Abstract
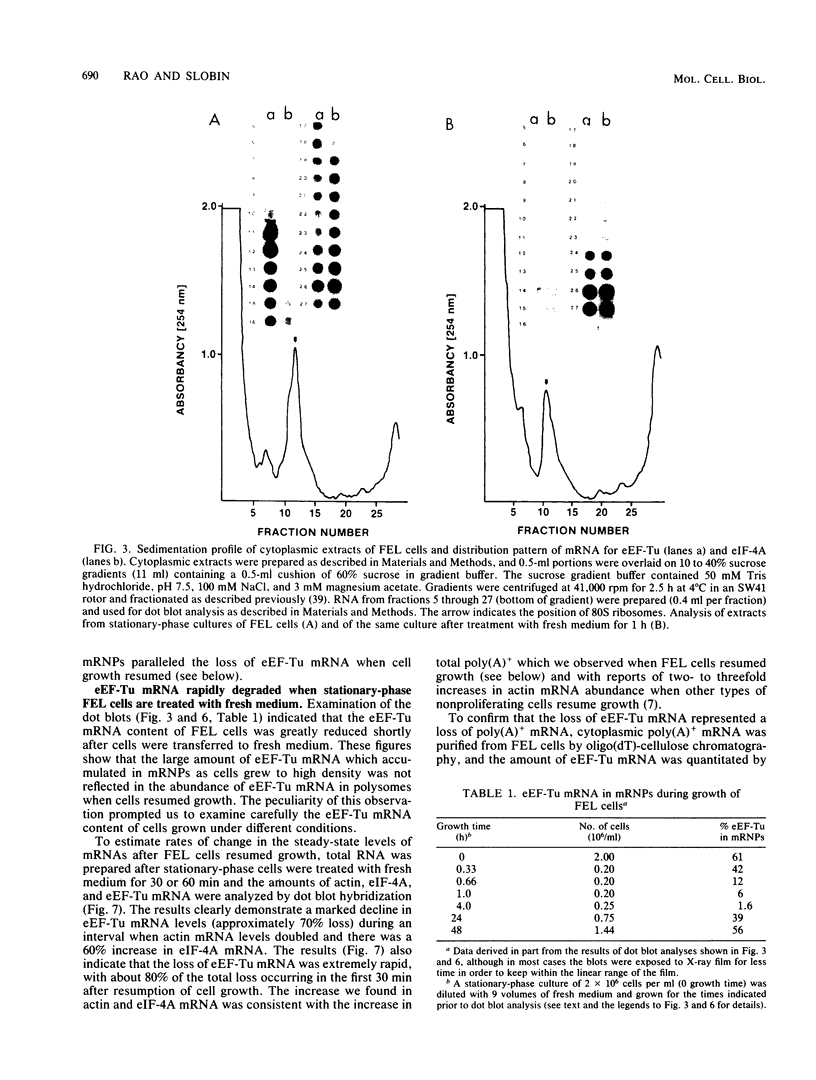
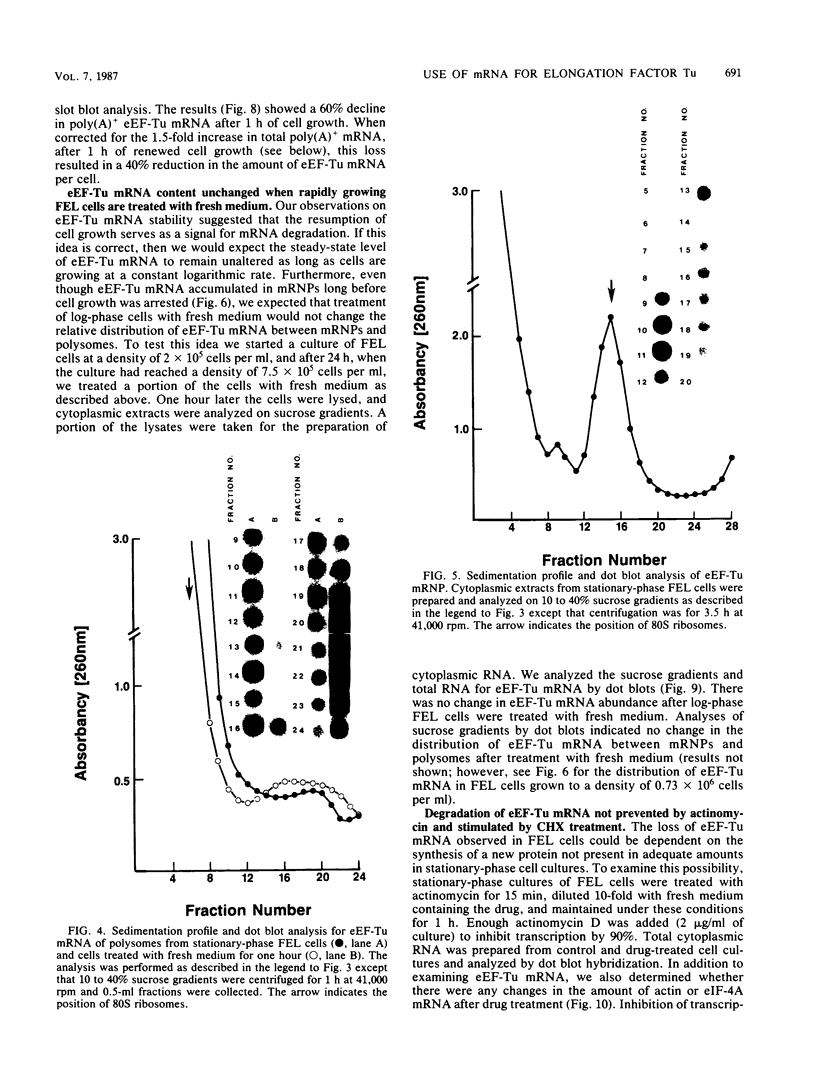
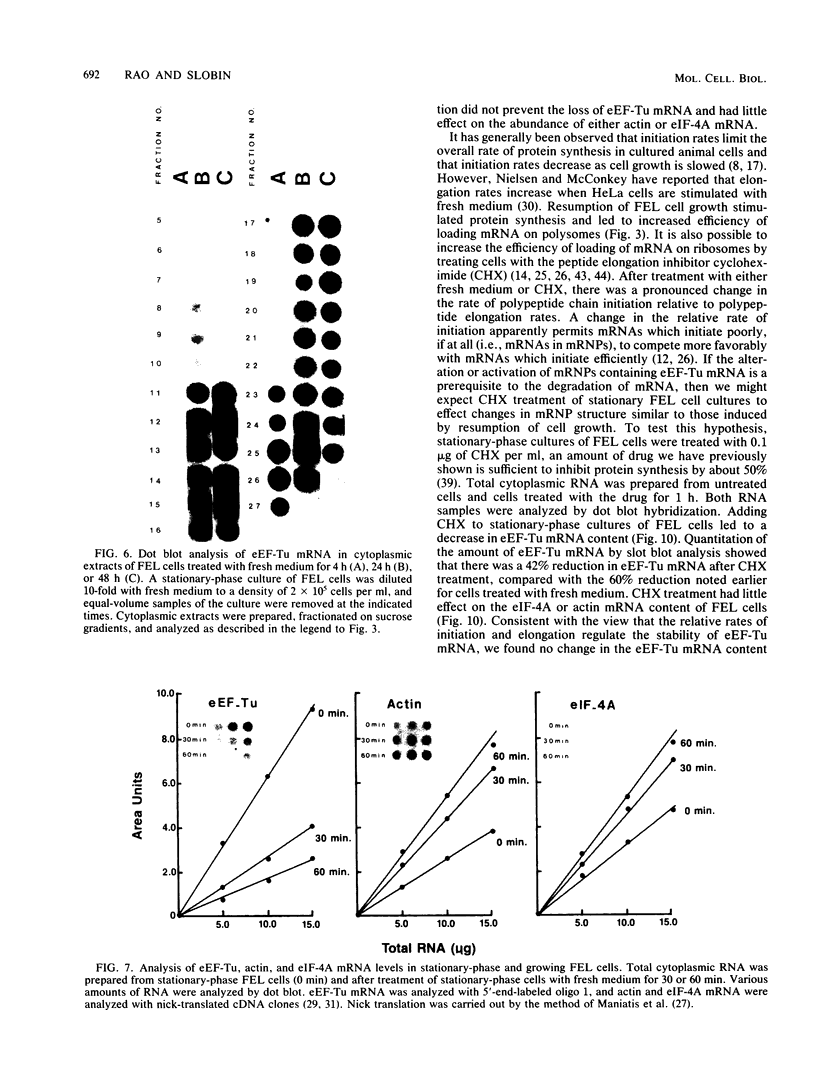
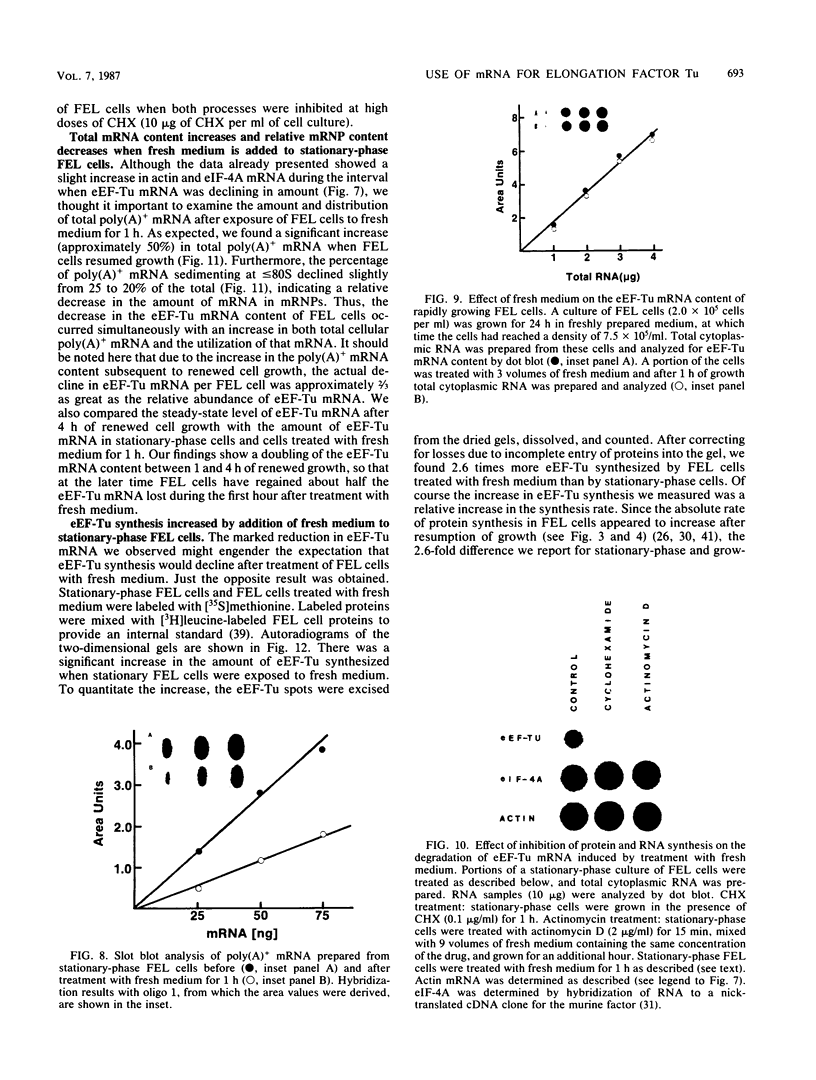
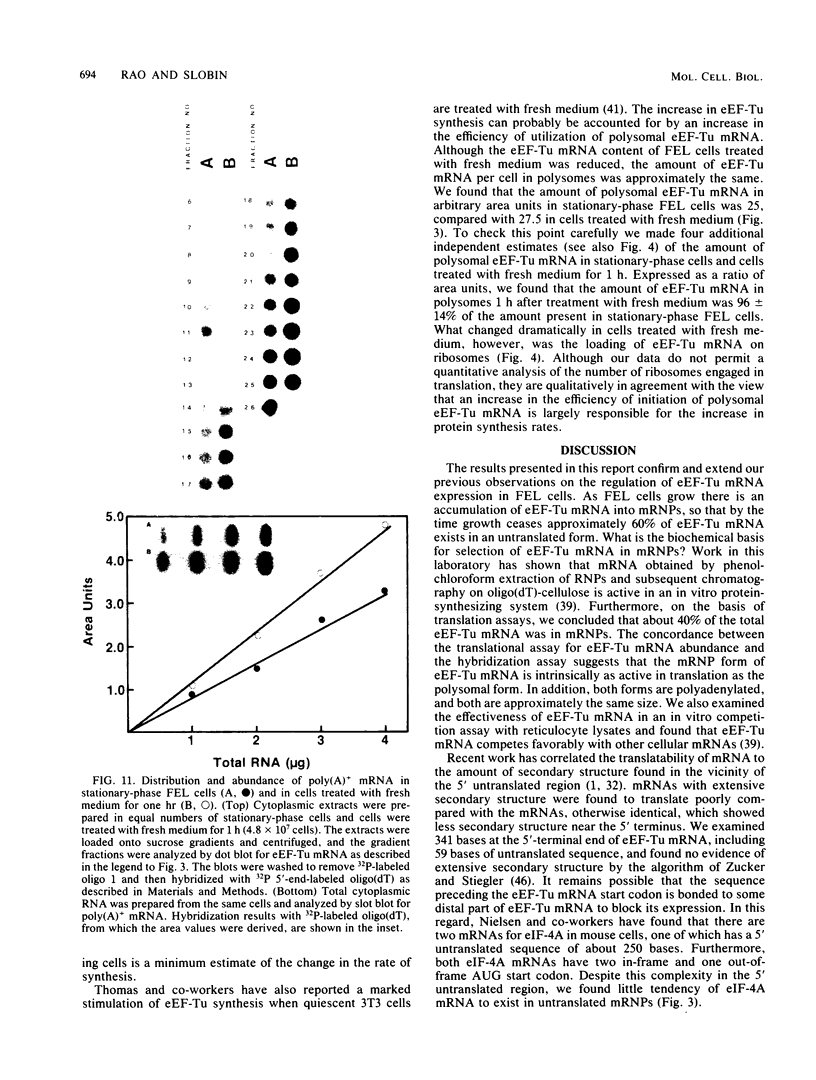
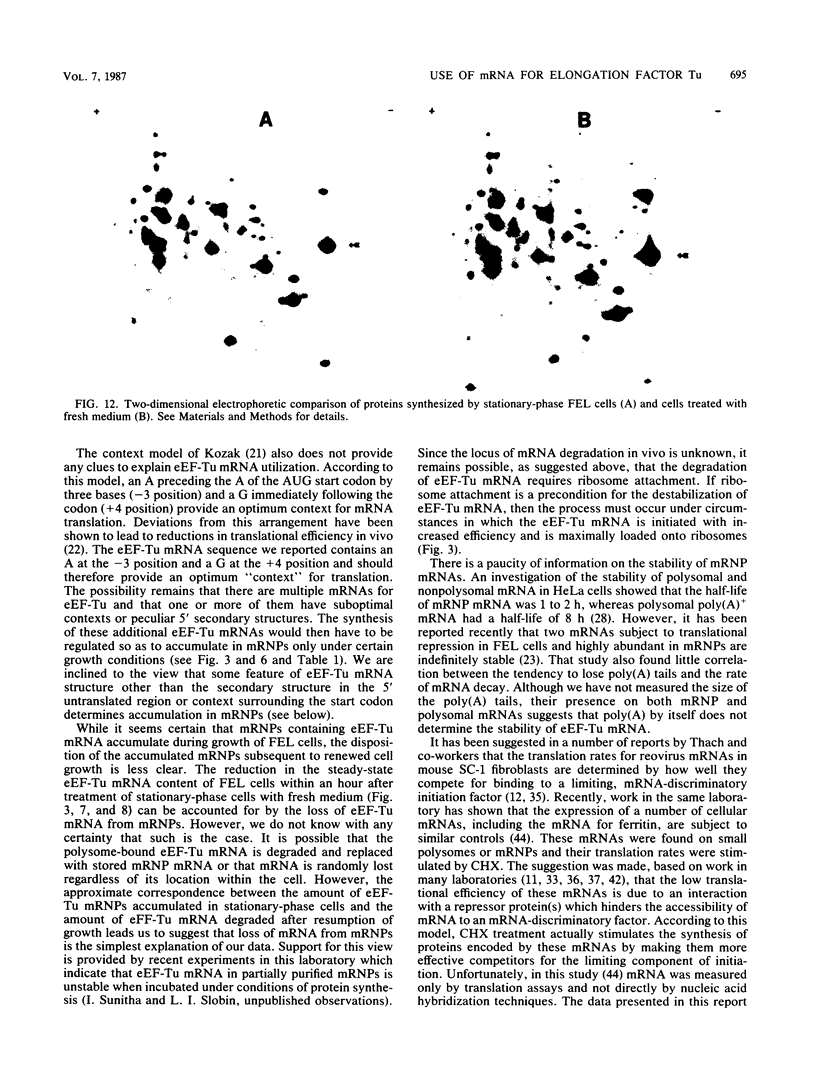
When Friend erythroleukemia cells were allowed to grow to stationary phase (2 X 10(6) to 3 X 10(6) cells per ml), approximately 60% of the mRNA for eucaryotic elongation factor Tu (eEF-Tu) sedimented at less than or equal to 80S, and most of the remaining factor mRNA was associated with small polysomes. Under the same growth conditions, greater than 90% of the mRNA for eucaryotic initiation factor 4A remained associated with polysomes. The association of eEF-Tu mRNA with polysomes changed dramatically when stationary-phase cells were treated with fresh medium. After 1 h in fresh medium, approximately 90% of eEF-Tu mRNA in Friend cells was found in heavy polysomes. Associated with the shift of eEF-Tu mRNA into heavy polysomes, we found at least a 2.6-fold increase in the synthesis of eEF-Tu in vivo as well as a remarkable 40% decrease in the total amount of eEF-Tu mRNA per cell. Our data raise the possibility that eEF-Tu mRNA that has accumulated in ribonucleoprotein particles in stationary-phase cells is degraded rather than reutilized for eEF-Tu synthesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baim S. B., Pietras D. F., Eustice D. C., Sherman F. A mutation allowing an mRNA secondary structure diminishes translation of Saccharomyces cerevisiae iso-1-cytochrome c. Mol Cell Biol. 1985 Aug;5(8):1839–1846. doi: 10.1128/mcb.5.8.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz M., Gurdon J. B. The heat-shock response in Xenopus oocytes is controlled at the translational level. Cell. 1982 Jul;29(3):811–819. doi: 10.1016/0092-8674(82)90443-3. [DOI] [PubMed] [Google Scholar]
- Brands J. H., Maassen J. A., van Hemert F. J., Amons R., Möller W. The primary structure of the alpha subunit of human elongation factor 1. Structural aspects of guanine-nucleotide-binding sites. Eur J Biochem. 1986 Feb 17;155(1):167–171. doi: 10.1111/j.1432-1033.1986.tb09472.x. [DOI] [PubMed] [Google Scholar]
- Cheley S., Anderson R. A reproducible microanalytical method for the detection of specific RNA sequences by dot-blot hybridization. Anal Biochem. 1984 Feb;137(1):15–19. doi: 10.1016/0003-2697(84)90339-7. [DOI] [PubMed] [Google Scholar]
- Croall D. E., Morrison M. R. Polysomal and non-polysomal messenger RNA in neuroblastoma cells. Lack of correlation between polyadenylation or initiation efficiency and messenger RNA location. J Mol Biol. 1980 Jul 15;140(4):549–564. doi: 10.1016/0022-2836(80)90270-3. [DOI] [PubMed] [Google Scholar]
- Dean M., Levine R. A., Ran W., Kindy M. S., Sonenshein G. E., Campisi J. Regulation of c-myc transcription and mRNA abundance by serum growth factors and cell contact. J Biol Chem. 1986 Jul 15;261(20):9161–9166. [PubMed] [Google Scholar]
- Duncan R., Hershey J. W. Regulation of initiation factors during translational repression caused by serum depletion. Abundance, synthesis, and turnover rates. J Biol Chem. 1985 May 10;260(9):5486–5492. [PubMed] [Google Scholar]
- Epel D. Protein synthesis in sea urchin eggs: a "late" response to fertilization. Proc Natl Acad Sci U S A. 1967 Apr;57(4):899–906. doi: 10.1073/pnas.57.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feramisco J. R., Smart J. E., Burridge K., Helfman D. M., Thomas G. P. Co-existence of vinculin and a vinculin-like protein of higher molecular weight in smooth muscle. J Biol Chem. 1982 Sep 25;257(18):11024–11031. [PubMed] [Google Scholar]
- Geoghegan T., Cereghini S., Brawerman G. Inactive mRNA-protein complexes from mouse sarcoma-180 ascites cells. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5587–5591. doi: 10.1073/pnas.76.11.5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godefroy-Colburn T., Thach R. E. The role of mRNA competition in regulating translation. IV. Kinetic model. J Biol Chem. 1981 Nov 25;256(22):11762–11773. [PubMed] [Google Scholar]
- Heumann R., Korsching S., Scott J., Thoenen H. Relationship between levels of nerve growth factor (NGF) and its messenger RNA in sympathetic ganglia and peripheral target tissues. EMBO J. 1984 Dec 20;3(13):3183–3189. doi: 10.1002/j.1460-2075.1984.tb02277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignotz G. G., Hokari S., DePhilip R. M., Tsukada K., Lieberman I. Lodish model and regulation of ribosomal protein synthesis by insulin-deficient chick embryo fibroblasts. Biochemistry. 1981 Apr 28;20(9):2550–2558. doi: 10.1021/bi00512a029. [DOI] [PubMed] [Google Scholar]
- Imaizumi-Scherrer M. T., Maundrell K., Civelli O., Scherrer K. Transcriptional and post-transcriptional regulation in duck erythroblasts. Dev Biol. 1982 Sep;93(1):126–138. doi: 10.1016/0012-1606(82)90246-9. [DOI] [PubMed] [Google Scholar]
- Infante A. A., Heilmann L. J. Distribution of messenger ribonucleic acid in polysomes and nonpolysomal particles of sea urchin embryos: translational control of actin synthesis. Biochemistry. 1981 Jan 6;20(1):1–8. doi: 10.1021/bi00504a001. [DOI] [PubMed] [Google Scholar]
- Jagus R., Anderson W. F., Safer B. The regulation of initiation of mammalian protein synthesis. Prog Nucleic Acid Res Mol Biol. 1981;25:127–185. doi: 10.1016/s0079-6603(08)60484-5. [DOI] [PubMed] [Google Scholar]
- Jenkins N. A., Kaumeyer J. F., Young E. M., Raff R. A. A test for masked message: the template activity of messenger ribonucleoprotein particles isolated from sea urchine eggs. Dev Biol. 1978 Apr;63(2):279–298. doi: 10.1016/0012-1606(78)90134-3. [DOI] [PubMed] [Google Scholar]
- Kay M. A., Jacobs-Lorena M. Selective translational regulation of ribosomal protein gene expression during early development of Drosophila melanogaster. Mol Cell Biol. 1985 Dec;5(12):3583–3592. doi: 10.1128/mcb.5.12.3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinniburgh A. J., McMullen M. D., Martin T. E. Distribution of cytoplasmic poly(A+)RNA sequences in free messenger ribonucleoprotein and polysomes of mouse ascites cells. J Mol Biol. 1979 Aug 25;132(4):695–708. doi: 10.1016/0022-2836(79)90383-8. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Krowczynska A., Yenofsky R., Brawerman G. Regulation of messenger RNA stability in mouse erythroleukemia cells. J Mol Biol. 1985 Jan 20;181(2):231–239. doi: 10.1016/0022-2836(85)90087-7. [DOI] [PubMed] [Google Scholar]
- Lee G. T., Engelhardt D. L. Growth-related fluctuation in messenger RNA utilization in animal cells. J Cell Biol. 1978 Oct;79(1):85–86. doi: 10.1083/jcb.79.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G. T., Engelhardt D. L. Peptide coding capacity of polysomal and non-polysomal messenger RNA during growth of animal cells. J Mol Biol. 1979 Apr 5;129(2):221–233. doi: 10.1016/0022-2836(79)90278-x. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Alpha and beta globin messenger ribonucleic acid. Different amounts and rates of initiation of translation. J Biol Chem. 1971 Dec 10;246(23):7131–7138. [PubMed] [Google Scholar]
- Mauron A., Spohr G. Kinetics of synthesis of cytoplasmic messenger-like RNA not associated with ribosomes in HeLa cells. Eur J Biochem. 1978 Jan 16;82(2):619–625. doi: 10.1111/j.1432-1033.1978.tb12058.x. [DOI] [PubMed] [Google Scholar]
- Minty A. J., Caravatti M., Robert B., Cohen A., Daubas P., Weydert A., Gros F., Buckingham M. E. Mouse actin messenger RNAs. Construction and characterization of a recombinant plasmid molecule containing a complementary DNA transcript of mouse alpha-actin mRNA. J Biol Chem. 1981 Jan 25;256(2):1008–1014. [PubMed] [Google Scholar]
- Nielsen P. J., McConkey E. H. Evidence for control of protein synthesis in HeLa cells via the elongation rate. J Cell Physiol. 1980 Sep;104(3):269–281. doi: 10.1002/jcp.1041040302. [DOI] [PubMed] [Google Scholar]
- Nielsen P. J., McMaster G. K., Trachsel H. Cloning of eukaryotic protein synthesis initiation factor genes: isolation and characterization of cDNA clones encoding factor eIF-4A. Nucleic Acids Res. 1985 Oct 11;13(19):6867–6880. doi: 10.1093/nar/13.19.6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Insertion mutagenesis to increase secondary structure within the 5' noncoding region of a eukaryotic mRNA reduces translational efficiency. Cell. 1985 Mar;40(3):515–526. doi: 10.1016/0092-8674(85)90200-4. [DOI] [PubMed] [Google Scholar]
- Preobrazhensky A. A., Spirin A. S. Informosomes and their protein components: the present state of knowledge. Prog Nucleic Acid Res Mol Biol. 1978;21:1–38. doi: 10.1016/s0079-6603(08)60265-2. [DOI] [PubMed] [Google Scholar]
- Rao T. R., Slobin L. I. Structure of the amino-terminal end of mammalian elongation factor Tu. Nucleic Acids Res. 1986 Mar 11;14(5):2409–2409. doi: 10.1093/nar/14.5.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A., Walden W. E., Brendler T., Zenger V. E., Thach R. E. Effect of medium hypertonicity on reovirus translation rates. An application of kinetic modeling in vivo. Biochemistry. 1985 Dec 17;24(26):7525–7532. doi: 10.1021/bi00347a004. [DOI] [PubMed] [Google Scholar]
- Ruzdijic S., Bag J., Sells B. H. Cross-linked proteins associated with a specific mRNA in the cytoplasm of HeLa cells. Eur J Biochem. 1984 Jul 16;142(2):239–245. doi: 10.1111/j.1432-1033.1984.tb08277.x. [DOI] [PubMed] [Google Scholar]
- Sinclair G. D., Dixon G. H. Purification and characterization of cytoplasmic protamine messenger ribonucleoprotein particles from rainbow trout testis cells. Biochemistry. 1982 Apr 13;21(8):1869–1877. doi: 10.1021/bi00537a026. [DOI] [PubMed] [Google Scholar]
- Singh L., Jones K. W. The use of heparin as a simple cost-effective means of controlling background in nucleic acid hybridization procedures. Nucleic Acids Res. 1984 Jul 25;12(14):5627–5638. doi: 10.1093/nar/12.14.5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobin L. I., Jordan P. Translational repression of mRNA for eucaryotic elongation factors in Friend erythroleukemia cells. Eur J Biochem. 1984 Nov 15;145(1):143–150. doi: 10.1111/j.1432-1033.1984.tb08533.x. [DOI] [PubMed] [Google Scholar]
- Tansey T. R., Ruderman J. V. Differential mRNA accumulation and translation during Spisula development. Dev Biol. 1983 Oct;99(2):338–351. doi: 10.1016/0012-1606(83)90284-1. [DOI] [PubMed] [Google Scholar]
- Thomas G., Thomas G., Luther H. Transcriptional and translational control of cytoplasmic proteins after serum stimulation of quiescent Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5712–5716. doi: 10.1073/pnas.78.9.5712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent A., Goldenberg S., Standart N., Civelli O., Imaizumi-Scherrer T., Maundrell K., Scherrer K. Potential role of mRNP proteins in cytoplasmic control of gene expression in duck erythroblasts. Mol Biol Rep. 1981 May 22;7(1-3):71–81. doi: 10.1007/BF00778736. [DOI] [PubMed] [Google Scholar]
- Walden W. E., Godefroy-Colburn T., Thach R. E. The role of mRNA competition in regulating translation. I. Demonstration of competition in vivo. J Biol Chem. 1981 Nov 25;256(22):11739–11746. [PubMed] [Google Scholar]
- Walden W. E., Thach R. E. Translational control of gene expression in a normal fibroblast. Characterization of a subclass of mRNAs with unusual kinetic properties. Biochemistry. 1986 Apr 22;25(8):2033–2041. doi: 10.1021/bi00356a030. [DOI] [PubMed] [Google Scholar]
- Yenofsky R., Bergmann I., Brawerman G. Messenger RNA species partially in a repressed state in mouse sarcoma ascites cells. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5876–5880. doi: 10.1073/pnas.79.19.5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]













