Abstract
Background
Preeclampsia is a hypertensive, multi-system pregnancy disorder whose pathophysiology remains unclear. Elevations in circulating soluble endoglin (sENG) and placental/blood ENG mRNA expression antedate the clinical onset of preeclampsia. This study investigated if endoglin (ENG) pathway genetic variation was also associated with the development of preeclampsia.
Methods
We used a case–control candidate gene association design. Data from 355 white (181 preeclampsia cases/174 controls) and 60 black (30 preeclampsia cases/30 controls) women matched on ancestry, age, and parity were analyzed. Tagging single nucleotide polymorphisms (tSNPs) and potentially functional SNPs in ENG, TGFβ1, TGFβR1, ALK1, and TGFβR2 were genotyped with iPLEX® and TaqMan®. Chi-square or Fisher’s exact tests were used to conduct allele/genotype/haplotype tests in white/black subgroups separately. Odds ratios were computed with binary logistic regression for tSNPs with significant genotype tests.
Results
Of the 49 SNPs evaluated, variation in two ENG tSNPs (rs11792480, rs10121110) and one TGFβR2 tSNP (rs6550005) was associated with preeclampsia in white women (P <0.05, each). In black women, variation in two TGFβ1 tSNPs (rs4803455, rs4803457), one TGFβR1 tSNP (rs10739778), and three TGFβR2 tSNPs (rs6550005, rs1346907, rs877572) was associated with preeclampsia (P <0.05, each). Further evaluation of ENG tSNP rs10121110 revealed that white women inheriting the AA genotype were 2.29 times more likely to develop preeclampsia compared to the GG genotype (P = 0.008, [99% CI: 1.02 to 5.13]). For black women, similar evaluation of TGFβ1 tSNP rs4803457 revealed women inheriting the CT genotype were 7.44 times more likely to develop preeclampsia than those with the CC genotype (P = 0.005, [99% CI: 1.19 to 46.41]).
Conclusions
ENG pathway genetic variation is associated with preeclampsia. Different ENG pathway genes may be involved in preeclampsia development among white and black women. Additional studies are needed to validate these findings and to determine if genetic variation in ENG pathway genes impacts ENG and sENG levels in preeclampsia.
Keywords: Endoglin, Genetic association study, Preeclampsia, SNP
Background
Preeclampsia is a multi-system disorder of pregnancy that complicates 3-5% of pregnancies [1] and is diagnosed by new onset hypertension and proteinuria after 20 weeks’ gestation [2,3]. The heterogeneous nature of preeclampsia suggests that multiple mechanisms lead to its development. Research has identified endoglin (ENG) as a mechanism that may contribute to preeclampsia in some women.
ENG is a trans-membrane glycoprotein that serves as a co-receptor of the transforming growth factor beta (TGFβ) signaling system [4]. It is expressed on vascular endothelial cells [5], synctyiotrophoblasts, and invasive cytotrophoblasts of cell columns [6]. ENG is involved in maintenance of vascular tone through regulation of nitric oxide dependent vasodilatation [7,8], and likely contributes to regulation of placental implantation and spiral artery remodeling during pregnancy [9,10]. Inhibition of ENG translation in first trimester human villous explants [9] or a human extravillous tropholast cell line [10] improves the invasive capacity of extravillous trophoblasts. Invasion of extravillous trophoblasts is proposed to be vital to uterine spiral artery remodeling, which increases placental perfusion during pregnancy [9,10].
Because systemic endothelial dysfunction and shallow placental implantation/spiral artery remodeling represent hallmark abnormalities of preeclampsia [11], ENG’s potential role has been investigated. In multiple studies, ENG gene expression (mRNA) is increased in placenta and/or cellular/non-cellular components of blood throughout pregnancy in women who develop preeclampsia [12-18]. Soluble endoglin (sENG), which is released into circulation after cleavage of trans-membrane ENG by matrix metalloproteinase-14 (MMP-14) [19], is also elevated in preeclampsia [20]. Our study proposed to test the role of ENG in preeclampsia by assessing if ENG pathway genetic variations are associated with preeclampsia.
Methods
Study population
Subjects were from the Prenatal Exposures and Preeclampsia Prevention (PEPP) study. Conducted at Magee-Womens Hospital of UPMC (Pittsburgh, PA), PEPP examines factors predisposing women to preeclampsia via two recruitment approaches. Women ages 14-44 were enrolled during early pregnancy (≤ 20 weeks’ gestation) and followed through delivery/postpartum while other women were enrolled at the labor/delivery unit due to suspected preeclampsia. Women with a history of chronic renal disease, hypertension, diabetes, or other disorders increasing risk of preeclampsia were excluded. Genomic DNA was extracted from peripherally collected venous blood samples. The University of Pittsburgh and Magee-Womens Hospital Institutional Review Board approved all aspects of PEPP and this study. We excluded subjects not consenting to genetic evaluation and subjects without a stored genetic sample.
Phenotype classifications
Preeclampsia was defined as gestational hypertension and blood pressure increase, proteinuria, and hyperuricemia. These criteria were reviewed by a panel of clinicians/researchers to determine preeclampsia diagnosis. The average of the last five blood pressures taken in the hospital prior to therapeutic intervention was compared to average blood pressure prior to 20 weeks’ gestation to establish the presence/absence of a relevant increase of blood pressure. Gestational hypertension was defined as a blood pressure ≥ 140 mmHg systolic and/or 90 mmHg diastolic AND an increase of blood pressure > 30 mmHg systolic and/or 15 mmHg diastolic after 20 weeks’ gestation. Proteinuria was defined as ≥ 300 mg/24 hours, ≥ 0.3 protein/creatinine ratio, ≥ 2+ on a random urine specimen, or ≥ 1+ on a catheterized urine specimen. Hyperuricemia was a serum uric acid concentration > 1 standard deviation from normal for gestational age [21]. Severe preeclampsia was preeclampsia plus ≥ 1 of the following: (a) systolic blood pressure ≥ 160 mmHg, (b) diastolic blood pressure ≥ 110 mmHg, (c) proteinuria ≥ 5 grams/24 hours, (d) elevated liver enzymes, or (e) platelet count ≤ 100,000. Hemolysis, elevated liver enzymes, and low platelets in subjects with preeclampsia indicated HELLP syndrome. The case group included PEPP subjects diagnosed with preeclampsia, severe preeclampsia, or HELLP syndrome.
Women with normal medical histories (e.g., without chronic renal disease, hypertension, diabetes) that did not meet criteria for preeclampsia, severe preeclampsia, or HELLP syndrome were designated as healthy controls. Controls delivering prematurely (< 37 weeks’ gestation at delivery) were excluded from our analysis. A total of 215 controls were 1:1 frequency matched to 215 cases on ancestry (self-reported race), age, and parity. Data on 355 white (181 cases/174 controls) and 60 black (30 cases/30 controls) women were analyzed. In the white case group, 161 (89.0%) subjects were diagnosed with PE, 19 (10.5%) subjects were diagnosed with severe PE, and 1 (0.6%) subject was diagnosed with HELLP syndrome. In the black case group, 27 (90%) subjects were diagnosed with PE, 2 (6.7%) subjects were diagnosed with severe PE, and 1 (3.3%) subject was diagnosed with HELLP syndrome.
Polymorphism selection
Genetic variability of the candidate genes and their regulatory regions was evaluated with tagging single nucleotide polymorphisms (tSNPs) selected from HapMap (HapMap Data Phase III/Rel#2, Feb09, on NCBI B36 assembly, dbSNP b126). Selection criteria of tSNPs included: minor allele frequency ≥20%, R2 cutoff = 0.8, and CEU ancestry. Forty-seven tSNPs were identified. These tSNPs and two potentially functional SNPs identified in the literature were studied. The UCSC Genome Browser [22] and Database of Single Nucleotide Polymorphisms were used to identify SNP nucleotide positions/genomic locations.
Genotyping methods
The 49 SNPs were genotyped with the iPLEX® Gold-SNP Genotyping assay (Sequenom® Inc, San Diego, CA) (Table 1). Five SNPs (rs1800468, rs10739778, rs6809777, rs3087465, rs8179181) not meeting data quality criteria (call rate < 86% or multi-allelic >2 alleles) with iPLEX® were genotyped by TaqMan® allelic discrimination (Applied Biosystems®).
Table 1.
tSNPS examined in iPLEX® assays
|
Assay 1 |
Assay 2 |
||
|---|---|---|---|
| Gene | rs numbers | Gene | rs numbers |
| Endoglin (ENG) |
rs10987746, rs10819309, rs10760505, rs11792480, rs10121110 |
TGFβR2 |
rs2043136, rs13075948, rs1346907, rs3773652, rs4955212, rs1155708, rs3773640, rs1036097, rs2082224, rs876688, rs744751, rs1078985, rs17025785, rs877572, rs5020833, rs6809777, rs6802220, rs9843942, rs6550005, rs3773644, rs3773645, rs13083813, rs12487185, rs13086588, rs6792117, rs3773663, rs4522809, rs11129420, rs995435, rs11924422 |
| Transforming growth factor beta 1 (TGFβ1) |
rs4803455, rs1800469, rs4803457, rs8179181, rs1800468, rs11466314 |
||
| Transforming growth factor beta receptor 1 (TGFβR1) |
rs6478974, rs420549, rs10739778 |
||
| Activin receptor like kinase 1 (ALK1) |
rs3759178, rs11169953, rs706819 |
||
| Transforming growth factor beta receptor 2 (TGFβR2) | rs749794, rs3087465 | ||
Note. The online Human GenoTyping Tools and MassARRAY® Assay Designer v4.0 software (http://www.mysequenom.com) were used to design the iPLEX® assays. To optimize the multiplex reactions, tSNPs were grouped into Assay 1 or Assay 2.
Genotype data reliability, haplotype assignment, and linkage disequilibrium estimation
Genotype reliability checks included comparing expected to observed homozygosity, comparing study and dbSNP allele frequencies, evaluating genotype call rates, including blind duplicates, double calling genotypes, and checking Hardy-Weinberg Equilibrium (HWE). HWE calculations were conducted with PLINK v1.07 [23] or an online HWE calculator. ENG haplotypes and pair-wise linkage disequilibrium (R2) were estimated in non-related white and black subgroups separately using PLINK. Further black subgroup haplotype analysis was not conducted due to small sample size and haplotype frequencies.
Statistical analysis
A power analysis was conducted with Quanto version 1.2.4 (Additional file 1).
White and black subgroups were evaluated separately. Demographic characteristics were compared between cases and controls. Continuous variables were assessed with independent samples t-tests, independent samples t-tests with unequal variances, or Mann–Whitney U tests. Categorical variables were assessed with the Mann–Whitney U test or the χ2 test of independence. Missing pre-pregnancy body mass index values were estimated with multiple imputation.
Deviations from HWE were assessed with a χ2 goodness-of-fit test or an exact test. For SNPs violating HWE (P < 0.05), HWE consistency was further assessed in cases and controls separately. We concluded that any SNP violating HWE in the entire group was due to enrichment for preeclampsia in cases and non-preeclampsia in controls and not due to genotyping error.
Associations between SNP alleles and preeclampsia status (allele test) were assessed with a χ2 test of independence. Associations between SNP genotypes and preeclampsia status (genotype test) were tested with a χ2 test of independence or Fisher’s exact test. SNPs with homozygote variant frequencies of < 10% in cases, controls, or both were dichotomized (homozygote wildtype; homozygote variant + heterozygote) before genotype testing. Given the exploratory nature of this study, we used a less stringent significance criterion of P < 0.05 to initially evaluate our genotype test results and identify potentially interesting hits. SNPs with significant findings (P < 0.05) on genotype tests were further analyzed with binary logistic regression (odds ratio and 99% CI). A 99% CI was selected for these analyses as a way to reduce the potential for Type 1 error due to multiple testing. Analyses were conducted with SPSS version 19 (SPSS Inc., Chicago, IL.).
The most probable ENG haplotypes estimated for each white subject were selected for analysis. Haplotype frequencies with < 10% in cases, controls, or both were collapsed into one category (Additional file 1: Table S1). A χ2 test of independence was used to determine if haplotype frequency distributions differed in cases and controls. Separate pair-wise comparisons of haplotype frequency distributions were also analyzed. The association between diplotypes, which were generated from ENG haplotypes, and preeclampsia status was assessed with a χ2 test of independence.
Results
Allele/genotype findings support association between ENG pathway genetic variation and preeclampsia
Tables 2 and 3 provide demographic/clinical characteristics for white and black subgroups and reveal the success of matching (ancestry, age, and parity) and the expected differences between women with preeclampsia and controls (blood pressure, gestational age at delivery, and BMI). Additional file 1: Tables S2-S5 provide descriptive information and allele/genotype test results.
Table 2.
White subgroup demographic and clinical characteristics
| Variable | Cases (n = 181) | Controls (n = 174) | p-value |
|---|---|---|---|
| Maternal age, years (M (SD)) |
28.3 (5.8) |
28.4 (5.7) |
0.87a |
| Gravida (Mdn (min-max)) |
1 (1-6) |
1 (1-8) |
0.08b |
| Nulliparous (n, %) |
146 (80.7%) |
139 (79.9%) |
0.85c |
| Gestational age at delivery, wks (M (SD)) |
36.1 (3.2) |
39.6 (1.1) |
< 0 .001d |
| Birthweight, grams (M (SD))e |
2497.5 (841.2) |
3481.6 (446.3) |
< 0.001d |
| Avg. SBP < 20 weeks’, mm Hg (M (SD))f |
116.6 (9.6) |
112.1 (7.5) |
< 0.001d |
| Avg. DBP < 20 weeks’, mm Hg (M (SD))f |
71.7 (7.2) |
68.1 (4.9) |
< 0.001d |
| Avg. SBP in labor, mm Hg (M (SD))g |
154.8 (13.9) |
120.4 (10.2) |
< 0.001d |
| Avg. DBP in labor, mm Hg (M (SD))h |
92.6 (8.0) |
72.3 (7.2) |
< 0. 001a |
| Pre-pregnancy BMI (Mdn (min-max))i | 25.8 (17-46) | 22.9 (16-37) | < 0.001b |
Abbreviations: (M (SD)), mean (standard deviation); (Mdn (min-max)), median (minimum-maximum); wks, weeks; Avg. SBP, average systolic blood pressure; mmHg, millimeters of mercury; Avg. DBP, average diastolic blood pressure; BMI, body mass index.
a Independent samples t-test.
b Mann–Whitney U test.
cχ2 test of independence.
d Independent samples t-test with unequal variance.
e Outlier removed in controls.
fn = 168 cases &n = 170 controls.
gn = 181 cases &n = 173 controls.
hn = 180 cases &n = 173 controls.
i BMI values imputed for n = 22 cases, sample size n = 178 cases &n = 172 controls.
Table 3.
Black subgroup demographic and clinical characteristics
| Variable | Cases (n = 30) | Controls (n = 30) | p-value |
|---|---|---|---|
| Maternal age, years (Mdn (min-max)) |
20.0 (14-37) |
20.0 (14-37) |
0.99a |
| Gravida (Mdn (min-max)) |
1 (1-6) |
1 (1-6) |
0.38a |
| Nulliparous (n, %) |
25 (83.3%) |
25 (83.3%) |
------- |
| Gestational age at delivery, wks (Mdn (min-max)) |
36.9 (27.4-40.0) |
40.6 (37.1-42.1) |
< 0.001a |
| Birthweight, grams (M (SD)) |
2313.9 (715.8) |
3388.8 (405.3) |
< 0.001b |
| Avg. SBP < 20 weeks’, mm Hg (M (SD))c |
113.3 (9.2) |
114.4 (6.8) |
0.60d |
| Avg. DBP < 20 weeks’, mm Hg (M (SD))c |
70.4 (6.5) |
69.2 (4.2) |
0.41d |
| Avg. SBP in labor, mm Hg (M (SD))e |
159.9 (17.8) |
120.7 (9.1) |
< 0.001b |
| Avg. DBP in labor, mm Hg (M (SD))e |
97.1 (10.4) |
72.6 (7.4) |
< 0.001d |
| Pre-pregnancy BMI (Mdn (min-max))f | 23.0 (17.7-38.4) | 25.8 (19.4-49.9) | 0.25a |
Abbreviations: (Mdn (min-max)), median (minimum-maximum); wks, weeks; (M (SD)), mean (standard deviation); Avg. SBP, average systolic blood pressure; mmHg, millimeters of mercury; Avg. DBP, average diastolic blood pressure; BMI, body mass index.
a Mann–Whitney U test.
b Independent samples t-test with unequal variances.
cn = 24 cases &n = 29 controls.
d Independent samples t-test.
en = 30 cases &n = 29 controls.
fn = 27 cases &n = 30 controls.
We examined if the proportion of alleles for each of the 49 SNPs (allele 1 or allele 2) differed in women with preeclampsia compared to controls. Using P < 0.05 to indicate statistical significance, allelic frequency distributions for two tSNPs in ENG (rs11792480: G/A alleles; rs10121110: A/G alleles) and one tSNP in TGFβR2 (rs6550005: G/A alleles) were significantly different in white cases and controls. The G allele of rs11792480 (71.7% vs. 63.0%, P = 0.01), the A allele of rs10121110 (66.0% vs. 55.3%, P = 0.004), and the G allele of rs6550005 (84.0% vs. 77.6%, P = 0.03) were overrepresented in white cases. Allelic distributions for all SNPs in TGFβR1, ALK1, and TGFβ1, along with the remaining SNPs in ENG and TGFβR2, were not significantly different in the white subgroup.
The genotype for ENG tSNP rs10121110 (AA vs. GG vs. AG) was also significantly associated with preeclampsia (P = 0.02) in the white subgroup. This association was further explored with binary logistic regression, and odds ratios were generated. Because of multiple testing, we evaluated logistic regression results with α = 0.01 (Table 4). Women inheriting the AA genotype for rs10121110 were 2.29 times more likely to develop preeclampsia compared to women inheriting the GG genotype (P = 0.008, [99% CI: 1.02 to 5.13]). The genotype for TGFβR2 tSNP rs6550005 (GG vs. AA + GA) was also significantly associated with preeclampsia in the white subgroup (P = 0.04), but further exploration of this association with logistic regression and a more stringent criterion for significance (P < 0.01) did not support this association (Table 4). Genotype tests for all SNPs in TGFβR1, ALK1, and TGFβ1, along with the remaining SNPs in ENG and TGFβR2, demonstrated no significant differences in whites.
Table 4.
Logistic regression for tSNPs with significant genotype tests
|
White Subgroup | ||||
|
Gene/tSNP |
Genotype Groups |
OR |
99% CI |
p-valuea |
|
ENG: rs10121110 |
AA vs. GG |
2.29 |
1.02 - 5.13 |
0.008 |
| AG vs. GG |
1.52 |
0.69 - 3.36 |
0.17 |
|
|
TGFBR2: rs6550005b |
GG vs. AA + GA |
1.60 |
0.89 - 2.87 |
0.04 |
|
Black Subgroup | ||||
|
Gene/tSNP |
Genotype Groups |
OR |
99% CI |
p-value |
|
TGFβ1: rs4803455 |
AA vs. CC |
0.39 |
0.06 - 2.61 |
0.20 |
| CA vs. CC |
3.04 |
0.56 - 16.33 |
0.09 |
|
|
TGFβ1:rs4803457 |
TT vs. CC |
3.50 |
0.50 - 24.53 |
0.10 |
| CT vs. CC |
7.44 |
1.19 - 46.41 |
0.005 |
|
| TGFβR1: rs10739778 | CC vs. AA |
0.19 |
0.02 - 2.08 |
0.07 |
| AC vs. AA | 0.24 | 0.05 - 1.09 | 0.02 | |
Abbreviations: tSNP, tagging single nucleotide polymorphism; OR, odds ratio; CI, confidence interval.
a α set at 0.01 because of multiple testing.
b SNP genotypes dichotomized (homozygote wildtype, homozygote variant + heterozygote) due to small homozygote variant frequencies in either cases, controls, or both.
In the black subgroup, allelic frequency distributions for one tSNP in TGFβR1 (rs10739778: A/C alleles) and three tSNPs in TGFβR2 (rs6550005: G/A alleles; rs1346907: C/T alleles; rs877572: G/C alleles) were significantly different in cases and controls. The A allele of rs10739778 (79.3% vs. 56.7%, P = 0.008), the A allele of rs6550005 (46.7% vs. 26.7%, P = 0.02), the C allele of rs1346907 (70.0% vs. 63.3%, P = 0.04), and the G allele of rs877572 (70.0% vs. 61.7%, P = .03) were overrepresented in black cases. Allelic distributions for all SNPs in ALK1, TGFβ1, and ENG, along with the remaining SNPs in TGFβR1, TGFβR2, were not significantly different in the black subgroup.
The genotype for TFGβ1 tSNP rs4803455 (CC vs. AA vs. CA) was significantly associated with preeclampsia (P = 0.01) in the black subgroup. However, evaluation of the association by binary logistic regression did not support this association at α = 0.01 (Table 4). TGFβ1 tSNP rs4803457 genotype (CC vs. TT vs. CT) was also significantly associated with preeclampsia in the black subgroup (P = 0.01). Further analysis of rs4803457 revealed that women inheriting the CT genotype were 7.44 times more likely to develop preeclampsia compared to women inheriting the CC genotype (P = 0.005, [99% CI: 1.19 to 46.41]). Lastly, the genotype for TGFβR1 tSNP rs10739778 (AA vs. CC vs. AC) was significantly associated with preeclampsia in the black subgroup (P = 0.03). However, further exploration with binary logistic regression did not support this association (Table 4). Genotype tests for all SNPs in ALK1, ENG, and TGFβR2, along with the remaining SNPs in TGFβR1, and TGFβ1, demonstrated no significant differences in the black subgroup.
ENG haplotype TACGA associated with preeclampsia in white subgroup
We estimated linkage disequilibrium and haplotypes for ENG in whites. Pairwise R2 values ranged from 0.007-0.64. For rs10121110 and rs11792480, which were both significantly associated with preeclampsia and are separated by about 4000 bases, R2 = 0.29. These results validate our tSNP selection criteria and indicate that haploblocks tagged by rs10121110 and rs11792480 are independently associated with preeclampsia.
Nineteen possible haplotypes were estimated across the ENG tSNPs (rs10987746, rs10819309, rs10760505, rs11792480, rs10121110). Seventeen of the haplotypes were present in whites (Additional file 1: Table S1). ENG haplotype distributions (CGTGA, TACAG, TACGA, and combined) were significantly different in cases and controls (χ2(3) = 8.26, P = 0.04). The TACGA allele, which contains the risk alleles from our significantly associated tSNPS, was over-represented in cases (χ2(1) = 5.23, P = 0.02) when compared to the other alleles combined. We also observed a 6.1% difference in TACGA frequency among whites and blacks (19.7% vs.13.6%). Diplotype analysis in whites found no significant differences in ENG diplotypes among cases and controls (χ2(4) = 7.28, P = 0.12).
Discussion
This study begins to examine the association between ENG pathway genetic variation and preeclampsia. In our white sample, variation in ENG and TGFβR2 appears to be associated with preeclampsia while variation in TGFβ1 (excluding rs8179181- not genotyped), TGFβR1, and ALK1 was not associated with preeclampsia. In our smaller black sample, pathway associations also appeared, but were different. As with whites, variation in TGFβR2 was associated with preeclampsia, but variation in ENG was not. In blacks, but not whites, pathway variation was suggested for TGFβ1 and TGFβR1. As with whites, variants in ALK1 were not associated with preeclampsia. These results suggest that ENG pathway genetic variation is associated with preeclampsia, with different pathway genes contributing to preeclampsia development in white and black women.
In this study we used a very strict definition of preeclampsia that included both incremental and absolute increases in blood pressure, as well as hyperuricemia. We believe such rigor is necessary for genetic studies of preeclampsia, but this approach limited sample sizes. Further, because of multiple comparisons, we explored significant genotype findings (P < 0.05) with binary logistic regression using a more stringent level of significance (α = 0.01), which again limits our definitive findings. Nonetheless, we believe that using this strategy of examining genotypic variation in pathway analysis to support the role of a potential pathogenic factor provides useful insights to guide further studies of specific pathway variation.
Are the pathway associations we have demonstrated biologically plausible? Although we do not know the functional consequences of these associations, several explanations could account for the association between ENG (rs10121110 and rs11792480) and TGFβR2 (rs6550005) genetic variation and preeclampsia in white women. Intronically located between the second and third exons, rs10121110 tags a genomic region that includes ENG’s promoter (Figure 1). Given rs10121110’s location, this tSNP potentially identifies a correlated promoter variant that has the ability to impact transcription factor access/binding (e.g., SP1 transcription factor, SMAD binding elements) [24] and subsequent transcription/translation of ENG.
Figure 1.
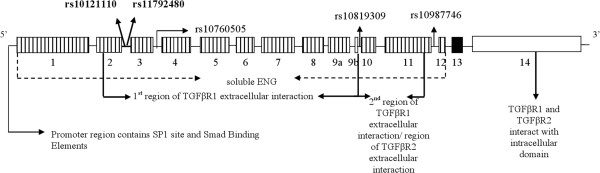
Endoglin gene structure.ENG extracellular domain exons (vertical lines), transmembrane domain exon (black), and the intracellular domain exon (white). tSNPs with significant allele &/or genotype tests are bolded. The following resources were utilized to create the figure [22,24-27].
Knockdown of ENG in a human extravillous trophoblast cell line with short hairpin RNA specific for ENG[10] or knockdown of first trimester human trophoblast villous explants with antisense endoglin nucleotides [9] improves extravillous trophoblast invasive capacity, which is essential to uterine spiral artery remodeling in pregnancy [9,10]. In preeclampsia, placental concentrations of ENG mRNA are elevated throughout pregnancy [12,16-18] and spiral artery remodeling is shallow [11]. Therefore, a variant within ENG’s promoter could increase expression of placental ENG, which could inhibit extravillous trophoblast invasion of the spiral arteries, leading to shallow implantation and reduced placental perfusion. In a setting with increased concentration of membrane-bound ENG receptors, mass action predicts that more will be cleaved by MMP-14, resulting in increased sENG, which is found in women with preeclampsia and has been suggested to cause endothelial dysfunction [18]. Studies examining ENG’s promoter, its transcription factors, and MMP-14 are needed to better understand mechanisms that drive observed differences in ENG and sENG.
ENG tSNP rs10121110 is also located between exons coding for ENG’s extracellular domain, as is rs11792480, the other tSNP associated with preeclampsia in whites (Figure 1). As part of TGFβ1’s signaling cascade, TGFβR1 interacts with amino acid residues 26-437 of ENG’s extracellular domain [25]. Only through interaction of ENG and type 1/2 receptors can ENG gain access to TGFβ1 [25]. Genetic variation within exons that code for the extracellular domain could therefore influence ENG’s ability to interact with TGFβR1, affecting ENG’s access to TGFβ1 and the transmission of TGFβ1 signals. Because TGFβ1 induces ENG expression [10] and stimulates ENG promoter activity [28], genetic variation that affects the degree of TGFβ1 transmission may also explain differences in ENG expression (mRNA) between women with/without preeclampsia. Studies examining genetic regions tagged by rs10121110 and rs11792480 may provide insight into ENG’s involvement in preeclampsia.
TGFβR2 tSNP rs6550005, which was associated with preeclampsia in both groups, is intronically located between the first two exons that lie adjacent to the TGFβR2 promoter. As a result, rs6550005 may tag a promoter variant that influences TGFβR2 transcription and translation. Because ENG only binds TGFβ1 ligand in the presence of type 1/2 signaling receptors [25], alteration in TGFβR2 transcription/translation could impact the number of TGFβR2 receptors available for ENG interaction and transmission of TGFβ1 ligand signaling. Furthermore, this association in whites and blacks potentially indicates that there is also one component of the ENG pathway that similarly contributes to PE development regardless of ancestry, but because the minor allele frequencies differed in whites and blacks (0.192 vs. 0.367), we did not combine the data from these two groups.
Our exploratory examination in black women revealed that different genes from different components of the ENG pathway (TGFβ1 and TGFβR1, but not ENG) were associated with preeclampsia compared to white women. These results suggest that the pathway’s involvement in preeclampsia may differ in blacks and whites. Interestingly, in a study comparing TGFβ1 mRNA and protein levels in nonpregnant black and white hypertensive subjects, TGFβ1 protein levels were significantly higher in blacks compared to whites (P < 0.001) [29].
One additional study has investigated the association between ENG and preeclampsia in white and black women using a pre-designed IBCv2 array (Illumina Inc, San Diego, CA) [30]. Unlike the significant associations found between ENG and preeclampsia in our white subgroup, their study failed to find significant associations in a much smaller white subgroup that was likely underpowered. Consistency in our findings from allele, genotype, and haplotype tests of ENG increases our confidence in our findings. In both studies, associations between ENG and preeclampsia were non-significant in blacks.
In addition to sample size, there are other limitations to our study. Multiallelic tSNPs rs8179181 (TGFβ1) and rs3087465 (TGFβR2) could not be genotyped despite multiple attempts with iPLEX® and TaqMan®, which limits our ability to fully evaluate TGFβ1 and TGFβR2 genetic variability. Our black subgroup was likely underpowered. This was not an issue in the white sample, which generated a power ranging from 0.898 to 0.999 (Additional file 1). Additionally, we selected tSNPs for CEU ancestry, which may have resulted in decreased informativeness in blacks since haploblocks tagged by tSNPs selected for CEU ancestry may differ from haploblocks tagged by tSNPs selected for African ancestry. Finally, we used self-reported race to match controls to cases on ancestry. Because this may not reliably account for population admixture, the use of ancestral informative markers in future studies represents a more robust approach that statistically accounts for population admixture.
Conclusions
In summary, our study demonstrated that ENG pathway genetic variation is associated with preeclampsia in white and black women. Our results further suggest that the pathway’s involvement in preeclampsia differs in whites and blacks, with ENG and TGFBR2 being associated in whites and TGFβ1, TGFβR1, and TGFβR2 being associated in blacks. Validation of results is needed to confirm these preliminary findings. Moreover, the ENG pathway tSNPs found to be significantly associated with preeclampsia likely represent surrogate markers, which tag genomic regions that contain causal variants. Focused examination of genomic regions (e.g., ENG promoter sequencing) tagged by these SNPs will further improve understanding of the ENG pathway’s role in preeclampsia.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MB participated in the conception and design of research, acquisition of data, analysis and interpretation of data, and drafting of the manuscript. JR participated in the acquisition of data/samples, analysis and interpretation of data, and critical revision of the manuscript for important intellectual content. SF participated in the interpretation of data and critical revision of the manuscript for important intellectual content. AJ participated in the recruitment of subjects, acquisition of data, and critical revision of the manuscript for important intellectual content. LT participated in the analysis and interpretation of data and critical revision of the manuscript for important intellectual content. YP participated in conception and design of research, interpretation of data, drafting the manuscript, and critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
Variation in endoglin pathway genes is associated with preeclampsia: A case-control candidate gene association study.
Contributor Information
Mandy J Bell, Email: mjb111@pitt.edu.
James M Roberts, Email: jroberts@mwri.magee.edu.
Sandra A Founds, Email: foundss@pitt.edu.
Arun Jeyabalan, Email: ajeyabalan@mail.magee.edu.
Lauren Terhorst, Email: terhorstl@ccbh.com.
Yvette P Conley, Email: yconley@pitt.edu.
Acknowledgements
This work was supported by the National Institutes of Health (T32NR009759, 1F31NR011379, P01HD30367), Eta Chapter of Sigma Theta Tau International Honor Society of Nursing, International Society of Nurses in Genetics, and Magee CRC grant #5M01RR00056.
We would like to thank Magee-Womens Research Institute and the PEPP cohort study for access to de-identified subject samples and data. We would like to thank Sandra DesLouches and Jake Richards from the University of Pittsburgh School of Nursing Genetics Laboratory for their assistance in sample management and data collection. We would like to thank David Lykins from Magee-Womens Research Institute for his assistance in sample acquisition and database management. We would like to thank Ryan Minster from the University of Pittsburgh Graduate School of Public Health (Human Genetics) for his assistance with haplotype analysis.
References
- Roberts JM, Cooper DW. Pathogenesis and genetics of pre-eclampsia. Lancet. 2001;357:53–56. doi: 10.1016/S0140-6736(00)03577-7. [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists. ACOG practice bulletin: diagnosis and management of preeclampsia and eclampsia (No. 33) Obstet Gynecol. 2002;99:159–167. doi: 10.1016/S0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- National Heart, Lung, and Blood Institute National High Blood Pressure Education Program. Report of the national high blood pressure education program working group on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- Cheifetz S, Bellón T, Calés C, Vera S, Bernabeu C, Massagué J, Letarte M. Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J Biol Chem. 1992;267:19027–19030. [PubMed] [Google Scholar]
- Gougos A, Letarte M. Primary structure of endoglin, an RGD-containing glycoprotein of human endothelial cells. J Biol Chem. 1990;265:8361–8364. [PubMed] [Google Scholar]
- St-Jacques S, Forte M, Lye SJ, Letarte M. Localization of endoglin, a transforming growth factor-β binding protein, and of CD44 and integrins in placenta during the first trimester of pregnancy. Biol Reprod. 1994;51:405–413. doi: 10.1095/biolreprod51.3.405. [DOI] [PubMed] [Google Scholar]
- Jerkic M, Rivas-Elena JV, Prieto M, Carrón R, Sanz-Rodriguez F, Pérez-Barriocanal F, Rodríguez-Barbero A, Bernabéu C, López-Novoa JM. Endoglin regulates nitric oxide-dependent vasodilatation. FASEB J. 2004;18:609–611. doi: 10.1096/fj.03-0197fje. [DOI] [PubMed] [Google Scholar]
- Toporsian M, Gros R, Kabir MG, Vera S, Govindaraju K, Eidelman DH, Husain M, Letarte M. A role for endoglin in coupling eNOS activity and regulating vascular tone revealed in hereditary hemorrhagic telangiectasia. Circ Res. 2005;96:684–692. doi: 10.1161/01.RES.0000159936.38601.22. [DOI] [PubMed] [Google Scholar]
- Caniggia I, Taylor CV, Ritchie JW, Lye SJ, Letarte M. Endoglin regulates trophoblast differentiation along the invasive pathway in human placental villous explants. Endocrinology. 1997;138:4977–4988. doi: 10.1210/en.138.11.4977. [DOI] [PubMed] [Google Scholar]
- Mano Y, Kotani T, Shibata K, Matsumura H, Tsuda H, Sumigama S, Yamamato E, Iwase A, Senga T, Kikkawa F. The loss of endoglin promotes the invasion of extravillous trophoblasts. Endocrinology. 2011;152:4386–4394. doi: 10.1210/en.2011-1088. [DOI] [PubMed] [Google Scholar]
- Roberts JM, Hubel CA. The two stage model of preeclampsia: variations on the theme. Placenta. 2009;23:S32–S37. doi: 10.1016/j.placenta.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina A, Sekizawa A, De Sanctis P, Purwosunu Y, Okai T, Cha DH, Kang JH, Vicenzi C, Tempesta A, Wibowo N, Valvassori L, Rizzo N. Gene expression in chorionic villous samples at 11 weeks’ gestation from women destined to develop preeclampsia. Prenat Diagn. 2008;28:956–961. doi: 10.1002/pd.2109. [DOI] [PubMed] [Google Scholar]
- Farina A, Zucchini C, Sekizawa A, Purwosunu Y, de Sanctis P, Santarsiero G, Rizzo N, Morano D, Okai T. Performance of messenger RNAs circulating in maternal blood in the prediction of preeclampsia at 10-14 weeks. Am J Obstet Gynecol. 2010;203:1.e1–1.e7. doi: 10.1016/j.ajog.2010.07.043. [DOI] [PubMed] [Google Scholar]
- Purwosunu Y, Sekizawa A, Yoshimura S, Farina A, Wibowo N, Nakamura M, Shimizu H, Okai T. Expression of angiogenesis-related genes in the cellular component of the blood of preeclamptic women. Reproductive Sciences. 2009;16:857–864. doi: 10.1177/1933719109336622. [DOI] [PubMed] [Google Scholar]
- Sekizawa A, Purwosunu Y, Farina A, Shimizu H, Nakamura M, Wibowo N, Rizzo N, Okai T. Prediction of pre-eclampsia by an analysis of placenta-derived cellular mRNA in the blood of pregnant women at 15-20 weeks of gestation. Br J Obstet Gynaecol. 2010;117:557–564. doi: 10.1111/j.1471-0528.2010.02491.x. [DOI] [PubMed] [Google Scholar]
- Sitras V, Paulssen RH, Gronaas H, Leirvik J, Hanssen TA, Vartun A, Acharya G. Differential placental gene expression in severe preeclampsia. Placenta. 2009;30:424–433. doi: 10.1016/j.placenta.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Tsai S, Hardison NE, James AH, Motsinger-Reif AA, Bischoff SR, Thames BH, Piedrahita JA. Transcriptional profiling of human placentas from pregnancies complicated by preeclampsia reveals disregulation of sialic acid acetylesterase and immune signaling pathways. Placenta. 2011;32:175–182. doi: 10.1016/j.placenta.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, Stillman IE, Roberts D, D’Amore PA, Epstein FH, Sellke FW, Romero R, Sukhatme VP, Karumanchi SA. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- Kaitu’u-Lino T, Palmer KR, Whitehead CL, Williams E, Lappas M, Tong S. MMP-14 is expressed in preeclamptic placentas and mediates release of soluble endoglin. Am J Pathol. 2012;180:888–894. doi: 10.1016/j.ajpath.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Rana S, Karumanchi SA, Levine RJ, Venkatesha S, Rauh-Hain JA, Tamez H, Thadhani R. Sequential changes in antiangiogenic factors in early pregnancy and risk developing preeclampsia. Hypertension. 2007;50:137–142. doi: 10.1161/HYPERTENSIONAHA.107.087700. [DOI] [PubMed] [Google Scholar]
- Lind T, Godfrey KA, Otun H, Philips PR. Changes in serum uric acid concentrations during normal pregnancy. Br J Obstet Gynaecol. 1984;91:128–132. doi: 10.1111/j.1471-0528.1984.tb05895.x. [DOI] [PubMed] [Google Scholar]
- Fujita PA, Rhead B, Zweig AS, Hinrichs AS, Karolchik D, Cline MS, Goldman M, Barber GP, Clawson H, Coelho A, Diekhans M, Dreszer TR, Giardine BM, Harte RA, Hillman-Jackson J, Hsu F, Kirkup V, Kuhn RM, Learned K, Li CH, Meyer LR, Pohl A, Raney BJ, Rosenbloom KR, Smith KE, Haussler D, Kent WJ. The UCSC genome browser database: update 2011. Nucleic Acids Res. 2011;39:D876–D882. doi: 10.1093/nar/gkq963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a toolset for whole-genome association and population-based linkage analysis. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botella LM, Sánchez-Elsner T, Rius C, Corbí A, Bernabéu C. Identification of a critical Sp1 site within the endoglin promoter and its involvement in the transforming growth factor-β stimulation. J Biol Chem. 2001;276:34486–34494. doi: 10.1074/jbc.M011611200. [DOI] [PubMed] [Google Scholar]
- Guerrero-Esteo M, Sánchez-Elsner T, Letamendia A, Bernabéu C. Extracellular and cytoplasmic domains of endoglin interact with the transforming growth factor-beta receptors I and II. J Biol Chem. 2002;277:29197–29209. doi: 10.1074/jbc.M111991200. [DOI] [PubMed] [Google Scholar]
- Bosler AD, Richards J, George C, Godmilow L, Ganguly A. Novel mutations in ENG and ACVRL1 identified in a series of 200 individuals undergoing clinical genetic testing for hereditary hemorrhagic telangiectasia (HHT): Correlation of genotype with phenotype. Hum Mut. 2006;27:667–675. doi: 10.1002/humu.20342. [DOI] [PubMed] [Google Scholar]
- Hawinkles L, Kuiper P, Wiercinska E, Verspaget HW, Liu Z, Pardali E, Sier CF, ten Dijke P. Matrix metalloproteinase-14 (MT1-MMP)-mediated endoglin shedding inhibits tumor angiogenesis. Cancer Res. 2010;70:4141–4150. doi: 10.1158/0008-5472.CAN-09-4466. [DOI] [PubMed] [Google Scholar]
- Ríus C, Smith JD, Almendro N, Langa C, Botella LM, Marchuk DA, Vary CP, Bernabéu C. Cloning of the promoter region of human endoglin, the target gene for hereditary hemorrhagic telangiectasia type 1. Blood. 1998;92:4677–4690. [PubMed] [Google Scholar]
- Suthanthiran M, Li B, Song JO, Ding R, Sharma VK, Schwartz JE, August P. Transforming growth factor-β1 hyperexpression in African-American hypertensives: a novel mediator of hypertension and/or target organ damage. Proc Natl Acad Sci U S A. 2000;97:3479–3484. doi: 10.1073/pnas.050420897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas SK, Morrison AC, Andrela CM, Elovitz MA. Allelic variations in angiogenic pathway genes are associated with preeclampsia. Am J Obstet Gynecol. 2010;202:445.e1–11. doi: 10.1016/j.ajog.2010.01.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Variation in endoglin pathway genes is associated with preeclampsia: A case-control candidate gene association study.


