Abstract
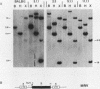
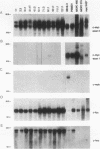
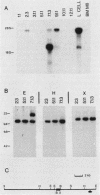
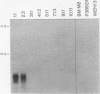
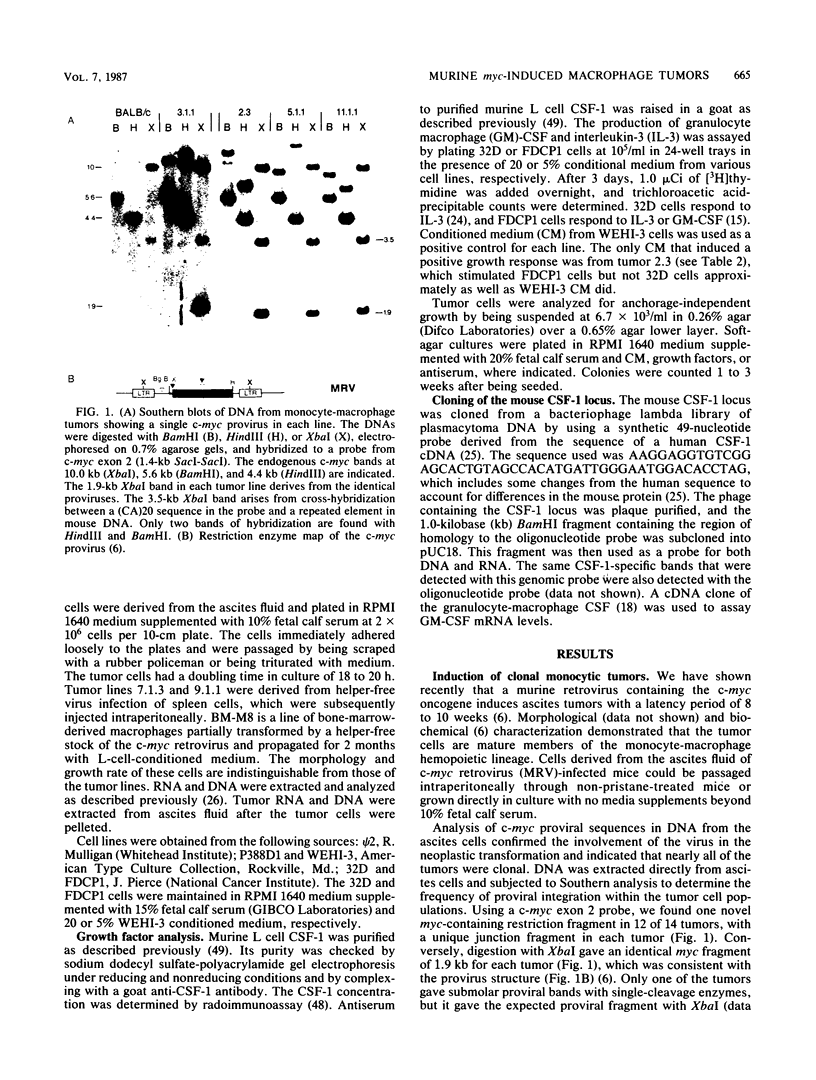
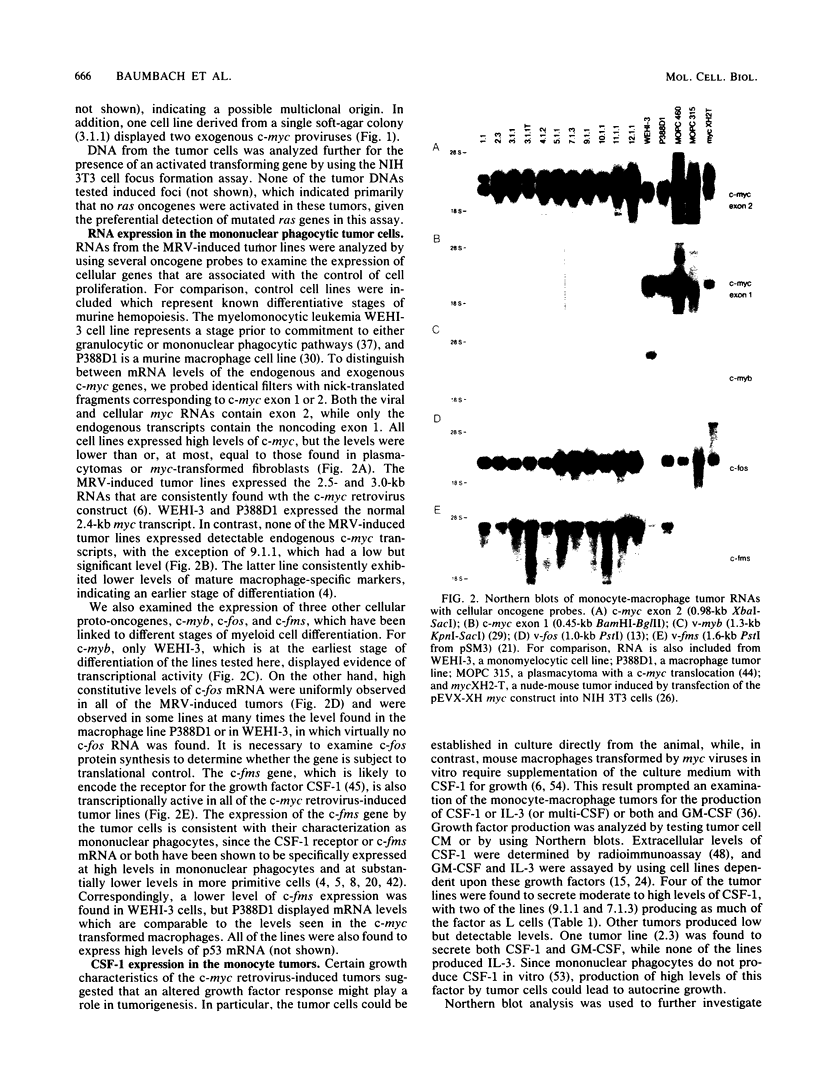
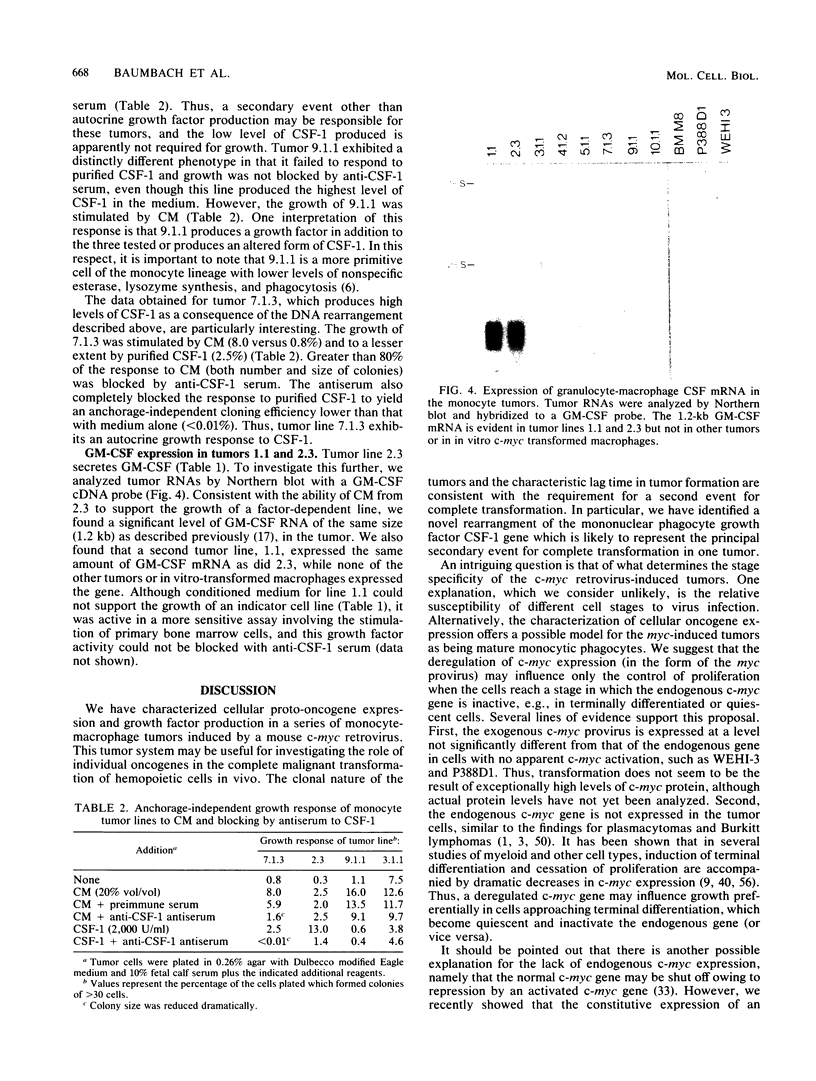
A mouse retrovirus containing the c-myc oncogene was found to induce tumors of mononuclear phagocytic cells in vivo. All tumors expressed the c-myc retroviral gene but not the endogenous c-myc gene (with one exception), and virtually all tumors were clonal with a unique proviral integration. This observation, coupled with a lag time in tumor formation, suggests that a second event, in addition to c-myc proviral integration, is necessary for the generation of neoplastic cells in vivo. All of the tumor cells expressed high levels of mRNA for both the putative colony-stimulating factor 1 (CSF-1) receptor (c-fms proto-oncogene product), as well as the c-fos proto-oncogene. Although all of the tumor cells proliferated in culture without the addition of exogenous CSF-1, which is required for the proliferation of primary macrophages partially transformed by the same c-myc retrovirus, several phenotypes were observed with respect to the expression of CSF-1 and granulocyte-macrophage CSF and to their growth factor responsiveness. The proliferation of one tumor, which secreted high levels of CSF-1, was blocked by specific anti-CSF-1 serum. This tumor was found to express altered CSF-1 mRNA and to have a DNA rearrangement at the CSF-1 locus. In this particular case, the data indicate that a CSF-1 gene rearrangement was the secondary event in development of the tumor. The pleiotropy of phenotypes among the other tumors indicated that there are a variety of other mechanisms for such secondary events which can be investigated with this system.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Gerondakis S., Webb E., Corcoran L. M., Cory S. Cellular myc oncogene is altered by chromosome translocation to an immunoglobulin locus in murine plasmacytomas and is rearranged similarly in human Burkitt lymphomas. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1982–1986. doi: 10.1073/pnas.80.7.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adkins B., Leutz A., Graf T. Autocrine growth induced by src-related oncogenes in transformed chicken myeloid cells. Cell. 1984 Dec;39(3 Pt 2):439–445. doi: 10.1016/0092-8674(84)90451-3. [DOI] [PubMed] [Google Scholar]
- Bartelmez S. H., Sacca R., Stanley E. R. Lineage specific receptors used to identify a growth factor for developmentally early hemopoietic cells: assay of hemopoietin-2. J Cell Physiol. 1985 Mar;122(3):362–369. doi: 10.1002/jcp.1041220305. [DOI] [PubMed] [Google Scholar]
- Bartelmez S. H., Stanley E. R. Synergism between hemopoietic growth factors (HGFs) detected by their effects on cells bearing receptors for a lineage specific HGF: assay of hemopoietin-1. J Cell Physiol. 1985 Mar;122(3):370–378. doi: 10.1002/jcp.1041220306. [DOI] [PubMed] [Google Scholar]
- Baumbach W. R., Keath E. J., Cole M. D. A mouse c-myc retrovirus transforms established fibroblast lines in vitro and induces monocyte-macrophage tumors in vivo. J Virol. 1986 Aug;59(2):276–283. doi: 10.1128/jvi.59.2.276-283.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betsholtz C., Westermark B., Ek B., Heldin C. H. Coexpression of a PDGF-like growth factor and PDGF receptors in a human osteosarcoma cell line: implications for autocrine receptor activation. Cell. 1984 Dec;39(3 Pt 2):447–457. doi: 10.1016/0092-8674(84)90452-5. [DOI] [PubMed] [Google Scholar]
- Byrne P. V., Guilbert L. J., Stanley E. R. Distribution of cells bearing receptors for a colony-stimulating factor (CSF-1) in murine tissues. J Cell Biol. 1981 Dec;91(3 Pt 1):848–853. doi: 10.1083/jcb.91.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J., Gray H. E., Pardee A. B., Dean M., Sonenshein G. E. Cell-cycle control of c-myc but not c-ras expression is lost following chemical transformation. Cell. 1984 Feb;36(2):241–247. doi: 10.1016/0092-8674(84)90217-4. [DOI] [PubMed] [Google Scholar]
- Collins S., Groudine M. Amplification of endogenous myc-related DNA sequences in a human myeloid leukaemia cell line. Nature. 1982 Aug 12;298(5875):679–681. doi: 10.1038/298679a0. [DOI] [PubMed] [Google Scholar]
- Coppola J. A., Cole M. D. Constitutive c-myc oncogene expression blocks mouse erythroleukaemia cell differentiation but not commitment. Nature. 1986 Apr 24;320(6064):760–763. doi: 10.1038/320760a0. [DOI] [PubMed] [Google Scholar]
- Corcoran L. M., Adams J. M., Dunn A. R., Cory S. Murine T lymphomas in which the cellular myc oncogene has been activated by retroviral insertion. Cell. 1984 May;37(1):113–122. doi: 10.1016/0092-8674(84)90306-4. [DOI] [PubMed] [Google Scholar]
- Dalla-Favera R., Wong-Staal F., Gallo R. C. Onc gene amplification in promyelocytic leukaemia cell line HL-60 and primary leukaemic cells of the same patient. Nature. 1982 Sep 2;299(5878):61–63. doi: 10.1038/299061a0. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Garland J., Scott D., Scolnick E., Metcalf D. Growth of factor-dependent hemopoietic precursor cell lines. J Exp Med. 1980 Oct 1;152(4):1036–1047. doi: 10.1084/jem.152.4.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson J., ar-Rushdi A., Drwinga H. L., Nowell P. C., Croce C. M. Transcriptional activation of the translocated c-myc oncogene in burkitt lymphoma. Proc Natl Acad Sci U S A. 1983 Feb;80(3):820–824. doi: 10.1073/pnas.80.3.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough N. M., Gough J., Metcalf D., Kelso A., Grail D., Nicola N. A., Burgess A. W., Dunn A. R. Molecular cloning of cDNA encoding a murine haematopoietic growth regulator, granulocyte-macrophage colony stimulating factor. 1984 Jun 28-Jul 4Nature. 309(5971):763–767. doi: 10.1038/309763a0. [DOI] [PubMed] [Google Scholar]
- Gough N. M., Metcalf D., Gough J., Grail D., Dunn A. R. Structure and expression of the mRNA for murine granulocyte-macrophage colony stimulating factor. EMBO J. 1985 Mar;4(3):645–653. doi: 10.1002/j.1460-2075.1985.tb03678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf T., Beug H. Avian leukemia viruses: interaction with their target cells in vivo and in vitro. Biochim Biophys Acta. 1978 Nov 17;516(3):269–299. doi: 10.1016/0304-419x(78)90011-2. [DOI] [PubMed] [Google Scholar]
- Guilbert L. J., Stanley E. R. Specific interaction of murine colony-stimulating factor with mononuclear phagocytic cells. J Cell Biol. 1980 Apr;85(1):153–159. doi: 10.1083/jcb.85.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampe A., Gobet M., Sherr C. J., Galibert F. Nucleotide sequence of the feline retroviral oncogene v-fms shows unexpected homology with oncogenes encoding tyrosine-specific protein kinases. Proc Natl Acad Sci U S A. 1984 Jan;81(1):85–89. doi: 10.1073/pnas.81.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hapel A. J., Lee J. C., Farrar W. L., Ihle J. N. Establishment of continuous cultures of thy1.2+, Lyt1+, 2-T cells with purified interleukin 3. Cell. 1981 Jul;25(1):179–186. doi: 10.1016/0092-8674(81)90242-7. [DOI] [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Keller J., Greenberger J. S., Henderson L., Yetter R. A., Morse H. C., 3rd Phenotypic characteristics of cell lines requiring interleukin 3 for growth. J Immunol. 1982 Oct;129(4):1377–1383. [PubMed] [Google Scholar]
- Kawasaki E. S., Ladner M. B., Wang A. M., Van Arsdell J., Warren M. K., Coyne M. Y., Schweickart V. L., Lee M. T., Wilson K. J., Boosman A. Molecular cloning of a complementary DNA encoding human macrophage-specific colony-stimulating factor (CSF-1). Science. 1985 Oct 18;230(4723):291–296. doi: 10.1126/science.2996129. [DOI] [PubMed] [Google Scholar]
- Keath E. J., Caimi P. G., Cole M. D. Fibroblast lines expressing activated c-myc oncogenes are tumorigenic in nude mice and syngeneic animals. Cell. 1984 Dec;39(2 Pt 1):339–348. doi: 10.1016/0092-8674(84)90012-6. [DOI] [PubMed] [Google Scholar]
- Kelekar A., Cole M. D. Tumorigenicity of fibroblast lines expressing the adenovirus E1a, cellular p53, or normal c-myc genes. Mol Cell Biol. 1986 Jan;6(1):7–14. doi: 10.1128/mcb.6.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Klempnauer K. H., Gonda T. J., Bishop J. M. Nucleotide sequence of the retroviral leukemia gene v-myb and its cellular progenitor c-myb: the architecture of a transduced oncogene. Cell. 1982 Dec;31(2 Pt 1):453–463. doi: 10.1016/0092-8674(82)90138-6. [DOI] [PubMed] [Google Scholar]
- Koren H. S., Handwerger B. S., Wunderlich J. R. Identification of macrophage-like characteristics in a cultured murine tumor line. J Immunol. 1975 Feb;114(2 Pt 2):894–897. [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Lang R. A., Metcalf D., Gough N. M., Dunn A. R., Gonda T. J. Expression of a hemopoietic growth factor cDNA in a factor-dependent cell line results in autonomous growth and tumorigenicity. Cell. 1985 Dec;43(2 Pt 1):531–542. doi: 10.1016/0092-8674(85)90182-5. [DOI] [PubMed] [Google Scholar]
- Leder P., Battey J., Lenoir G., Moulding C., Murphy W., Potter H., Stewart T., Taub R. Translocations among antibody genes in human cancer. Science. 1983 Nov 18;222(4625):765–771. doi: 10.1126/science.6356357. [DOI] [PubMed] [Google Scholar]
- Little C. D., Nau M. M., Carney D. N., Gazdar A. F., Minna J. D. Amplification and expression of the c-myc oncogene in human lung cancer cell lines. Nature. 1983 Nov 10;306(5939):194–196. doi: 10.1038/306194a0. [DOI] [PubMed] [Google Scholar]
- Marcu K. B., Harris L. J., Stanton L. W., Erikson J., Watt R., Croce C. M. Transcriptionally active c-myc oncogene is contained within NIARD, a DNA sequence associated with chromosome translocations in B-cell neoplasia. Proc Natl Acad Sci U S A. 1983 Jan;80(2):519–523. doi: 10.1073/pnas.80.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D., Moore M. A., Warner N. L. Colony formation in vitro by myelomonocytic leukemic cells. J Natl Cancer Inst. 1969 Oct;43(4):983–1001. [PubMed] [Google Scholar]
- Mougneau E., Lemieux L., Rassoulzadegan M., Cuzin F. Biological activities of v-myc and rearranged c-myc oncogenes in rat fibroblast cells in culture. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5758–5762. doi: 10.1073/pnas.81.18.5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri S., Kahn P., Graf T. Quail embryo fibroblasts transformed by four v-myc-containing virus isolates show enhanced proliferation but are non tumorigenic. EMBO J. 1983;2(12):2385–2389. doi: 10.1002/j.1460-2075.1983.tb01750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitsma P. H., Rothberg P. G., Astrin S. M., Trial J., Bar-Shavit Z., Hall A., Teitelbaum S. L., Kahn A. J. Regulation of myc gene expression in HL-60 leukaemia cells by a vitamin D metabolite. Nature. 1983 Dec 1;306(5942):492–494. doi: 10.1038/306492a0. [DOI] [PubMed] [Google Scholar]
- Sariban E., Mitchell T., Kufe D. Expression of the c-fms proto-oncogene during human monocytic differentiation. Nature. 1985 Jul 4;316(6023):64–66. doi: 10.1038/316064a0. [DOI] [PubMed] [Google Scholar]
- Schrader J. W., Crapper R. M. Autogenous production of a hemopoietic growth factor, persisting-cell-stimulating factor, as a mechanism for transformation of bone marrow-derived cells. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6892–6896. doi: 10.1073/pnas.80.22.6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen-Ong G. L., Keath E. J., Piccoli S. P., Cole M. D. Novel myc oncogene RNA from abortive immunoglobulin-gene recombination in mouse plasmacytomas. Cell. 1982 Dec;31(2 Pt 1):443–452. doi: 10.1016/0092-8674(82)90137-4. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Rettenmier C. W., Sacca R., Roussel M. F., Look A. T., Stanley E. R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985 Jul;41(3):665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- Siden E. J., Baltimore D., Clark D., Rosenberg N. E. Immunoglobulin synthesis by lymphoid cells transformed in vitro by Abelson murine leukemia virus. Cell. 1979 Feb;16(2):389–396. doi: 10.1016/0092-8674(79)90014-x. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Autocrine growth factors and cancer. 1985 Feb 28-Mar 6Nature. 313(6005):745–747. doi: 10.1038/313745a0. [DOI] [PubMed] [Google Scholar]
- Stanley E. R. Colony-stimulating factor (CSF) radioimmunoassay: detection of a CSF subclass stimulating macrophage production. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2969–2973. doi: 10.1073/pnas.76.6.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E. R. The macrophage colony-stimulating factor, CSF-1. Methods Enzymol. 1985;116:564–587. doi: 10.1016/s0076-6879(85)16044-1. [DOI] [PubMed] [Google Scholar]
- Stanton L. W., Watt R., Marcu K. B. Translocation, breakage and truncated transcripts of c-myc oncogene in murine plasmacytomas. Nature. 1983 Jun 2;303(5916):401–406. doi: 10.1038/303401a0. [DOI] [PubMed] [Google Scholar]
- Steffen D. Proviruses are adjacent to c-myc in some murine leukemia virus-induced lymphomas. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2097–2101. doi: 10.1073/pnas.81.7.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub R., Kirsch I., Morton C., Lenoir G., Swan D., Tronick S., Aaronson S., Leder P. Translocation of the c-myc gene into the immunoglobulin heavy chain locus in human Burkitt lymphoma and murine plasmacytoma cells. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7837–7841. doi: 10.1073/pnas.79.24.7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tushinski R. J., Oliver I. T., Guilbert L. J., Tynan P. W., Warner J. R., Stanley E. R. Survival of mononuclear phagocytes depends on a lineage-specific growth factor that the differentiated cells selectively destroy. Cell. 1982 Jan;28(1):71–81. doi: 10.1016/0092-8674(82)90376-2. [DOI] [PubMed] [Google Scholar]
- Vennström B., Kahn P., Adkins B., Enrietto P., Hayman M. J., Graf T., Luciw P. Transformation of mammalian fibroblasts and macrophages in vitro by a murine retrovirus encoding an avian v-myc oncogene. EMBO J. 1984 Dec 20;3(13):3223–3229. doi: 10.1002/j.1460-2075.1984.tb02282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin E. H., Wong-Staal F., Gelmann E. P., Dalla-Favera R., Papas T. S., Lautenberger J. A., Eva A., Reddy E. P., Tronick S. R., Aaronson S. A. Expression of cellular homologues of retroviral onc genes in human hematopoietic cells. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2490–2494. doi: 10.1073/pnas.79.8.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ar-Rushdi A., Nishikura K., Erikson J., Watt R., Rovera G., Croce C. M. Differential expression of the translocated and the untranslocated c-myc oncogene in Burkitt lymphoma. Science. 1983 Oct 28;222(4622):390–393. doi: 10.1126/science.6414084. [DOI] [PubMed] [Google Scholar]