Abstract
We propose to enhance the axial resolution of photoacoustic microscopy (PAM) by reducing the speed of sound within the imaging region of interest. With silicone oil immersion, we have achieved a finest axial resolution of 5.8 μm for PAM, as validated by phantom experiments. The axial resolution was also enhanced in vivo when mouse ears injected with silicone oil were imaged. When tissue-compatible low-speed liquid becomes available, this approach may find broad applications in PAM as well as in other imaging modalities, such as photoacoustic computed tomography and ultrasound imaging.
Photoacoustic microscopy (PAM) is an emerging three-dimensional (3D) imaging technology with the unique advantage of imaging optical absorption of biomolecules structurally and functionally.1 In typical PAM, a laser pulse irradiates the optical absorbers, and the excited photoacoustic wave is detected by an ultrasonic transducer. In optical-resolution PAM,2, 3 the spot size of the optical focus determines the lateral resolution. To resolve absorbers in the axial direction, the arrival time of the photoacoustic signal is converted to depth. Hence, the width of the temporal impulse response of the PAM system determines the axial resolution. While submicron lateral resolution has been achieved for PAM,4, 5 the finest axial resolution still remains on the micron level,6, 7 much poorer than the lateral resolution.
The axial resolution of PAM, if the impulse response of the PAM system has a Gaussian envelope, can be estimated as 0.88 c/B,6 where c is the speed of sound and B is the bandwidth of the PAM system. To enhance the axial resolution, previous work has concentrated on increasing the bandwidth B by using a very broadband ultrasonic transducer6 or optical resonance acoustic sensor.7 However, as the bandwidth increases, the penetration depth of PAM becomes quite limited because the acoustic attenuation coefficient of soft tissue is nearly proportional to the frequency. Alternatively, nonlinear optical effects have been explored as another mechanism to provide optically determined axial resolution for PAM, such as in two-photon-absorption PAM.8, 9 Although promising, this technique is expected to have relatively low detection sensitivity due to the inefficiency of these nonlinear effects within the safety limit of laser intensity, and its 3D image acquisition is lengthened by the additional depth scanning.
Here, we propose to enhance the axial resolution of PAM by reducing the speed of sound c. Since an ultrasonic transducer detects the time-resolved signal, reducing the speed of sound would increase the time interval between two objects with a given spatial distance, thereby shortening the smallest resolvable distance between objects. Note that the time interval between two objects in the photoacoustic signal is determined by the speed of sound of the medium between them (ignoring acoustic scattering and reflection). In contrast, the speed of sound of the medium between the objects and the ultrasonic transducer determines the “time delay” to both signals from the two objects. Therefore, our method aims to reduce the speed of sound inside the imaging region of interest instead of the surrounding coupling medium (typically water or ultrasonic gel). This procedure can be realized by immersing the region of interest in a liquid that has a relatively low speed of sound. Our approach is analogous to the oil immersion used to increase the lateral resolution in optical microscopy.10 In both cases, the acoustic or optical wavelength is decreased as the sound or light speed is lower in the immersion liquids.
The selection of immersion liquid is critical. With a lower speed of sound, the immersion liquid is expected to have a different acoustic impedance Z (= ρc, where ρ is the density) from that of the surrounding medium. The acoustic impedance mismatch will induce acoustic reflection at the interface, decreasing the detected signal amplitude and generating reverberation. In most biomedical applications of PAM, the acoustic impedances of the imaged soft tissues and the coupling water are about 1.6 MRayl and 1.5 MRayl, respectively, while the speeds of sound are approximately 1.5 × 103 m/s. To demonstrate the principle of our method, here, we choose silicone oil (85421, Sigma-Aldrich) as the immersion liquid, whose speed of sound is about 1.1 × 103 m/s and acoustic impedance is about 1.1 MRayl. Thus, the axial resolution is expected to be enhanced by ∼1.4 times, while the acoustic impedance mismatch is relatively low (the amplitude reflection coefficient between the silicone oil and water is 0.16). Moreover, silicone oil is non-toxic and has been used in medical applications, such as in eye injection for managing complicated retinal detachments11, 12 and in soft tissue injection for tissue augmentation.13, 14 Without carrying out systematic biological studies, we believe that silicone oil is a relatively simple and safe choice for injection into biological tissues to reduce the speed of sound.
For experimental demonstration, we used a previously reported PAM system,6 which has achieved the finest axial resolution heretofore, reaching 7.6 μm. The PAM system, shown in Fig. 1, is briefly described here. Laser pulses at 532 nm wavelength from a tunable optical parametric oscillator (OPO) laser (NT242-SH, Ekspla) were focused into the sample by a 0.32 NA objective (Phaco 1, Leitz Wetzlar), providing ∼0.8 μm lateral resolution. The imaging region of interest of the sample was immersed in silicone oil. The excited photoacoustic waves from the sample were detected by a focused ultrasonic transducer (125-MHz central frequency, 100-MHz bandwidth; V2062, Olympus NDT), which was immersed in water for coupling. The photoacoustic signals were amplified and digitized at 1 GS/s. The sample was mechanically scanned in 2D to generate a 3D image, while each time-resolved photoacoustic signal was deconvolved with the PAM system impulse response to further improve the axial resolution (as the effective bandwidth of the PAM system was increased).6 The image acquisition speed was limited by the laser pulse repetition rate of 1 KHz.
Figure 1.
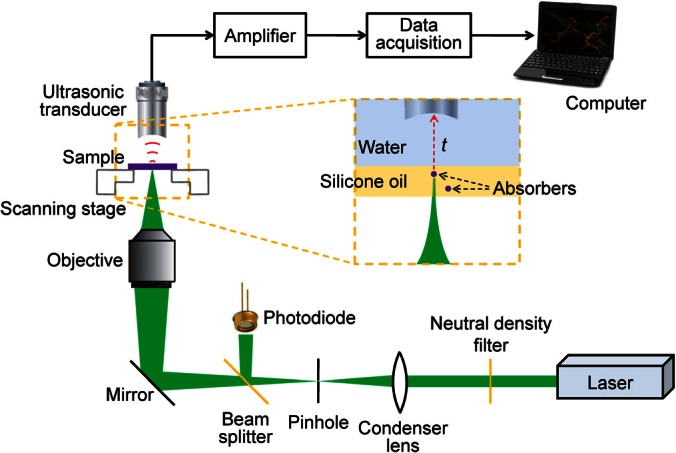
Schematic of the PAM system. The inset with a dashed line boundary shows the absorbers immersed in silicone oil and the ultrasonic transducer immersed in water. The acoustic flight time t from the absorbers to the ultrasonic transducer can be converted to depth based on the speeds of sound in the two media.
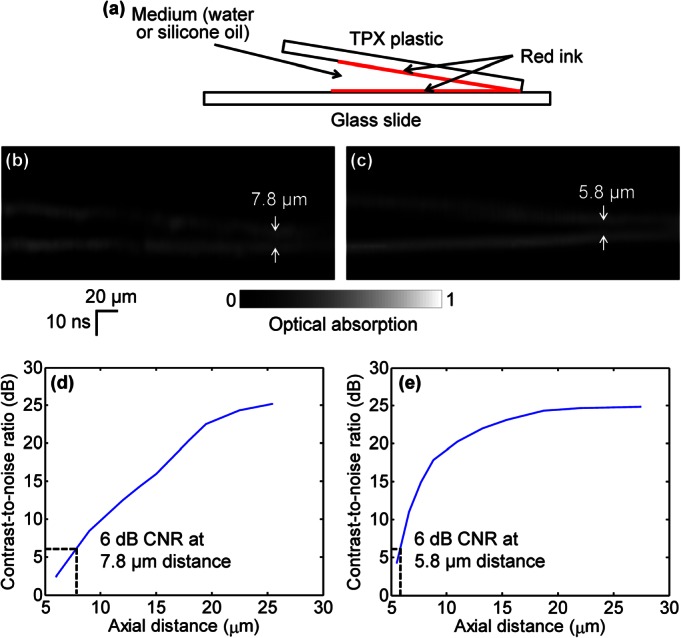
We designed a phantom experiment to demonstrate the axial resolution improvement by reducing speed of sound. As shown in Fig. 2a, two layers of red ink for imaging were smeared on a polymethylpentene (TPX) plastic sheet (upper, matching well with water in acoustic impedance) and a glass slide (lower), respectively. A small angle between the TPX plastic and the glass slide provided a continuously variable distance between the two layers, which enabled quantitative measurement of axial resolution. The gap between the two layers was filled with either water or silicone oil for comparison, and the space between the two layers and the ultrasonic transducer was filled with water for coupling. B-scan images of the water-filled sample and the silicone-oil-filled sample are shown in Figs. 2b, 2c, respectively. In both images, the vertical direction is plotted in the units of time. It can be seen that the bottom layer of ink, which was placed horizontally, appears oblique in Fig. 2c. This is because that as the thickness of the silicone oil in the gap increases, the photoacoustic signal from the bottom layer takes longer to travel to the ultrasonic transducer due to the slower speed of sound in silicone oil compared with water. For the same reason, the two layers can be separated more clearly. The contrast-to-noise ratio (CNR) versus the axial distance between the two layers of the water-filled sample and the silicone-oil-filled sample are shown in Figs. 2d, 2e, respectively. Here, in each 1D image of the two layers, the contrast is defined as the difference in amplitude between the smaller peak of the two layers and the intermediate valley; the noise is defined as the standard deviation of the background amplitude. The axial resolution, defined as the axial distance with 6 dB CNR, is 7.8 μm for the water-filled sample. The axial resolution is improved to 5.8 μm by silicone oil immersion, which is close to the theoretical prediction of 7.8 μm/1.5 × 1.1 ≈ 5.7 μm.
Figure 2.
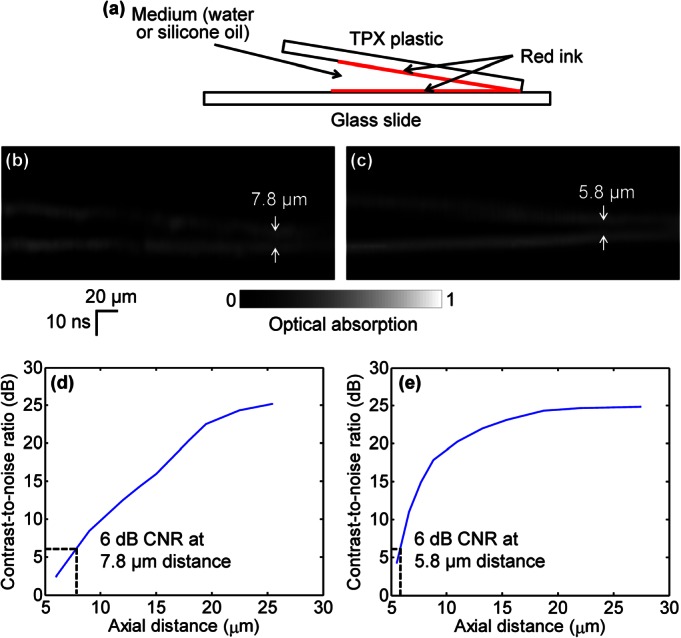
Axial resolution of PAM. (a) Two layers of red ink are smeared on a TPX plastic sheet (upper) and a glass slide (lower), respectively, for imaging. The gap between the two layers is filled with water or silicone oil. B-scan images of the water-filled sample (b) and the silicone-oil-filled sample (c). The CNR versus the axial distance between the two layers of the water-filled sample (d) and the silicone-oil-filled sample (e). The axial resolutions, defined as the axial distance with 6 dB CNR, are 7.8 μm for the water-filled sample and 5.8 μm for the silicone-oil-filled sample, respectively.
We also showed potential biomedical applications of our method by injecting silicone oil into a mouse ear to enhance the axial resolution in vivo. Approximately 30 μl of silicone oil was injected into a nude mouse ear and allowed to diffuse for 30 min. The blood vessels in the silicone-oil-diffused area of the ear were imaged with the same laser intensity in vivo before and 30 min after injection, as shown in Figs. 3a, 3b, 3c, 3d. The top-view maximum-amplitude-projection images are very similar before and after injection, but the side-view images demonstrate the improvement in axial resolution by injecting silicone oil. The amplitudes along the dashed profiles in Figs. 3c, 3d are shown in Figs. 3e, 3f, respectively, with their overlay shown in Fig. 3g, which further demonstrate that the blood vessels are resolved more clearly with silicone oil. In Fig. 3g, the time scale of the profile from Fig. 3f has been adjusted to maximize the correlation coefficient between the two profiles (maximum at 0.82). Based on the ratio between the time scales of the two profiles, the average speed of sound was estimated to be ∼1.3 × 103 m/s in the post-injection mouse ear, an environment mixed with silicone oil and water. Due to the acoustic impedance mismatch between silicone oil and water, the CNR in post-injection images is about 2 dB lower than that in pre-injection images.
Figure 3.
In vivo PAM images of a mouse ear. Top-view PAM images before (a) and after (b) injection of silicone oil. Side-view PAM images before (c) and after (d) injection of silicone oil. (e) Normalized PA amplitude along the dashed line in (c). (f) Normalized PA amplitude along the dashed line in (d). Corresponding features in (e) and (f) are labeled with numbers. (g) Overlay of (e) and (f) with the corresponding time axes, as indicated by the arrows.
In summary, we have demonstrated the feasibility of enhancing the axial resolution of PAM by using an immersion liquid to reduce the speed of sound. With silicone oil immersion, we have achieved a finest axial resolution of 5.8 μm, and with silicone oil injection, we improved the axial resolution in imaging mouse ears in vivo. It is possible to further improve the axial resolution, at the cost of detection sensitivity, by using an immersion liquid with a lower speed of sound, such as fluorosilicone oil (7.6 × 102 m/s) or tallow (3.9 × 102 m/s). Theoretically, tallow can help achieve an axial resolution of 2.0 μm for non-biological samples. For biomedical applications, we will seek more low-speed biocompatible immersion liquids. Our method can potentially be used in other imaging modalities, such as photoacoustic computed tomography and ultrasound imaging.
Acknowledgments
This work was sponsored in part by National Institutes of Health Grant Nos. DP1 EB016986 (NIH Director's Pioneer Award), R01 CA134539, U54 CA136398, R01 CA157277, and R01 CA159959. L.W. has a financial interest in Microphotoacoustics, Inc. and Endra, Inc., which, however, did not support this work.
References
- Wang L. V. and Hu S., Science 335(6075 ), 1458 (2012). 10.1126/science.1216210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslov K., Zhang H. F., Hu S., and Wang L. V., Opt. Lett. 33(9 ), 929 (2008). 10.1364/OL.33.000929 [DOI] [PubMed] [Google Scholar]
- Xie Z., Jiao S., Zhang H. F., and Puliafito C. A., Opt. Lett. 34(12 ), 1771 (2009). 10.1364/OL.34.001771 [DOI] [PubMed] [Google Scholar]
- Zhang C., Maslov K., and Wang L. V., Opt. Lett. 35(19 ), 3195 (2010). 10.1364/OL.35.003195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Maslov K., Hu S., Chen R., Zhou Q., Shung K. K., and Wang L. V., J. Biomed. Opt. 17(2 ), 020501 (2012). 10.1117/1.JBO.17.2.020501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Maslov K., Yao J., and Wang L. V., J. Biomed. Opt. 17(11 ), 116016 (2012). 10.1117/1.JBO.17.11.116016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z., Chen S.-L., Ling T., Guo L. J., Carson P. L., and Wang X., Opt. Express 19(10 ), 9027 (2011). 10.1364/OE.19.009027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka Y., Nambu M., and Takamatsu T., Opt. Express 19(14 ), 13365 (2011). 10.1364/OE.19.013365 [DOI] [PubMed] [Google Scholar]
- Lai Y.-H., Chang C.-F., Cheng Y.-H., and Sun C.-K., Proc. SPIE 8581, 85812R (2013). 10.1117/12.2002831 [DOI] [Google Scholar]
- Pawley J. B., Handbook of Biological Confocal Microscopy (Springer, New York, 2006), p. 145. [Google Scholar]
- Federman J. L. and Schubert H. D., Ophthalmology 95(7 ), 870 (1988). [DOI] [PubMed] [Google Scholar]
- Tognetto D., Minutola D., Sanguinetti G., and Ravalico G., Ophthalmology 112(9 ), 1574 (2005). 10.1016/j.ophtha.2005.04.013 [DOI] [PubMed] [Google Scholar]
- Rohrich R. J. and Potter J. K., Plast. Reconstr. Surg. 113(4 ), 1239 (2004). 10.1097/01.PRS.0000118022.71802.78 [DOI] [PubMed] [Google Scholar]
- Chasan P. E., Plast. Reconstr. Surg. 120(7 ), 2034 (2007). 10.1097/01.prs.0000267580.92163.33 [DOI] [PubMed] [Google Scholar]


