Abstract
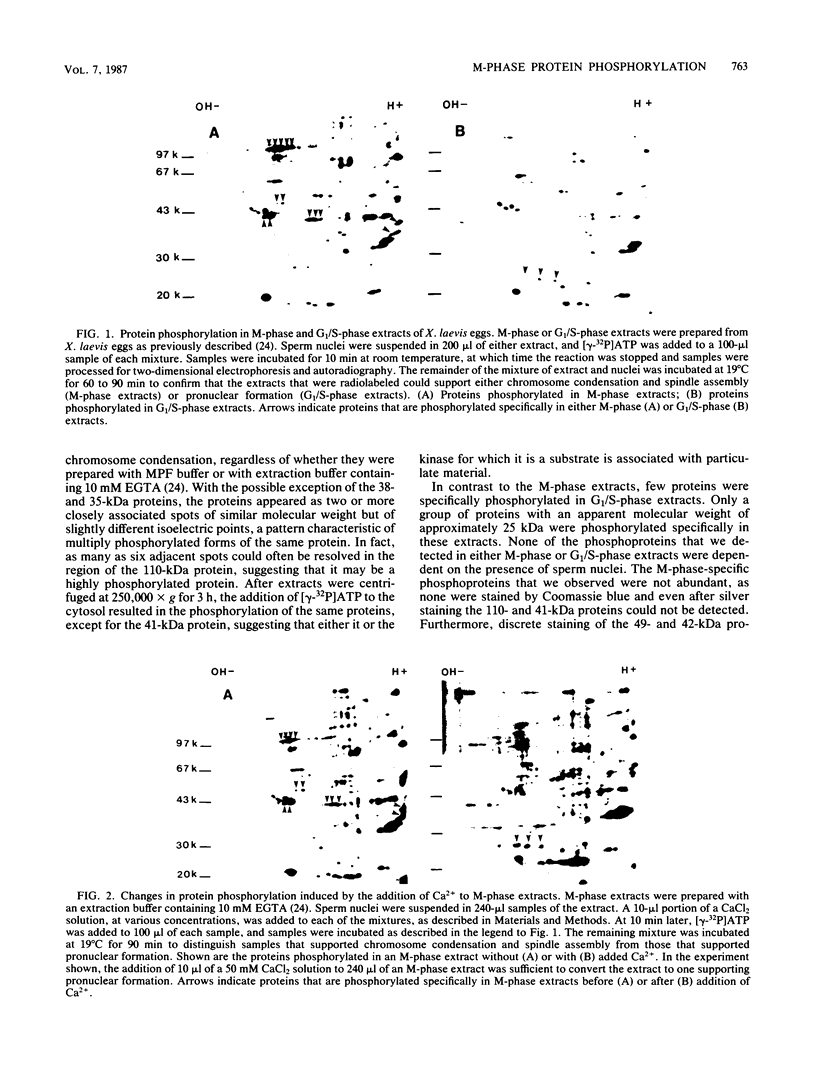
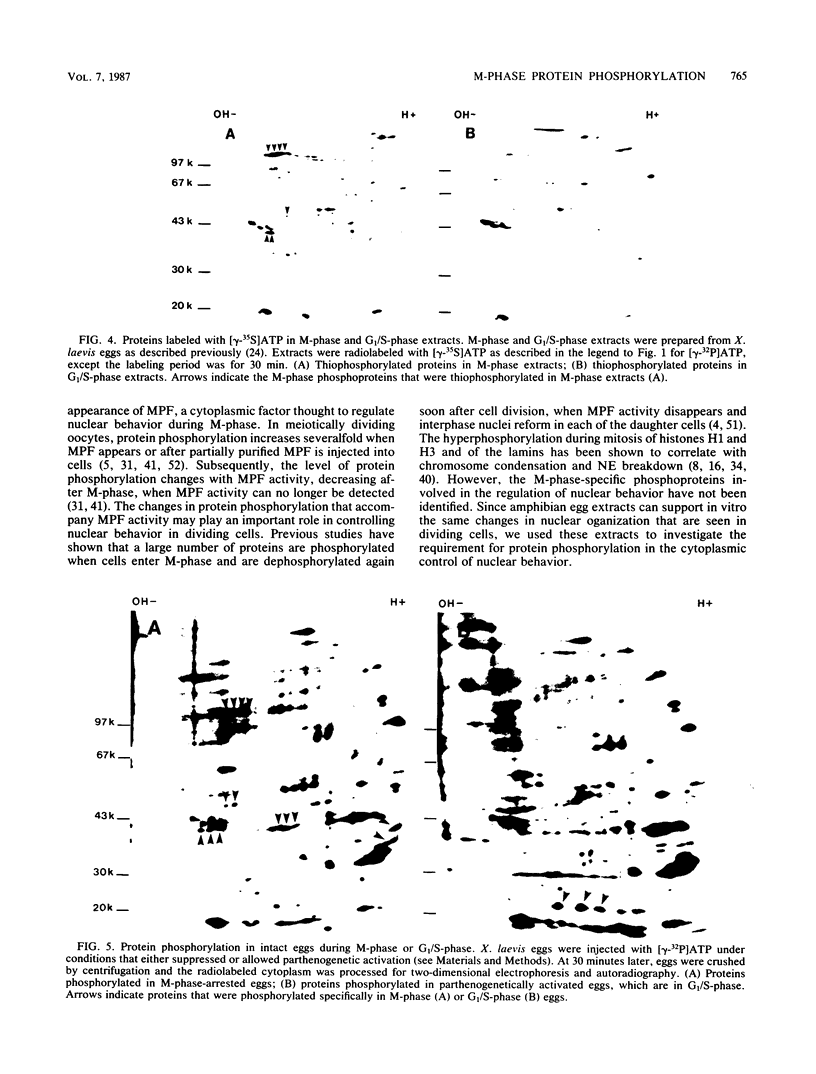
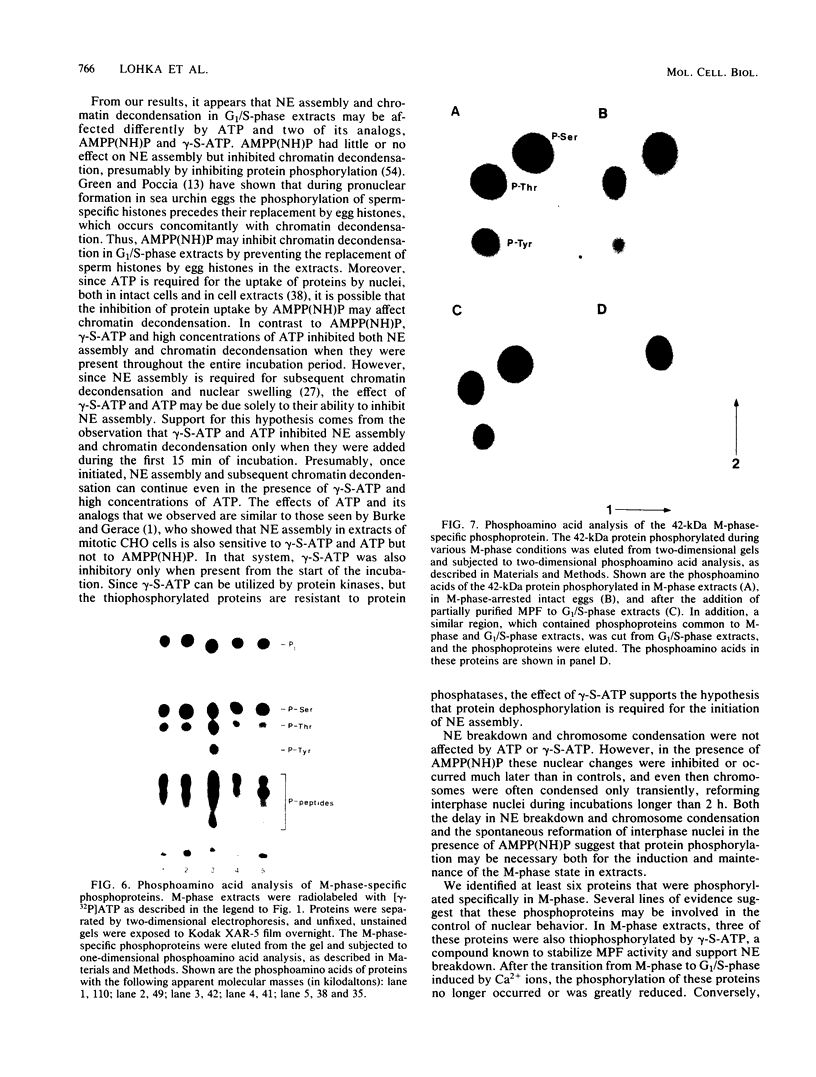
Cytoplasmic extracts of metaphase (M-phase)-arrested Xenopus laevis eggs support nuclear envelope breakdown and chromosome condensation in vitro. Induction of nuclear breakdown is inhibited by AMPP(NH)P, a nonhydrolyzable ATP analog, but not by ATP or gamma-S-ATP, a hydrolyzable ATP analog, suggesting that protein phosphorylation may be required for M-phase nuclear events in vitro. By addition of [gamma-32P]ATP, we have identified in cytoplasmic extracts and in intact eggs at least six phosphoproteins that are present during M-phase but absent in G1/S-phase. These phosphoproteins also appear in response to partially purified preparations of maturation-promoting factor. A subset of these proteins are thiophosphorylated by gamma-S-ATP under conditions that promote nuclear envelope breakdown and chromosome condensation. Each of these proteins is phosphorylated on serine and threonine, and one, a 42-kilodalton protein, is also phosphorylated on tyrosine both in extracts and in intact eggs. These results indicate that activation of protein kinases accounts for at least part of the increased phosphorylation in M-phase and that both protein-serine-threonine kinases and protein-tyrosine kinases may play a role in controlling M-phase nuclear behavior.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burke B., Gerace L. A cell free system to study reassembly of the nuclear envelope at the end of mitosis. Cell. 1986 Feb 28;44(4):639–652. doi: 10.1016/0092-8674(86)90273-4. [DOI] [PubMed] [Google Scholar]
- Busa W. B., Nuccitelli R. An elevated free cytosolic Ca2+ wave follows fertilization in eggs of the frog, Xenopus laevis. J Cell Biol. 1985 Apr;100(4):1325–1329. doi: 10.1083/jcb.100.4.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Davis F. M., Tsao T. Y., Fowler S. K., Rao P. N. Monoclonal antibodies to mitotic cells. Proc Natl Acad Sci U S A. 1983 May;80(10):2926–2930. doi: 10.1073/pnas.80.10.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorée M., Peaucellier G., Picard A. Activity of the maturation-promoting factor and the extent of protein phosphorylation oscillate simultaneously during meiotic maturation of starfish oocytes. Dev Biol. 1983 Oct;99(2):489–501. doi: 10.1016/0012-1606(83)90298-1. [DOI] [PubMed] [Google Scholar]
- Drury K. Method for the preparation of active maturation promoting factor (MPF) from in vitro matured oocytes of Xenopus laevis. Differentiation. 1978 May 26;10(3):181–186. doi: 10.1111/j.1432-0436.1978.tb00962.x. [DOI] [PubMed] [Google Scholar]
- Forbes D. J., Kirschner M. W., Newport J. W. Spontaneous formation of nucleus-like structures around bacteriophage DNA microinjected into Xenopus eggs. Cell. 1983 Aug;34(1):13–23. doi: 10.1016/0092-8674(83)90132-0. [DOI] [PubMed] [Google Scholar]
- Gerace L., Blobel G. The nuclear envelope lamina is reversibly depolymerized during mitosis. Cell. 1980 Jan;19(1):277–287. doi: 10.1016/0092-8674(80)90409-2. [DOI] [PubMed] [Google Scholar]
- Gerhart J., Wu M., Cyert M., Kirschner M. M-phase promoting factors from eggs of Xenopus laevis. Cytobios. 1985;43(174S):335–347. [PubMed] [Google Scholar]
- Gerhart J., Wu M., Kirschner M. Cell cycle dynamics of an M-phase-specific cytoplasmic factor in Xenopus laevis oocytes and eggs. J Cell Biol. 1984 Apr;98(4):1247–1255. doi: 10.1083/jcb.98.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green G. R., Poccia D. L. Phosphorylation of sea urchin sperm H1 and H2B histones precedes chromatin decondensation and H1 exchange during pronuclear formation. Dev Biol. 1985 Mar;108(1):235–245. doi: 10.1016/0012-1606(85)90026-0. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B. Changes in somatic cell nuclei inserted into growing and maturing amphibian oocytes. J Embryol Exp Morphol. 1968 Nov;20(3):401–414. [PubMed] [Google Scholar]
- Gurdon J. B. Injected nuclei in frog oocytes: fate, enlargement, and chromatin dispersal. J Embryol Exp Morphol. 1976 Dec;36(3):523–540. [PubMed] [Google Scholar]
- Hermann J., Bellé R., Tso J., Ozon R. Stabilization of the maturation promoting factor (MPF) from Xenopus laevis oocytes. Protection against calcium ions. Cell Differ. 1983 Oct;13(2):143–148. doi: 10.1016/0045-6039(83)90106-9. [DOI] [PubMed] [Google Scholar]
- Johnson R. A., Walseth T. F. The enzymatic preparation of [alpha-32P]ATP, [alpha-32P]GTP, [32P]cAMP, and [32P]cGMP, and their use in the assay of adenylate and guanylate cyclases and cyclic nucleotide phosphodiesterases. Adv Cyclic Nucleotide Res. 1979;10:135–167. [PubMed] [Google Scholar]
- Katagiri C., Moriya M. Spermatozoan response to the toad egg matured after removal of germinal vesicle. Dev Biol. 1976 May;50(1):235–241. doi: 10.1016/0012-1606(76)90080-4. [DOI] [PubMed] [Google Scholar]
- Kishimoto T., Kanatani H. Cytoplasmic factor responsible for germinal vesicle breakdown and meiotic maturation in starfish oocyte. Nature. 1976 Mar 25;260(5549):321–322. doi: 10.1038/260321a0. [DOI] [PubMed] [Google Scholar]
- Kishimoto T., Kondo H. Extraction and preliminary characterization of maturation-promoting factor from starfish oocytes. Exp Cell Res. 1986 Apr;163(2):445–452. doi: 10.1016/0014-4827(86)90075-3. [DOI] [PubMed] [Google Scholar]
- Kishimoto T., Kuriyama R., Kondo H., Kanatani H. Generality of the action of various maturation-promoting factors. Exp Cell Res. 1982 Jan;137(1):121–126. doi: 10.1016/0014-4827(82)90014-3. [DOI] [PubMed] [Google Scholar]
- Lohka M. J., Maller J. L. Induction of nuclear envelope breakdown, chromosome condensation, and spindle formation in cell-free extracts. J Cell Biol. 1985 Aug;101(2):518–523. doi: 10.1083/jcb.101.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohka M. J., Masui Y. Effects of Ca2+ ions on the formation of metaphase chromosomes and sperm pronuclei in cell-free preparations from unactivated Rana pipiens eggs. Dev Biol. 1984 Jun;103(2):434–442. doi: 10.1016/0012-1606(84)90331-2. [DOI] [PubMed] [Google Scholar]
- Lohka M. J., Masui Y. Formation in vitro of sperm pronuclei and mitotic chromosomes induced by amphibian ooplasmic components. Science. 1983 May 13;220(4598):719–721. doi: 10.1126/science.6601299. [DOI] [PubMed] [Google Scholar]
- Lohka M. J., Masui Y. Roles of cytosol and cytoplasmic particles in nuclear envelope assembly and sperm pronuclear formation in cell-free preparations from amphibian eggs. J Cell Biol. 1984 Apr;98(4):1222–1230. doi: 10.1083/jcb.98.4.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller J. L. Regulation of amphibian oocyte maturation. Cell Differ. 1985 Jun;16(4):211–221. doi: 10.1016/0045-6039(85)90570-6. [DOI] [PubMed] [Google Scholar]
- Maller J. L., Smith D. S. Two-dimensional polyacrylamide gel analysis of changes in protein phosphorylation during maturation of Xenopus oocytes. Dev Biol. 1985 May;109(1):150–156. doi: 10.1016/0012-1606(85)90355-0. [DOI] [PubMed] [Google Scholar]
- Maller J., Wu M., Gerhart J. C. Changes in protein phosphorylation accompanying maturation of Xenopus laevis oocytes. Dev Biol. 1977 Jul 15;58(2):295–312. doi: 10.1016/0012-1606(77)90093-8. [DOI] [PubMed] [Google Scholar]
- Masui Y., Clarke H. J. Oocyte maturation. Int Rev Cytol. 1979;57:185–282. doi: 10.1016/s0074-7696(08)61464-3. [DOI] [PubMed] [Google Scholar]
- Miake-Lye R., Kirschner M. W. Induction of early mitotic events in a cell-free system. Cell. 1985 May;41(1):165–175. doi: 10.1016/0092-8674(85)90071-6. [DOI] [PubMed] [Google Scholar]
- Nelkin B., Nichols C., Vogelstein B. Protein factor(s) from mitotic CHO cells induce meiotic maturation in Xenopus laevis oocytes. FEBS Lett. 1980 Jan 14;109(2):233–238. doi: 10.1016/0014-5793(80)81094-5. [DOI] [PubMed] [Google Scholar]
- Newmeyer D. D., Lucocq J. M., Bürglin T. R., De Robertis E. M. Assembly in vitro of nuclei active in nuclear protein transport: ATP is required for nucleoplasmin accumulation. EMBO J. 1986 Mar;5(3):501–510. doi: 10.1002/j.1460-2075.1986.tb04239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ottaviano Y., Gerace L. Phosphorylation of the nuclear lamins during interphase and mitosis. J Biol Chem. 1985 Jan 10;260(1):624–632. [PubMed] [Google Scholar]
- Peaucellier G., Dorée M., Picard A. Rise and fall of protein phosphorylation during meiotic maturation in oocytes of Sabellaria alveolata (polychaete annelid). Dev Biol. 1984 Dec;106(2):267–274. doi: 10.1016/0012-1606(84)90224-0. [DOI] [PubMed] [Google Scholar]
- Smith L. D., Ecker R. E. The interaction of steroids with Rana pipiens Oocytes in the induction of maturation. Dev Biol. 1971 Jun;25(2):232–247. doi: 10.1016/0012-1606(71)90029-7. [DOI] [PubMed] [Google Scholar]
- Sorensen R. A., Cyert M. S., Pedersen R. A. Active maturation-promoting factor is present in mature mouse oocytes. J Cell Biol. 1985 May;100(5):1637–1640. doi: 10.1083/jcb.100.5.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhardt R. A., Epel D., Carroll E. J., Jr, Yanagimachi R. Is calcium ionophore a universal activator for unfertilised eggs? Nature. 1974 Nov 1;252(5478):41–43. doi: 10.1038/252041a0. [DOI] [PubMed] [Google Scholar]
- Stick R., Hausen P. Changes in the nuclear lamina composition during early development of Xenopus laevis. Cell. 1985 May;41(1):191–200. doi: 10.1016/0092-8674(85)90073-x. [DOI] [PubMed] [Google Scholar]
- Sunkara P. S., Wright D. A., Rao P. N. Mitotic factors from mammalian cells induce germinal vesicle breakdown and chromosome condensation in amphibian oocytes. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2799–2802. doi: 10.1073/pnas.76.6.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman W. J., Smith L. D. The cyclic behavior of a cytoplasmic factor controlling nuclear membrane breakdown. J Cell Biol. 1978 Jul;78(1):R15–R22. doi: 10.1083/jcb.78.1.r15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Buscaglia M., Ferrez M., Weiller S., Boulet A., Fabre F., Baulieu E. E. Mise en évidence d'une activité "MPF" chez Saccharomyces cerevisiae. C R Seances Acad Sci III. 1982 Dec 20;295(13):787–790. [PubMed] [Google Scholar]
- Westwood J. T., Church R. B., Wagenaar E. B. Changes in protein phosphorylation during the cell cycle of Chinese hamster ovary cells. J Biol Chem. 1985 Aug 25;260(18):10308–10313. [PubMed] [Google Scholar]
- Wu M., Gerhart J. C. Partial purification and characterization of the maturation-promoting factor from eggs of Xenopus laevis. Dev Biol. 1980 Oct;79(2):465–477. doi: 10.1016/0012-1606(80)90131-1. [DOI] [PubMed] [Google Scholar]
- YoungLai E. V., Godeau F., Mulvihill B., Baulieu E. E. Effects of cholera toxin and actinomycin on synthesis of [35s]methionine-labeled proteins during progesterone-induced maturation of Xenopus laevis oocytes. Dev Biol. 1982 May;91(1):36–42. doi: 10.1016/0012-1606(82)90005-7. [DOI] [PubMed] [Google Scholar]
- Yount R. G., Babcock D., Ballantyne W., Ojala D. Adenylyl imidodiphosphate, an adenosine triphosphate analog containing a P--N--P linkage. Biochemistry. 1971 Jun 22;10(13):2484–2489. doi: 10.1021/bi00789a009. [DOI] [PubMed] [Google Scholar]
- Ziegler D., Masui Y. Control of chromosome behavior in amphibian oocytes. I. The activity of maturing oocytes inducing chromosome condensation in transplanted brain nuclei. Dev Biol. 1973 Dec;35(2):283–292. doi: 10.1016/0012-1606(73)90024-9. [DOI] [PubMed] [Google Scholar]