Abstract
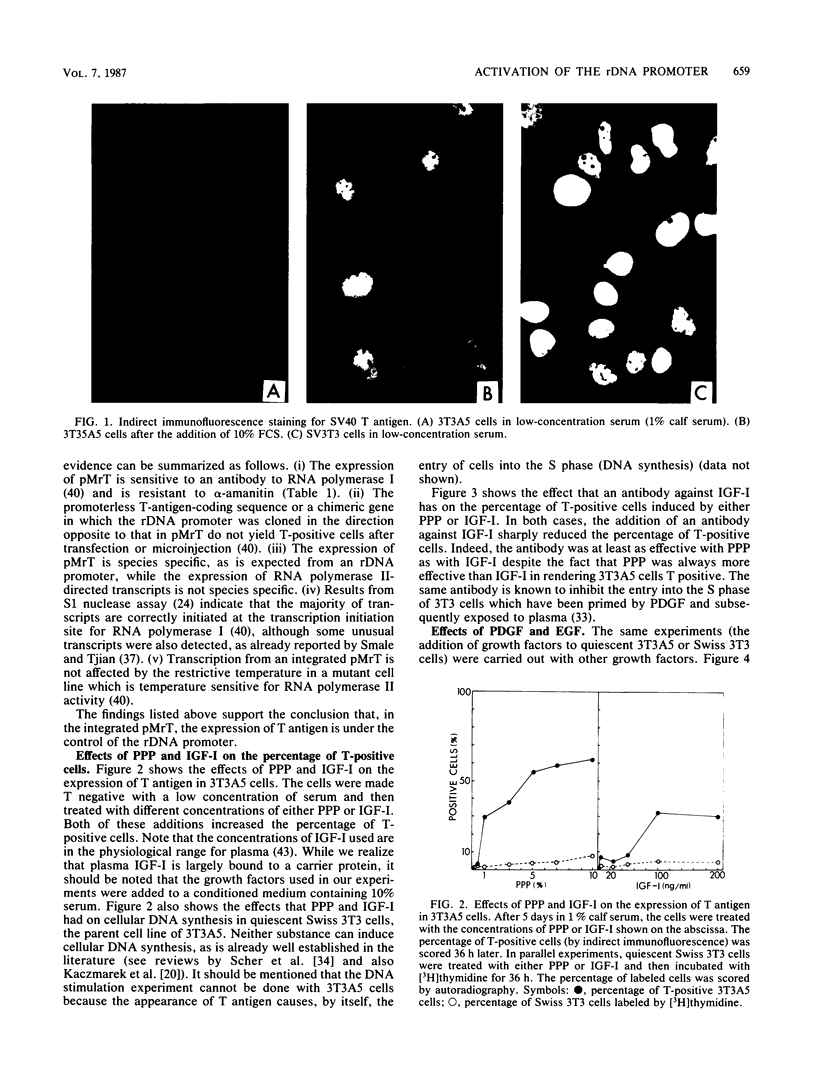
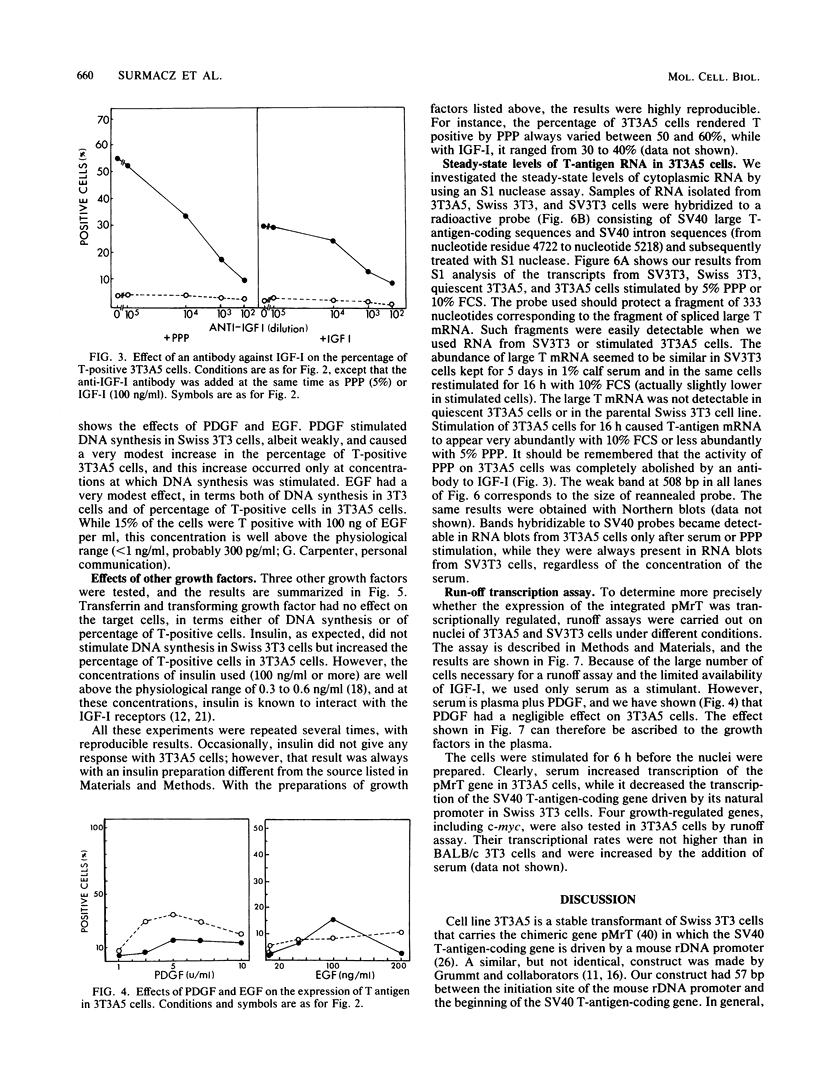
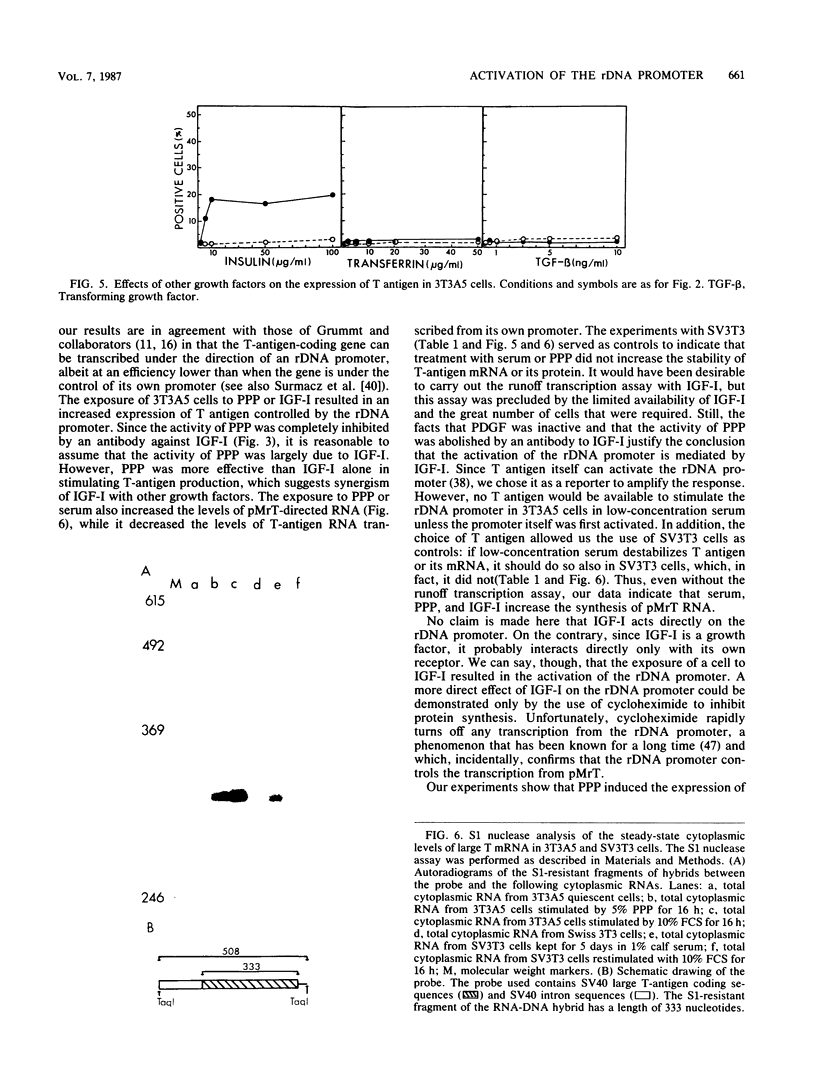
We constructed a stable cell line, 3T3A5, which carried a chimeric gene in which the simian virus 40 T-antigen-coding gene was under the control of the mouse ribosomal DNA promoter. These cells expressed T antigen when they were growing exponentially in 10% fetal calf serum, but they all became T negative when incubated for 5 days in low-concentration serum. The readdition of serum or platelet-poor plasma again induced the expression of T antigen, which was accompanied by an increase in steady-state levels of the corresponding RNA. Among the various growth factors tested for their ability to induce T-antigen expression in 3T3A5 cells, only insulinlike growth factor I (IGF-I) could induce T antigen at physiological concentrations. The effect of IGF-I or platelet-poor plasma was abolished by an antibody to IGF-I. Other growth factors, like insulin and epidermal growth factor, could induce the expression of T antigen in 3T3A5 cells, but only at concentrations far above the physiological range. Other growth factors were totally ineffective. These results indicate that exposure of cells to IGF-I can activate transcription from the ribosomal DNA promoter.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baserga R. Growth in size and cell DNA replication. Exp Cell Res. 1984 Mar;151(1):1–5. doi: 10.1007/978-3-642-67986-5_1. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Gerhard D. S., Fong N. M., Sanchez-Pescador R., Rall L. B. Isolation of the human insulin-like growth factor genes: insulin-like growth factor II and insulin genes are contiguous. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6450–6454. doi: 10.1073/pnas.82.19.6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin T. L. Virus-specific RNA in cells productively infected or transformed by polyoma virus. J Mol Biol. 1966 Apr;16(2):359–373. doi: 10.1016/s0022-2836(66)80179-1. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Brissenden J. E., Ullrich A., Francke U. Human chromosomal mapping of genes for insulin-like growth factors I and II and epidermal growth factor. 1984 Aug 30-Sep 5Nature. 310(5980):781–784. doi: 10.1038/310781a0. [DOI] [PubMed] [Google Scholar]
- Campisi J., Pardee A. B. Post-transcriptional control of the onset of DNA synthesis by an insulin-like growth factor. Mol Cell Biol. 1984 Sep;4(9):1807–1814. doi: 10.1128/mcb.4.9.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D. R., Parfett C. L., Denhardt D. T. Transcriptional regulation of two serum-induced RNAs in mouse fibroblasts: equivalence of one species to B2 repetitive elements. Mol Cell Biol. 1985 Nov;5(11):3280–3288. doi: 10.1128/mcb.5.11.3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel D. A., Samanta H., Brawner M. E., Lengyel P. Interferon action: transcriptional control of a gene specifying a 56,000-Da protein in Ehrlich ascites tumor cells. Virology. 1985 Apr 30;142(2):389–397. doi: 10.1016/0042-6822(85)90346-0. [DOI] [PubMed] [Google Scholar]
- Fleischer S., Grummt I. Expression of an mRNA coding gene under the control of an RNA polymerase I promoter. EMBO J. 1983;2(12):2319–2322. doi: 10.1002/j.1460-2075.1983.tb01740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flier J. S., Usher P., Moses A. C. Monoclonal antibody to the type I insulin-like growth factor (IGF-I) receptor blocks IGF-I receptor-mediated DNA synthesis: clarification of the mitogenic mechanisms of IGF-I and insulin in human skin fibroblasts. Proc Natl Acad Sci U S A. 1986 Feb;83(3):664–668. doi: 10.1073/pnas.83.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J. M., Chung E. Y., Darnell J. E., Jr Gene expression during liver regeneration. J Mol Biol. 1984 Oct 15;179(1):37–53. doi: 10.1016/0022-2836(84)90305-x. [DOI] [PubMed] [Google Scholar]
- Galanti N., Jonak G. J., Soprano K. J., Floros J., Kaczmarek L., Weissman S., Reddy V. B., Tilghman S. M., Baserga R. Characterization and biological activity of cloned simian virus 40 DNA fragments. J Biol Chem. 1981 Jun 25;256(12):6469–6474. [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Grummt I., Skinner J. A. Efficient transcription of a protein-coding gene from the RNA polymerase I promoter in transfected cells. Proc Natl Acad Sci U S A. 1985 Feb;82(3):722–726. doi: 10.1073/pnas.82.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer E., Darnell J. E., Jr The primary transcription unit of the mouse beta-major globin gene. Cell. 1981 Feb;23(2):585–593. doi: 10.1016/0092-8674(81)90154-9. [DOI] [PubMed] [Google Scholar]
- Jansen M., van Schaik F. M., Ricker A. T., Bullock B., Woods D. E., Gabbay K. H., Nussbaum A. L., Sussenbach J. S., Van den Brande J. L. Sequence of cDNA encoding human insulin-like growth factor I precursor. Nature. 1983 Dec 8;306(5943):609–611. doi: 10.1038/306609a0. [DOI] [PubMed] [Google Scholar]
- Kaczmarek L., Hyland J. K., Watt R., Rosenberg M., Baserga R. Microinjected c-myc as a competence factor. Science. 1985 Jun 14;228(4705):1313–1315. doi: 10.1126/science.4001943. [DOI] [PubMed] [Google Scholar]
- King G. L., Kahn C. R., Rechler M. M., Nissley S. P. Direct demonstration of separate receptors for growth and metabolic activities of insulin and multiplication-stimulating activity (an insulinlike growth factor) using antibodies to the insulin receptor. J Clin Invest. 1980 Jul;66(1):130–140. doi: 10.1172/JCI109826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leof E. B., Wharton W., van Wyk J. J., Pledger W. J. Epidermal growth factor (EGF) and somatomedin C regulate G1 progression in competent BALB/c-3T3 cells. Exp Cell Res. 1982 Sep;141(1):107–115. doi: 10.1016/0014-4827(82)90073-8. [DOI] [PubMed] [Google Scholar]
- Levis R., Hazelrigg T., Rubin G. M. Effects of genomic position on the expression of transduced copies of the white gene of Drosophila. Science. 1985 Aug 9;229(4713):558–561. doi: 10.1126/science.2992080. [DOI] [PubMed] [Google Scholar]
- Lopata M. A., Sollner-Webb B., Cleveland D. W. Surprising S1-resistant trimolecular hybrids: potential complication in interpretation of S1 mapping analyses. Mol Cell Biol. 1985 Oct;5(10):2842–2846. doi: 10.1128/mcb.5.10.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. G., Sollner-Webb B. Transcription of mouse rRNA genes by RNA polymerase I: in vitro and in vivo initiation and processing sites. Cell. 1981 Nov;27(1 Pt 2):165–174. doi: 10.1016/0092-8674(81)90370-6. [DOI] [PubMed] [Google Scholar]
- Monjardino J. P. The effect of alpha-amanitin on the synthesis of polyoma specific RNA. Biochem Biophys Res Commun. 1978 Feb 28;80(4):1049–1058. doi: 10.1016/0006-291x(78)91352-9. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Expression of a bacterial gene in mammalian cells. Science. 1980 Sep 19;209(4463):1422–1427. doi: 10.1126/science.6251549. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POPE J. H., ROWE W. P. DETECTION OF SPECIFIC ANTIGEN IN SV40-TRANSFORMED CELLS BY IMMUNOFLUORESCENCE. J Exp Med. 1964 Aug 1;120:121–128. doi: 10.1084/jem.120.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pledger W. J., Stiles C. D., Antoniades H. N., Scher C. D. Induction of DNA synthesis in BALB/c 3T3 cells by serum components: reevaluation of the commitment process. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4481–4485. doi: 10.1073/pnas.74.10.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Russell W. E., Van Wyk J. J., Pledger W. J. Inhibition of the mitogenic effects of plasma by a monoclonal antibody to somatomedin C. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2389–2392. doi: 10.1073/pnas.81.8.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher C. D., Shepard R. C., Antoniades H. N., Stiles C. D. Platelet-derived growth factor and the regulation of the mammalian fibroblast cell cycle. Biochim Biophys Acta. 1979 Aug 10;560(2):217–241. doi: 10.1016/0304-419x(79)90020-9. [DOI] [PubMed] [Google Scholar]
- Seuwen K., Adam G. Only one of the two signals required for initiation of the cell cycle is associated with cellular accumulation of ribosomal RNA. Biochem Biophys Res Commun. 1983 Nov 30;117(1):223–230. doi: 10.1016/0006-291x(83)91564-4. [DOI] [PubMed] [Google Scholar]
- Seuwen K., Steiner U., Adam G. Cellular content of ribosomal RNA in relation to the progression and competence signals governing proliferation of 3T3 and SV40-3T3 cells. Exp Cell Res. 1984 Sep;154(1):10–24. doi: 10.1016/0014-4827(84)90664-5. [DOI] [PubMed] [Google Scholar]
- Smale S. T., Tjian R. Transcription of herpes simplex virus tk sequences under the control of wild-type and mutant human RNA polymerase I promoters. Mol Cell Biol. 1985 Feb;5(2):352–362. doi: 10.1128/mcb.5.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soprano K. J., Dev V. G., Croce C. M., Baserga R. Reactivation of silent rRNA genes by simian virus 40 in human-mouse hybrid cells. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3885–3889. doi: 10.1073/pnas.76.8.3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles C. D., Capone G. T., Scher C. D., Antoniades H. N., Van Wyk J. J., Pledger W. J. Dual control of cell growth by somatomedins and platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1279–1283. doi: 10.1073/pnas.76.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
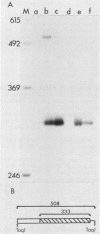
- Surmacz E., Rønning O., Kaczmarek L., Baserga R. Regulation of the expression of the SV40 T-antigen coding gene under the control of an rDNA promoter. J Cell Physiol. 1986 Jun;127(3):357–365. doi: 10.1002/jcp.1041270303. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Tricoli J. V., Rall L. B., Scott J., Bell G. I., Shows T. B. Localization of insulin-like growth factor genes to human chromosomes 11 and 12. 1984 Aug 30-Sep 5Nature. 310(5980):784–786. doi: 10.1038/310784a0. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wilkinson J. K., Sollner-Webb B. Transcription of Xenopus ribosomal RNA genes by RNA polymerase I in vitro. J Biol Chem. 1982 Dec 10;257(23):14375–14383. [PubMed] [Google Scholar]
- Yang H. C., Pardee A. B. Insulin-like growth factor I regulation of transcription and replicating enzyme induction necessary for DNA synthesis. J Cell Physiol. 1986 Jun;127(3):410–416. doi: 10.1002/jcp.1041270309. [DOI] [PubMed] [Google Scholar]
- Yu F. L., Feigelson P. The rapid turnover of RNA polymerase of rat liver nucleolus, and of its messenger RNA. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2833–2837. doi: 10.1073/pnas.69.10.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]