Abstract
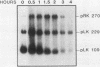
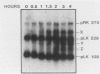
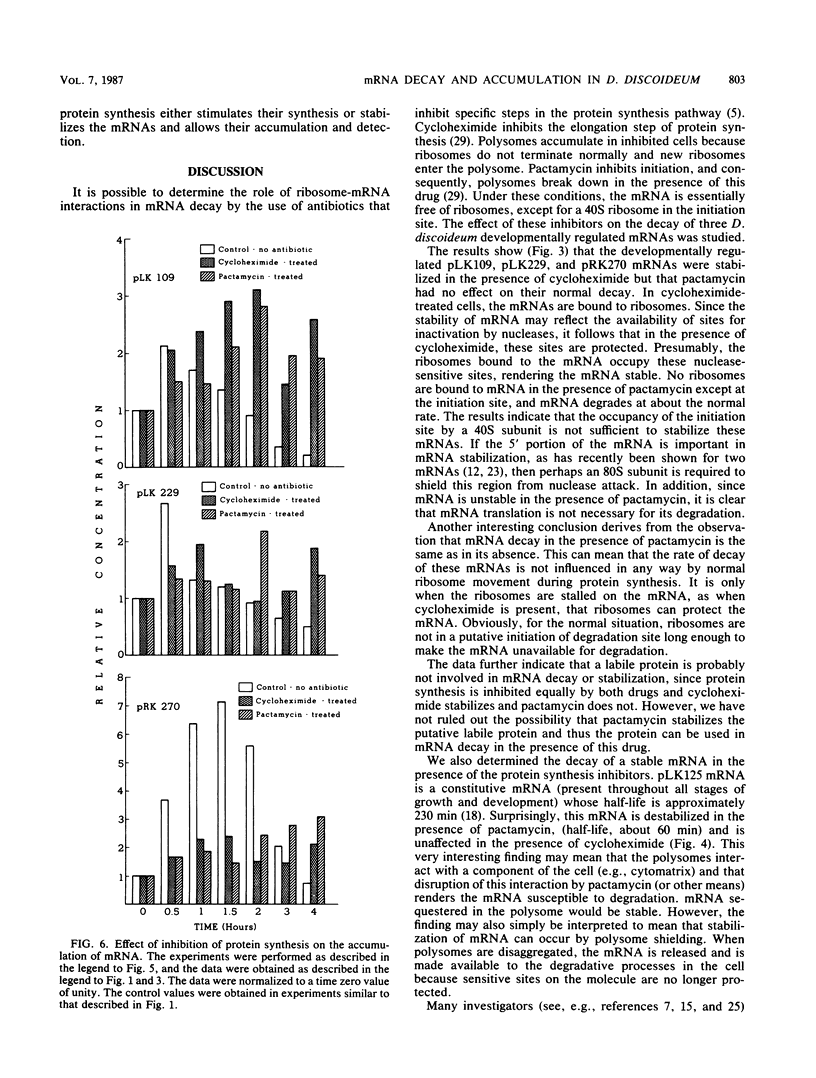
Spore germination in Dictyostelium discoideum is a particularly suitable model for studying the regulation of gene expression, since developmentally regulated changes in both protein and mRNA synthesis occur during the transition from dormant spore to amoeba. The previous isolation of three cDNA clones specific for mRNA developmentally regulated during spore germination allowed for the quantitation of the specific mRNAs during this process. The three mRNAs specific to clones pLK109, pLK229, and pRK270 have half-lives much shorter (minutes) than those of constitutive mRNAs (hours). Using spore germination as a model, we studied the roles of ribosome-mRNA interactions and protein synthesis in mRNA degradation by using antibiotics that inhibit specific reactions in protein biosynthesis. Cycloheximide inhibits the elongation step of protein synthesis. Polysomes accumulate in inhibited cells because ribosomes do not terminate normally and new ribosomes enter the polysome, eventually saturating the mRNA. Pactamycin inhibits initiation, and consequently polysomes break down in the presence of this drug. Under this condition, the mRNA is essentially free of ribosomes. pLK109, pLK229, and pRK270 mRNAs were stabilized in the presence of cycloheximide, but pactamycin had no effect on their normal decay. Since it seems likely that stability of mRNA reflects the availability of sites for inactivation by nucleases, it follows that in the presence of cycloheximide, these sites are protected, presumably by occupancy by ribosomes. No ribosomes are bound to mRNA in the presence of pactamycin, and therefore mRNA degrades at about the normal rate. The data further indicate that a labile protein is probably not involved in mRNA decay or stabilization, since protein synthesis is inhibited equally by both antibiotics. We conclude that it may be important to use more than one type of protein synthesis inhibitor to evaluate whether protein synthesis is required for mRNA decay. The effect of protein synthesis inhibition on mRNA synthesis and accumulation was also studied. mRNA synthesis continues in the presence of inhibitors, albeit at a diminished rate relative to that of the uninhibited control.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Affara N. A., Robert B., Jacquet M., Buckingham M. E., Gros F. Changes in gene expression during myogenic differentiation. I. Regulation of messenger RNA sequences expressed during myotube formation. J Mol Biol. 1980 Jul 15;140(4):441–458. doi: 10.1016/0022-2836(80)90264-8. [DOI] [PubMed] [Google Scholar]
- Baker E. J., Keller L. R., Schloss J. A., Rosenbaum J. L. Protein synthesis is required for rapid degradation of tubulin mRNA and other deflagellation-induced RNAs in Chlamydomonas reinhardi. Mol Cell Biol. 1986 Jan;6(1):54–61. doi: 10.1128/mcb.6.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belasco J. G., Beatty J. T., Adams C. W., von Gabain A., Cohen S. N. Differential expression of photosynthesis genes in R. capsulata results from segmental differences in stability within the polycistronic rxcA transcript. Cell. 1985 Jan;40(1):171–181. doi: 10.1016/0092-8674(85)90320-4. [DOI] [PubMed] [Google Scholar]
- Bird R. C., Jacobs F. A., Sells B. H. Stability of histone mRNAs is related to their location in polysomes. Biochem Cell Biol. 1986 Feb;64(2):99–105. doi: 10.1139/o86-017. [DOI] [PubMed] [Google Scholar]
- Cohen P. S., Lynch K. R., Walsh M. L., Hill J. M., Ehnis H. L. Use of antibiotics to determine ribosome-messenger RNA interactions necessary for in vivo stability of specific messengers. J Mol Biol. 1977 Aug 25;114(4):569–573. doi: 10.1016/0022-2836(77)90179-6. [DOI] [PubMed] [Google Scholar]
- Cox R. F. Estrogen withdrawal in chick oviduct. Selective loss of high abundance classes of polyadenylated messenger RNA. Biochemistry. 1977 Jul 26;16(15):3433–3443. doi: 10.1021/bi00634a022. [DOI] [PubMed] [Google Scholar]
- Dani C., Blanchard J. M., Piechaczyk M., El Sabouty S., Marty L., Jeanteur P. Extreme instability of myc mRNA in normal and transformed human cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7046–7050. doi: 10.1073/pnas.81.22.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowbenko D. J., Ennis H. L. Regulation of protein synthesis during spore germination in Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1791–1795. doi: 10.1073/pnas.77.4.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis H. L. Nogalamycin inhibits ribonucleic acid synthesis in growing and developing cells of the slime mold Dictyostelium discoideum. Antimicrob Agents Chemother. 1981 Apr;19(4):657–665. doi: 10.1128/aac.19.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis H. L., Sussman M. Mutants of Dictyostelium discoideum defective in spore germination. J Bacteriol. 1975 Oct;124(1):62–64. doi: 10.1128/jb.124.1.62-64.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri J. G., Ennis H. L. Developmental changes in RNA and protein synthesis during germination of Dictyostelium discoideum spores. Dev Biol. 1978 Nov;67(1):189–201. doi: 10.1016/0012-1606(78)90308-1. [DOI] [PubMed] [Google Scholar]
- Gorski K., Roch J. M., Prentki P., Krisch H. M. The stability of bacteriophage T4 gene 32 mRNA: a 5' leader sequence that can stabilize mRNA transcripts. Cell. 1985 Dec;43(2 Pt 1):461–469. doi: 10.1016/0092-8674(85)90176-x. [DOI] [PubMed] [Google Scholar]
- Guyette W. A., Matusik R. J., Rosen J. M. Prolactin-mediated transcriptional and post-transcriptional control of casein gene expression. Cell. 1979 Aug;17(4):1013–1023. doi: 10.1016/0092-8674(79)90340-4. [DOI] [PubMed] [Google Scholar]
- Heintz N., Sive H. L., Roeder R. G. Regulation of human histone gene expression: kinetics of accumulation and changes in the rate of synthesis and in the half-lives of individual histone mRNAs during the HeLa cell cycle. Mol Cell Biol. 1983 Apr;3(4):539–550. doi: 10.1128/mcb.3.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer E., Sekeris C. E. Cycloheximide causes increased accumulation of translatable mRNA for tyrosine aminotransferase and tryptophan oxygenase in livers of cortisol-treated rats. Eur J Biochem. 1978 May 16;86(2):547–554. doi: 10.1111/j.1432-1033.1978.tb12338.x. [DOI] [PubMed] [Google Scholar]
- Hynes N. E., Groner B., Sippel A. E., Jeep S., Wurtz T., Nguyen-Huu M. C., Giesecke K., Schütz G. Control of cellular content of chicken egg white protein specific RNA during estrogen administration and withdrawal. Biochemistry. 1979 Feb 20;18(4):616–624. doi: 10.1021/bi00571a011. [DOI] [PubMed] [Google Scholar]
- Kelly L. J., Kelly R., Ennis H. L. Characterization of cDNA clones specific for sequences developmentally regulated during Dictyostelium discoideum spore germination. Mol Cell Biol. 1983 Nov;3(11):1943–1948. doi: 10.1128/mcb.3.11.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R., Kelly L. J., Ennis H. L. Dictyostelium discoideum mRNAs developmentally regulated during spore germination have short half-lives. Mol Cell Biol. 1985 Jan;5(1):133–139. doi: 10.1128/mcb.5.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krowczynska A., Yenofsky R., Brawerman G. Regulation of messenger RNA stability in mouse erythroleukemia cells. J Mol Biol. 1985 Jan 20;181(2):231–239. doi: 10.1016/0022-2836(85)90087-7. [DOI] [PubMed] [Google Scholar]
- Linial M., Gunderson N., Groudine M. Enhanced transcription of c-myc in bursal lymphoma cells requires continuous protein synthesis. Science. 1985 Dec 6;230(4730):1126–1132. doi: 10.1126/science.2999973. [DOI] [PubMed] [Google Scholar]
- Lüscher B., Stauber C., Schindler R., Schümperli D. Faithful cell-cycle regulation of a recombinant mouse histone H4 gene is controlled by sequences in the 3'-terminal part of the gene. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4389–4393. doi: 10.1073/pnas.82.13.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiarotti G., Lefebvre P., Lodish H. F. Differences in the stability of developmentally regulated mRNAs in aggregated and disaggregated Dictyostelium discoideum cells. Dev Biol. 1982 Jan;89(1):82–91. doi: 10.1016/0012-1606(82)90296-2. [DOI] [PubMed] [Google Scholar]
- Morris T., Marashi F., Weber L., Hickey E., Greenspan D., Bonner J., Stein J., Stein G. Involvement of the 5'-leader sequence in coupling the stability of a human H3 histone mRNA with DNA replication. Proc Natl Acad Sci U S A. 1986 Feb;83(4):981–985. doi: 10.1073/pnas.83.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson G., Belasco J. G., Cohen S. N., von Gabain A. Growth-rate dependent regulation of mRNA stability in Escherichia coli. Nature. 1984 Nov 1;312(5989):75–77. doi: 10.1038/312075a0. [DOI] [PubMed] [Google Scholar]
- Olson P. S., Thompson E. B., Granner D. K. Regulation of hepatoma tissue culture cell tyrosine aminotransferase messenger ribonucleic acid by dexamethasone. Biochemistry. 1980 Apr 15;19(8):1705–1711. doi: 10.1021/bi00549a029. [DOI] [PubMed] [Google Scholar]
- Pennica D., Cohen P. S., Ennis H. L. Mechanism of vesicular stomatitis virus mRNA decay. Arch Biochem Biophys. 1981 May;208(2):403–408. doi: 10.1016/0003-9861(81)90525-7. [DOI] [PubMed] [Google Scholar]
- Sive H. L., Heintz N., Roeder R. G. Regulation of human histone gene expression during the HeLa cell cycle requires protein synthesis. Mol Cell Biol. 1984 Dec;4(12):2723–2734. doi: 10.1128/mcb.4.12.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles C. D., Lee K. L., Kenney F. T. Differential degradation of messenger RNAs in mammalian cells. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2634–2638. doi: 10.1073/pnas.73.8.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volloch V., Housman D. Stability of globin mRNA in terminally differentiating murine erythroleukemia cells. Cell. 1981 Feb;23(2):509–514. doi: 10.1016/0092-8674(81)90146-x. [DOI] [PubMed] [Google Scholar]