of DNA in UV-irradiated human fibroblasts. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1088–1094. doi: 10.1016/0006-291x(81)90730-0. [DOI] [PubMed] [Google Scholar]
- Soreq H., Miskin R. Plasminogen activator in the rodent brain. Brain Res. 1981 Jul 20;216(2):361–374. doi: 10.1016/0006-8993(81)90138-4. [DOI] [PubMed] [Google Scholar]
- Strickland S., Beers W. H. Studies on the role of plasminogen activator in ovulation. In vitro response of granulosa cells to gonadotropins, cyclic nucleotides, and prostaglandins. J Biol Chem. 1976 Sep 25;251(18):5694–5702. [PubMed] [Google Scholar]
- Strickland S., Reich E., Sherman M. I. Plasminogen activator in early embryogenesis: enzyme production by trophoblast and parietal endoderm. Cell. 1976 Oct;9(2):231–240. doi: 10.1016/0092-8674(76)90114-8. [DOI] [PubMed] [Google Scholar]
- Timson J. Hydroxyurea. Mutat Res. 1975;32(2):115–132. doi: 10.1016/0165-1110(75)90002-0. [DOI] [PubMed] [Google Scholar]
- Tyrrell R. M. Specific toxicity of aphidicolin to ultraviolet-irradiated excision proficient human skin fibroblasts. Carcinogenesis. 1983;4(3):327–329. doi: 10.1093/carcin/4.3.327. [DOI] [PubMed] [Google Scholar]
- Villeponteau B., Lundell M., Martinson H. Torsional stress promotes the DNAase I sensitivity of active genes. Cell. 1984 Dec;39(3 Pt 2):469–478. doi: 10.1016/0092-8674(84)90454-9. [DOI] [PubMed] [Google Scholar]
- Waters R. Aphidicolin: an inhibitor of DNA repair in human fibroblasts. Carcinogenesis. 1981;2(8):795–797. doi: 10.1093/carcin/2.8.795. [DOI] [PubMed] [Google Scholar]