Abstract
Elevated plasma fibrinogen is a prothrombotic risk factor for cardiovascular disease (CVD). Recent small studies report that fibrinogen oxidative modifications, specifically tyrosine residue nitration, can occur in inflammatory states and may modify fibrinogen function. HDL cholesterol is inversely related to CVD and suggested to reduce the oxidation of LDL cholesterol, but whether these antioxidant functions extend to fibrinogen modifications is unknown. We used a recently validated ELISA to quantify nitrated fibrinogen during experimental human endotoxemia (N=23) and in a cohort of healthy adults (N=361) who were characterized for inflammatory and HDL parameters as well as subclinical atherosclerosis measures, carotid artery intima-medial thickness (IMT) and coronary artery calcification (CAC). Fibrinogen nitration increased following endotoxemia and directly correlated with accelerated ex vivo plasma clotting velocity. In the observational cohort, nitrated fibrinogen was associated with levels of CRP and serum amyloid A. Nitrated fibrinogen levels were not lower with increasing HDL cholesterol and did not associate with IMT and CAC. In humans, fibrinogen nitration was induced during inflammation and was correlated with markers of inflammation and clotting function but not HDL cholesterol or subclinical atherosclerosis in our modest sample. Inflammation-induced fibrinogen nitration may be a risk factor for promoting CVD events.
Keywords: Fibrinogen, Nitration, Intima-medial thickness, Coronary artery calcification, High-density lipoprotein (HDL)
Introduction
Plasma fibrinogen levels are independent predictors of cardiovascular disease (CVD) [1]. Recently, an oxidative modification of human fibrinogen, nitration of tyrosine residues, was reported to result in acceleration of fibrin clot formation in vitro and ex vivo[2]. Thus, nitration of fibrinogen may confer cardiovascular disease (CVD) risk beyond that related to increased fibrinogen levels. Whether inflammation causes fibrinogen nitration in humans or relates to human atherosclerotic CVD or its risk factors remains unknown.
HDL cholesterol (HDL-C) is inversely related to CVD in humans [3]. Limited data suggest a role for HDL in reducing the oxidation of LDL cholesterol, but whether HDL's antioxidant functions extend to additional atherothrombotic molecules, such as fibrinogen, is unknown. Recent studies in mice lacking ApoA-I, the major protein thought to contribute to HDL's antioxidant activities, showed elevated levels of nitrated fibrinogen in the plasma of these animals [2], while proteomic studies have demonstrated a physical association between HDL and fibrinogen [4].
First, we hypothesized that in healthy humans, experimental endotoxemia would induce fibrinogen nitration coincident with increased capacity for fibrin clot formation. Second, we speculated that in an asymptomatic cohort, Penn HDL INflammation and Oxidation (PHINOX) sample, resting plasma levels of nitrated fibrinogen would be associated inversely with HDL-C (reflecting HDL antioxidant activity), but positively with levels of inflammatory CVD risk biomarkers as well as carotid artery intima-medial thickness (IMT) and coronary artery calcification (CAC), established measures of subclinical atherosclerosis and CVD risk [5–7]. The combination of these human studies provides independent but complementary experimental and epidemiological evidence supporting an inflammatory basis for fibrinogen nitration in humans.
Methods
Clinical studies
Acute human endotoxemia study sample and protocol
As part of an ongoing study, 23 young (aged 18 to 45), healthy men and women were recruited. Exclusion criteria included any significant medical history, any abnormality at physical examination or laboratory testing, and use of any medication except oral contraceptives. Following initial telephone screening, participants came to the Clinical and Translational Research Center at the University of Pennsylvania after an overnight fast. At screening, subjects reviewed and signed an Institutional Review Board-approved consent form, completed a questionnaire history and physical exam, and had blood collected for lipids, chemistries, and blood count. Within 4 to 6 weeks, eligible subjects were admitted for a 30-h stay. The protocol included blood sampling at multiple time points for biomarker measurement before and after the intravenous bolus administration of 1.0 ng/kg of human-research-grade endotoxin (PDS No. 67801, lot No. CC-RE-LOT-1+2) (LPS). Subjects returned 2 days after discharge for additional blood sampling at 72 h following LPS.
Chronic study sample and protocol
The PHINOX study was designed to examine inflammatory and oxidant factors related to HDL-C and subclinical atherosclerosis in healthy adults. Exclusion criteria included LDL cholesterol >200 mg/dl, triglycerides >400 mg/dl, and use of HDL-C-raising medications.
Following telephone screening, fasting participants came to the Clinical and Translational Research Center, where they provided informed, Institutional Review Board-approved consent, completed a health questionnaire, and had vital signs measured and blood collected for lipids, chemistries, and blood count. Within 30 days, eligible subjects returned for baseline studies including physical examination, blood draw for serum and plasma separation, and measurement of carotid IMT by ultrasound and CAC by electron beam tomography. A subset of the PHINOX study sample has completed a predefined 2-year return visit that included repeat measurement of carotid IMT (N=282) and, for subjects with baseline CAC scores >10 (N=94), follow-up measurement. This PHINOX sample has the power to detect moderate-large, but not small-moderate, variability in a quantitative trait (e.g., IMT or transformed coronary calcium scores); e.g., 80% power, at alpha 0.05, to detect ∼4.3% trait variability in gender-, age-, and race-adjusted models and ∼5.5% trait variability in fully adjusted models.
Measurement of carotid IMT and CAC
Bilateral B mode carotid artery imaging was performed using a SONOS 5500ultrasound imager (Philips; Eindhoven, Netherlands) and linear array 5.5/7.5 MHz probe. The posterior wall of the distal CCA, 1 cm below the bifurcation, was the site selected for measurements because the least variability in measurement has been reported for this area [8]. Quality of IMT images was rated by a blinded reader on an A-B-C scale, with C grade being eliminated (3.0% of all measurements). The averages of the maximal left and right IMT readings were used for analyses. CAC scores were determined during a single breath-hold, according to the method of Agatston, from 40 continuous 3-mm-thick computed tomograms collected on an Imatron C-150 XP/LXP Evolution EBCT (Imatron; San Francisco, CA) [9,10].
Laboratory studies
Plasma lipoprotein, inflammatory and metabolic markers
Total LDL, HDL, cholesterol, and triglycerides were isolated by the ultracentrifugation technique (beta-quantification) in a Center for Disease Control certified lipid laboratory. Glucose, cholesterol, triglycerides, and apolipoproteins A-I and B were measured enzymatically (Wako Diagnostics; Richmond, VA). As previously described [11] plasma levels of tumor necrosis factor alpha (TNF), interleukin-6 (IL-6) (Linco Multiplex ELISAs on Luminex IS100; Austin TX), C-reactive protein (high-sensitivity latex turbidimetric immunoassay; Wako Ltd., Osaka Japan), insulin (RIA; Linco Research; St. Charles, MO), and serum amyloid A (SAA) (ELISA; Biosource; Carlsbad, CA) were measured according to manufacturers' guidelines.
ELISA for nitrotyrosine concentration on plasma fibrinogen
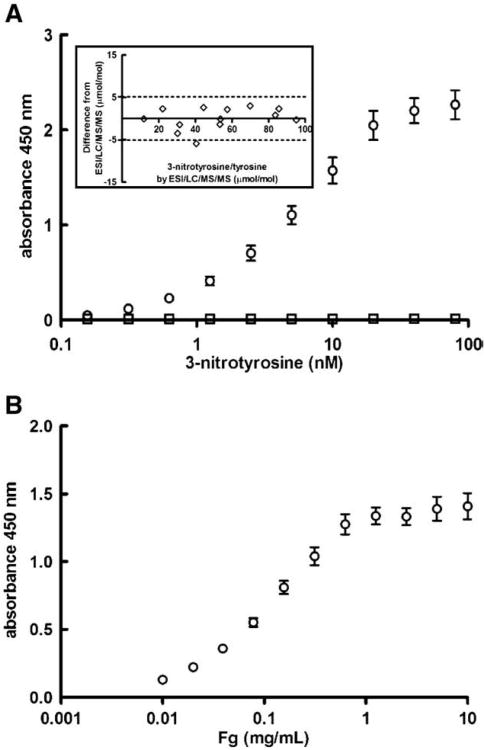
Ninety-six-well plates (maxisorb, Nunc, Rochester, NY) were coated with 50 μl of 10 μg/ml anti-nitrotyrosine antibodies, described in detail elsewhere [12], in 50 mM carbonate/bicarbonate buffer, pH 9, and incubated overnight at 4°C with (competition plates), or without, 25 μM of the nitrated peptide used to raise these antibodies. Plates were blocked with 200 μl 3% (w/vol) BSA (Roche, Nutley, NJ) in 50 mM Tris, 150 mM NaCl, 0.05% Tween 20 (vol/vol) (TBS-T) for 2 h at 37°C. Blocking buffer was aspirated, the competition plate was filled with 50 μl/well of 50 μM nitrated peptide in 1% BSA-TBS-T, the noncompetition plate was coated with 1% BSA-TBS-T and both were incubated for 90 min at 37°C. Plasma samples collected in citrate (50 μl), diluted 1/20 and 1/30 in 1% BSA-TBS-T, were added in triplicate. Serial dilutions (50 μl) of in vitro nitrated fibrinogen (nitrotyrosine 0.16 – 80 nM) were included for the standard curve. The plates were incubated at 37°C for 2 h and washed 3 times with 200 μl/well of TBS-T, 100 μl/well of 0.7 μg/ml rabbit anti-human fibrinogen polyclonal antibody (DAKO, Carpinteria, CA) conjugated with HRP (Pierce, Rockford, IL) in 1% BSA-TBS-T was added, and the plates were incubated for 2 h at room temperature. The assay was developed with 100 μl/well TMB substrate (Pierce) incubated for 10 min at 37°C. The reaction was stopped with 2 M sulfuric acid and the absorbance measured at 450 nm (Spectramax 250, Molecular Devices, Sunnyvale, CA). The dynamic range for this assay extended from 1.25 to 10 nM (Fig. 1A). The intra- and interassay variations were 15 and 14%, respectively.
Fig. 1.
Fibrinogen nitration quantification by ELISA. (A) Plates were coated with polyclonal anti-nitrotyrosine antibodies and bound fibrinogen was detected with a polyclonal anti-human fibrinogen antibody that recognizes all chains of fibrinogen. The average ± SEM of 10 standard curves in the absence (○) and presence (□) of the 3-nitrotyrosine peptide used to raise the anti-nitrotyrosine antibodies is shown. Inset: The assay was validated by comparing the 3-nitrotyrosine/tyrosine ratio acquired with the ELISA with the ratio acquired by the established LC/ESI/MS/MS analysis in affinity-purified fibrinogen of the same individuals. The insert illustrates the results of Bland-Altman analysis described in the text. (B) To quantify plasma fibrinogen levels, plates were coated with a monoclonal fibrinogen antibody that recognizes the α-chain of native, oxidized, and nitrated human fibrinogen and bound fibrinogen was detected with the same antibody as in panel A. The average ± SEM of 10 standard curves is presented.
In order to generate nitration fibrinogen standards for this assay, fibrinogen was nitrated by repeated (N=3) addition of 0.1 mM peroxynitrite to 2 mg/ml human fibrinogen solution in 100 mM phosphate, 100 μM DTPA, 25 mM sodium bicarbonate, pH 8 [13]. Excess salt was removed by passing the solution through a PD-10 column (Amersham Biosciences, Piscataway, NJ) and the nitrated fibrinogen was recovered with TBS. Nitrotyrosine concentration on fibrinogen was calculated by measuring absorbance at 450 nm in 0.1 N NaOH using ε=4400 M−1 cm−1 at 25°C.
Validation of nitrated-fibrinogen ELISA
The fibrinogen nitrotyrosine/tyrosine ratio for 14 individuals was measured with the newly developed ELISA as well as an established gold standard assay—high-performance liquid chromatography with on-line electrospray ionization tandem mass spectrometry (LC/ESI/MS/MS)—using stable isotope dilution methodology, as described previously [14]. The Bland-Altman analysis (Fig. 1A) supports a strong agreement between the two assays; in 95% of the cases the values acquired by the ELISA fall within 5 μmol/mol difference from the LC/ MS/MS values. Specifically, the bias was 0.029 ± 2.62 μmol/mol and the 95% confidence interval extended from 5.11 to 5.17 μmol/mol.
ELISA for plasma fibrinogen concentration
Ninety-six-well plates were coated with 100 μl of 10 μg/ml mouse monoclonal anti-human fibrinogen antibody 23C4A9, in 50 mM carbonate/bicarbonate buffer, pH 9, and incubated overnight at 4°C. This antibody was developed in our laboratory using standard techniques and recognizes the α-chain of native, oxidized, and nitrated human fibrinogen molecules. Liquid was aspirated and the plates were blocked with 200 μl 3% BSA-TBS-T for 2 h at 37°C. Plasma collected in citrate (100 μl), diluted in 1% BSA-TBS-T (1/15,000 and 1/20,000), as well as serial dilutions of fibrinogen standard (0.002 – 10 μg/ml), was added and incubated for 1 h at 37°C. Plates were washed three times with 200 μl/well TBS-T, and 100 μl/well of 0.7 μg/ml of rabbit anti-human fibrinogen antibody (DAKO, Carpinteria, CA) conjugated with HRP (Pierce)in 1% BSA-TBS-T was added and incubated for 1 h at room temperature. The assay was developed as described above. The linear range for this assay extended from 0.02 to 0.625 μg/ml (Fig. 1B). The precision and reproducibility of this ELISA were assessed in 10 repetitive analyses of a quality control plasma sample. The intra- and interassay variations were 10 and 9%, respectively.
Plasma clotting assays
Plasma clotting assays were performed as described previously with minor modifications [14]. Briefly, fibrinogen concentration was adjusted to 0.5 mg/ml with TBS to a final volume of 100 μl and clotting was initiated by the simultaneous addition of 0.1 U/ml human α-thrombin and 2.5 mM CaCl2. Absorbance at 350 nm was then monitored in a plate reader (Spectramax 250, Molecular Devices, Sunnyvale, CA) every 30 s for 60 min. The initial velocity (V0) of clot formation was calculated as the slope of the curve immediately after the lag phase.
Statistical analysis
Comparisons of nitrated fibrinogen concentrations and clotting velocity before and 72 h following LPS administration were made using paired sample t tests. Associations between nitrated fibrinogen and clotting rate were determined using Spearman's correlations and linear regression correcting for fibrinogen concentration.
In PHINOX, outcome variables with nonnormal distributions were natural log transformed for modeling. Crude correlations of nitrated fibrinogen with other continuous variables used Spearman's correlations. Comparison of biomarker levels between groups with low vs normal HDL-C was assessed by Kruskal Wallis testing. Multivariable linear regression was used to examine the association of nitrated fibrinogen and fibrinogen with CVD risk factors, IMT and CAC. Models were adjusted incrementally for: (a) age, gender, and race, and then (b) age, gender, race, body mass index, exercise (< 1×/week vs ≥ 1×/week), systolic blood pressure, and LDL cholesterol. Additional adjustment was performed when modeling nitrated fibrinogen in order to control for the confounding effect of plasma fibrinogen levels. Results of linear regression models are presented as beta values—the change in level of dependent variables (e.g., nitrated fibrinogen or IMT) for a log unit increase in the level of independent variables (e.g., CVD risk factors), except for BMI (5 units) and waist circumference (10 cm). The PHINOX sample was determined to have 80% power to detect ∼4.3% trait variability in gender-, age-, and race-adjusted models and ∼5.5% trait variability in fully adjusted models.
Statistical analyses were performed by S.P.H, M.P.R., and M.G.T. using SPSS 15.0 for Windows (SPSS Inc.; Chicago, IL) and Stata 9.0 software (Stata Corp; College Station, TX) and all authors had access to primary clinical data.
Results
Acute endotoxemia studies
Study subjects were young, healthy, normotensive, nonobese, and without lipid abnormalities (Table 1). As expected based on previous studies [11,15], endotoxin induced a cytokine-mediated, mild, transient, febrile illness in all subjects with modest increases from baseline in temperature (97.9 to 99.5 F) and heart rate (65 to 94), that largely resolved by 8 h following LPS. We and others have published the time course of cytokine and inflammatory protein responses to endotoxin administration. During the first 24-h, a marked transient induction of TNFα is followed by a gradual increase in the acute phase protein CRP, and stably elevated plasma MPO (Supplemental Figs. A–C).
Table 1. Characteristics of the human endotoxemia study sample.
| Females (N=16) | Males (N=7) | |
|---|---|---|
|
| ||
| Median (interquartile range) | ||
| Age, years | 26 (24 to 30) | 22 (19 to 24) |
| Caucasian ethnicity (%) | 94 | 100 |
| Body mass index, kg/m2 | 22.8 (22.0 to 25.0) | 27.4 (25.5 to 30.2) |
| Waist circumference, cm | 77 (70 to 88) | 96 (90 to 104) |
| Blood pressure, mm Hg | ||
| Systolic | 106 (97 to 112) | 112 (108 to 125) |
| Diastolic | 57 (51 to 65) | 69 (60 to 73) |
| Fasting glucose, mg/dl | 79 (71 to 85) | 84 (77 to 87) |
| Total cholesterol, mg/dl | 161 (143 to 177) | 155 (134 to 159) |
| HDL cholesterol, mg/dl | 59 (51 to 70) | 48 (40 to 50) |
| LDL cholesterol, mg/dl | 79 (68 to 106) | 92 (86 to 98) |
| Triglycerides, mg/dl | 79 (68 to 95) | 71 (45 to 87) |
Acute inflammation induces fibrinogen nitration and accelerates fibrin polymerization
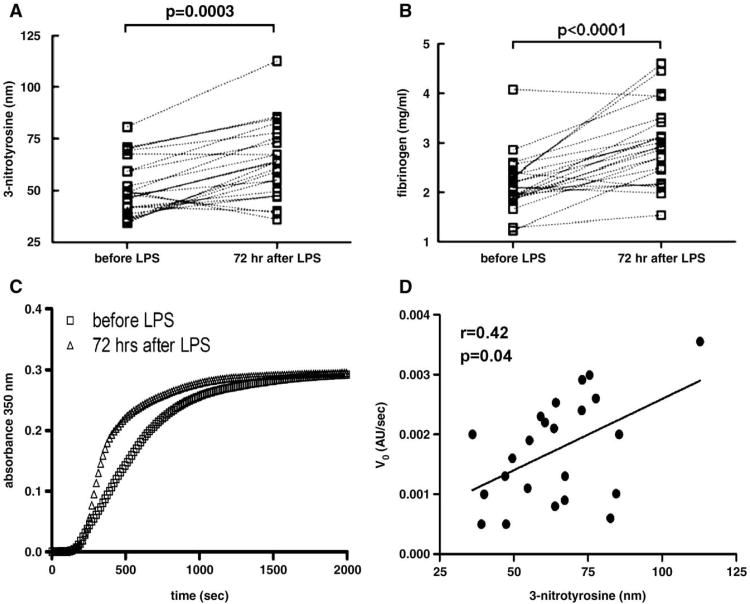
We performed an initial time-course exploration during endotoxemia in six subjects (Supplemental Fig. D) in which we noted a late increase in fibrinogen nitration at 72h following LPS and thus focused on this later time point for further study. At this time in the full sample (N=23), experimental endotoxemia significantly increased fibrinogen nitration (Fig. 2A) and, as expected, total plasma fibrinogen levels (Fig. 2B). To assess the effect of endotoxemia on plasma clotting properties irrespective of variations in fibrinogen levels, we performed clotting assays in plasma samples taken before and 72 h after LPS infusion, after normalizing samples to identical fibrinogen concentrations. In this analysis, the maximum turbidity, which is indicative of the average fiber size and is primarily dependent on fibrinogen concentration, did not change; the initial velocity, however, which denotes the rate of polymerization, was greater after endotoxemia (pre; 0.00127±0.0007 AU/s vs post; 0.00174±0.0009 AU/s, P=0.029; Fig. 2C). The change in initial velocity which reflects the process of fibrin lateral aggregation an event that follows the initial nucleation and the formation of half-staggered, double-stranded protofibrils, in the samples after 72 h of endotoxin challenge correlated with the levels of fibrinogen nitration (Spearman's r=0.42, P=0.04; Fig. 2D).
Fig. 2.
Effects of human endotoxemia on plasma fibrinogen, nitrated fibrinogen, and ex vivo fibrin clotting kinetics. (A) Paired t test statistical comparison before and 72 h after LPS administration reveals a significant increase in nitrated fibrinogen levels after the inflammatory stimulus. (B) The same analysis reveals a significant increase in the levels of fibrinogen in plasma. (C) The fibrinogen concentration was adjusted to 0.5 mg/ml with TBS and fibrin clot formation was initiated by the simultaneous addition of calcium and thrombin. The curves represent the average ± SEM of 22 individual samples before (□) and 72 h after (Δ) LPS injection and demonstrate an increased velocity of fibrin clot formation after LPS (P=0.029). (D) Spearman correlation between levels of fibrinogen nitration and initial velocity of fibrin clot formation 72 h after endotoxin (r=0.42, P=0.04, N=23).
Study of nitrated fibrinogen, CVD risk factors, and atherosclerosis in PHINOX
Three hundred sixty-one participants were recruited as part of the PHINOX cohort and had baseline plasma measurements (Table 2). Subjects were generally healthy, normotensive, and without lipid abnormalities, but were slightly overweight. Of 359 subjects with CAC data, the majority (63.7%) had CAC scores of zero, and less than 20% had CAC > 75th percentile for age and gender. Two hundred eighty-one subjects had high-quality ultrasound data for quantification of carotid IMT. As expected, there were significant differences between genders in levels of SAA (P<0.001), CRP (P=0.005), fibrinogen (P=0.001), and CAC (P=0.011) (Table 2).
Table 2. Characteristics of the Penn HDL INflammation and OXidation (PHINOX) cohort.
| Females (N=169) | Males (N=192) | |
|---|---|---|
|
| ||
| Median (interquartile range) | ||
| Age, years | 54 (48 to 61) | 49 (42 to 55) |
| Caucasian ethnicity (%) | 75 | 83 |
| Body mass index, kg/m2 | 26.6 (23.3 to 31.7) | 27.4 (25.5 to 30.2) |
| Waist circumference, cm | 89 (82 to 99) | 96 (90 to 104) |
| Blood pressure, mm Hg | ||
| Systolic | 123 (111 to 132) | 124 (116 to 133) |
| Diastolic | 75 (69 to 81) | 77 (72 to 84) |
| Fasting glucose, mg/dl | 73 (65 to 83) | 75 (66 to 84) |
| Total cholesterol, mg/dl | 202 (184 to 227) | 198 (169 to 218) |
| HDL cholesterol, mg/dl | 62 (49 to 74) | 48 (38 to 61) |
| Apolipoprotein A-I, mg/dl | 142 (125 to 160) | 124 (107 to 143) |
| Apolipoprotein A-II, mg/dl | 35 (32 to 39) | 33 (30 to 37) |
| LDL cholesterol, mg/dl | 120 (104 to 140) | 119 (101 to 142) |
| Apolipoprotein B, mg/dl | 83 (73 to 94) | 87 (75 to 98) |
| Triglycerides, mg/dl | 80 (60 to 122) | 91 (64 to 139) |
| C-reactive protein, mg/L | 1.6 (0.8 to 3.0) | 1.0 (0.6 to 2.1) |
| Serum amyloid A, μg/ml | 15.7 (8.6 to 26.2) | 9.3 (5.3 to 16.2) |
| Fibrinogen, mg/ml | 2.4 (2.0 to 2.9) | 2.2 (1.9 to 2.6) |
| Nitrated fibrinogen, nM | 43.1 (36.4 to 50.0) | 40.8 (34.8 to 49.0) |
| Coronary artery calcification | ||
| Mean score (±SD) | 83 ± 570 | 115 ±552 |
| Median score (IQR) | 0 (0 – 11.6) | 0 (0 to 42.3) |
| Common carotid artery intima-media thickness, (mm) | 0.68 (0.59 to 0.76) | 0.65 (0.57 to 0.75) |
Nitrated fibrinogen correlates with inflammatory markers independent of total fibrinogen
Nitrated fibrinogen levels were significantly, albeit modestly, correlated with total plasma fibrinogen. Levels also correlated with the inflammatory markers, SAA and CRP, but were not related to lipid or metabolic parameters (Table 3A). Remarkably, nitrated fibrinogen remained significantly associated with CRP and SAA in multivariable models, after adjusting for CVD risk factors, and even after further controlling for plasma levels of fibrinogen (Table 3B). In contrast, fibrinogen levels were associated with LDL-C, in addition to CRP and SAA, in both crude and adjusted models (Tables 3A and B).
Table 3. Table 3A: Correlations of cardiovascular risk factors with fibrinogen and nitrated fibrinogen.
| Spearman's correlation coefficients | ||
|---|---|---|
|
| ||
| Fibrinogen | Nitrated fibrinogen | |
| HDL cholesterol | 0.021 | 0.085 |
| Apolipoprotein A-I | −0.050 | 0.022 |
| Apolipoprotein A-II | 0.059 | 0.012 |
| LDL cholesterol | 0.069 | 0.027 |
| Apolipoprotein B | 0.093 | 0.023 |
| Triglycerides | −0.022 | −0.019 |
| C-reactive protein | 0.300§ | 0.174† |
| Serum amyloid A | 0.341§ | 0.242§ |
| Body mass index | 0.224§ | 0.017 |
| Waist circumference | 0.175‡ | 0.079 |
| Insulin | 0.214§ | −0.022 |
|
| ||
| P values: † ≤ 0.01; ‡ ≤ 0.001, § ≤ 0.0001. | ||
| Table 3B Multivariable associations of cardiovascular risk factors with fibrinogen and nitrated fibrinogen | |||||
|---|---|---|---|---|---|
|
| |||||
| Covariate | Regression coefficients (95% confidence interval) | ||||
|
| |||||
| Fibrinogen | Nitrated fibrinogen | ||||
|
|
|
||||
| Model 1 | Model 2 | Model 1 | Model 2 | Model 3 | |
| Ln-HDL cholesterol | −0.20 (−0.44 to 0.05) | 1.30 (−2.84 to 5.44) | |||
| Ln-apolipoprotein A-I | −0.52 (−0.93 to −0.10)* | 0.22 (−6.95 to 7.38) | |||
| Ln-apolipoprotein A-II | 0.12 (−0.33 to 0.57) | 0.45 (−7.15 to 8.05) | |||
| Ln-LDL cholesterol | 0.31 (0.04 to 0.59)* | 0.32 (0.04 to 0.61)* | 2.53 (-2.28 to 7.33) | ||
| Ln-apolipoprotein B | 0.44 (0.08 to 0.81)* | 3.41 (-2.61 to 9.43) | |||
| Ln-triglycerides | 0.030 (−0.10 to 0.16) | 0.29 (−1.93 to 2.50) | |||
| Ln-C reactive protein | 0.28 (0.16 to 0.40)§ | 0.14 (0.05 to 0.23)† | 4.0 (2.03 to 5.86)§ | 4.84 (2.58 to 7.09)‡ | 3.63 (0.71 to 4.78)† |
| Ln-serum amyloid A | 0.22 (0.13 to 0.31)§ | 0.12 (0.05 to 0.20)‡ | 2.86 (1.39 to 4.32)§ | 2.83 (1.23 to 4.43)‡ | 2.14 (0.60 to 3.67)† |
| Waist circumference | 0.10 (0.05 to 0.15)‡ | 0.69 (−0.24 to 1.62) | |||
| Ln-insulin | 0.22 (0.09 to 0.35)‡ | 0.68 (−1.51 to 2.87) | |||
Results of linear regression modeling are presented as the change in level of dependent variables (fibrinogen, nitrated fibrinogen) for a one unit increase in level of independent variables. Model 1 was adjusted for age, gender, and race; Model 2 for age, gender, race BMI, blood pressure, LDL cholesterol, and exercise; Model 3 also included fibrinogen values. P values:
< 0.05,
≤ 0.01;
≤ 0.001,
≤ 0.0001.
Nitrated fibrinogen levels are not related to HDL-C, apolipoprotein A-I, or measures of subclinical atherosclerosis
Nitrated fibrinogen levels were not associated with HDL-C, apoA-I, or apoA-II in crude or adjusted multivariable models (Tables 3A and B). Indeed, there was no difference in nitrated fibrinogen levels (39.7 vs 40.9 nM, P=0.37) between low (mean HDL-C 39 ± 6; N=103) and normal HDL-C (mean HDL-C 64±16 mg/dl; N = 257) groups. As expected, CRP levels tended to be higher in subjects with low HDL-C relative to those with normal HDL-C (1.65 mg/dl vs 1.17 mg/dl, P = 0.025). There was a modest inverse association of plasma fibrinogen levels with apoA-I, but not HDL-C, after controlling for age, gender, and race, although this association was attenuated in fully adjusted models (Table 3B).
Plasma fibrinogen levels (Spearman's r=0.17, P<0.01), but not nitrated fibrinogen (r=−0.09, P=0.18), were correlated with carotid IMT. In adjusted models, neither fibrinogen nor nitrated fibrinogen were associated with IMT. Furthermore, there was no relationship between levels of nitrated fibrinogen with CAC scores in crude or adjusted models (r=0.03, P=0.61). Finally, in the subjects with follow-up IMT and CAC data, nitrated fibrinogen level at baseline did not predict change in IMT (P=0.47) or CAC (P=0.45) over 2 years.
Discussion
We present complementary experimental and epidemiological evidence in two studies that support an inflammatory basis for fibrinogen nitration in humans. We report, for the first time, that experimental human inflammation induces fibrinogen nitration and accelerated fibrin polymerization. Further, in the asymptomatic, community-based PHINOX sample, circulating levels of nitrated fibrinogen correlated with inflammatory CVD risk biomarkers, CRP and SAA, but not with traditional CVD risk factors, including HDL-C. Finally, nitrated fibrinogen levels did not relate to validated measures of subclinical atherosclerosis.
Inflammation, fibrinogen nitration, and risk for clotting
Fibrinogen, a building block for thrombus formation, increases in the plasma during the acute phase reaction and is an independent risk factor for clinical CVD [16]. Furthermore, emerging data suggest that nitration of fibrinogen, which in this study was associated with accelerated fibrin polymerization, is also increased in inflammatory states and chronic disease [3,14,17,18]. Experimental data support the concept that fibrinogen nitration is likely to increase in proatherogenic chronic inflammatory states due to the formation of reactive nitrogen species [19], although previously no human data existed demonstrating an inflammatory basis for this modification. Our endotoxemia model of low-grade inflammation provides proof of concept that activation of innate immunity in vivo promotes nitration of fibrinogen in humans. Fibrinogen nitration peaks late, after induction of acute phase proteins and specifically after increases in MPO, which has been implicated in posttranslational nitration of proteins [20,21], although we cannot prove that MPO is the causal mechanism in this human model. Additional studies are required also to examine change in nitrated fibrinogen over time in diverse clinical settings and in larger samples.
Our endotoxemia model also suggests that inflammation modulates fibrin polymerization coincident with nitration of fibrinogen. Indeed, the inflammatory stimulus resulted in significant modulation of plasma clotting properties which could not be attributed exclusively to the changes in fibrinogen concentration. Thrombin-induced initiation of clotting in plasma samples with the same fibrinogen concentration showed that the rate of fibrin polymerization correlated with nitrated fibrinogen levels independent of level of total fibrinogen. These novel in vivo findings are supported by previously published in vitro and ex vivo studies [2,14,22,23] suggesting that posttranslational oxidative damage of fibrinogen, in the form of tyrosine nitration, is promoted by an inflammatory environment and is associated with prothrombotic changes in the plasma coagulation profile. Further, in healthy community-based PHINOX participants, we observed positive correlations of nitrated fibrinogen with CRP and SAA that were also independent of circulating fibrinogen levels. Our findings suggest that inflammation-driven nitration of fibrinogen is associated with a prothrombotic state in humans.
Fibrinogen nitration and HDL
HDL-C may retard atherosclerosis through anti-inflammatory and antioxidant actions, in addition to promotion of reverse cholesterol transport [24–26]. HDL is increasingly recognized as a lipoprotein modulator of both innate immune and thrombotic cascades [27,28]. In fact, plasma fibrinogen levels are inversely related to HDL-C [16] and, in PHINOX, we found a modest inverse association of fibrinogen with ApoA-I, the major apolipoprotein on HDL. A recent proteomic analysis of HDL found that fibrinogen is physically associated with HDL particles [4]. We hypothesized, therefore, that such an association could protect fibrinogen from oxidant-mediated damage such as tyrosine moiety nitration, through direct HDL antioxidant actions. However, in contrast to positive associations with acute phase proteins, nitrated fibrinogen did not inversely correlate with levels of HDL-C or apolipoprotein A-I, and did not vary across predefined groups with low versus normal/high HDL-C levels. Similarly, Shishehbor and colleagues found no association between HDL-C and total plasma protein nitrotyrosine levels, although they did not assay fibrinogen nitration specifically [29]. These findings suggest that HDL's putative antioxidant functions do not extend to attenuation of inflammation-induced fibrinogen nitration, at least in healthy asymptomatic humans.
Fibrinogen nitration and the burden of subclinical atherosclerosis
Cardiovascular risk factors promote clinical CVD through diverse mechanisms including atherogenesis, plaque instability, and acute thrombosis [30]. In the PHINOX cohort, we explored one aspect of CVD in examining the relationship of nitrated fibrinogen to quantitative measures of subclinical atherosclerosis, in the absence of the confounding effects of established clinical CVD or prior thrombosis. Despite modest correlations with inflammatory biomarkers of CVD risk, plasma nitrated fibrinogen did not demonstrate any relationship to subclinical atherosclerosis, either at baseline or in follow-up imaging. The PHINOX study sample, however, was underpowered to detect small-moderate associations with the burden of atherosclerosis.
Our findings also do not exclude a causal relationship between nitrated fibrinogen and clinical CVD events through promotion of plaque instability or thrombosis [2]. Notably, fibrinogen itself appears to have a more robust association with acute clinical CVD events than with direct measures of subclinical atherosclerosis [31–34]. Preliminary studies do, in fact, suggest that increased levels of nitrated fibrinogen are found in patients with clinical CAD and cigarette smokers, both recognized conditions with heightened prothrombotic risk [14,17]. Our findings complement and extend these early clinical studies and suggest that fibrinogen nitration is more likely to play a role in acute atherothrombosis than in initiation or acceleration of atherosclerosis.
Our studies have limitations. Induction of fibrinogen nitration during human inflammation and association with accelerated plasma clot kinetics is correlative and does not prove causality. The PHINOX study is largely cross-sectional, cannot define mechanisms, and was not designed to address relationships with clinical CVD events. The PHINOX sample had limited power to detect small associations. It is unlikely, however, that we failed to detect clinically important relationships of nitrated fibrinogen with HDL parameters, although larger studies are warranted to seek more modest associations with atherosclerosis as well as to examine prospectively the relationship to acute clinical CVD events.
In summary, the data provide the first evidence for an inflammation-related origin of nitrated fibrinogen during acute human inflammation and in asymptomatic subjects. Nitration of fibrinogen is associated with enhanced ex vivo plasma clotting but does not appear to be modulated by HDL-C. Fibrinogen nitration may alter fibrin polymerization kinetics independent of fibrinogen levels and therefore may be a risk factor for clinical atherothrombotic CVD, particularly in acute or chronic inflammatory states. Large longitudinal clinical studies are warranted to further examine relationships with atherosclerosis and clinical cardiovascular events, to determine if such relationships are independent of established risk factors and to define if nitrated fibrinogen data improve discrimination and classification for risk prediction purposes.
Supplementary Material
Acknowledgments
This work was supported by a Clinical and Translational Science Award (RFA-RM-06-002) and a General Clinical Research Center (M01 RR00040), both from the NCRR/NIH to the University of Pennsylvania and by Special Center of Research in Atherosclerosis (P50 HL70128) and Vascular Injury (HL P50-083799) from NHLBI/NIH. S.H. is a Doris Duke Clinical Research Fellow. M.P.R. is supported by HL RO1-073278, HL P50-083799 and the W.W. Smith Charitable Trust (No. H0204). M. C. is supported by K23 HL077146. H.I. is supported by grants from the National Institutes of Health P50-083799, P01 HL 079063, and ES013508 NIEHS Center of Excellence in Environmental Toxicology.
Abbreviations
- CVD
cardiovascular disease
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- LPS
lipopolysaccharide/endotxoin
- IMT
intima-medial thickness
- CAC
coronary artery calcification
- PHINOX
Penn HDL INflammation and Oxidation
- CRP
C-reactive protein
- SAA
serum amyloid A
- CAD
coronary artery disease
- MPO
myeloperoxidase
Footnotes
There are no conflicts of interest.
Appendix A. Supplementary data: Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.freeradbiomed.2009.07.025.
References
- 1.Maresca G, Di Blasio A, Marchioli R, Di Minno G. Measuring plasma fibrinogen to predict stroke and myocardial infarction: an update. Arterioscler Thromb Vasc Biol. 1999;19:1368–1377. doi: 10.1161/01.atv.19.6.1368. [DOI] [PubMed] [Google Scholar]
- 2.Parastatidis I, Thomson L, Fries DM, Moore RE, Tohyama J, Fu X, Hazen SL, Heijnen HF, Dennehy MK, Liebler DC, Rader DJ, Ischiropoulos H. Increased protein nitration burden in the atherosclerotic lesions and plasma of apolipoprotein A-I-deficient mice. Circ Res. 2007;101:368–376. doi: 10.1161/CIRCRESAHA.107.157537. [DOI] [PubMed] [Google Scholar]
- 3.Castelli WP, Garrison RJ, Wilson PW, Abbott RD, Kalousdian S, Kannel WB. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham study. JAMA. 1986;256:2835–2838. [PubMed] [Google Scholar]
- 4.Vaisar T, Pennathur S, Green PS, Gharib SA, Hoofnagle AN, Cheun MC, Byun J, Vuletic S, Kassim S, Singh P, Chea H, Knopp RH, Brunzell J, Geary R, Chait A, Zhao XQ, Elkon K, Marcovina S, Ridker P, Oram JF, Heinecke JW. Shotgun proteomics implicates protease inhibition and complement activation in the anti-inflammatory properties of HDL. J Clin Invest. 2007;117:746–756. doi: 10.1172/JCI26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 6.Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary artery calcium areas by electron beam computed tomography and coronary atherosclerotic plaque area: a histopathologic correlative study. Circulation. 1995;92:2157–2162. doi: 10.1161/01.cir.92.8.2157. [DOI] [PubMed] [Google Scholar]
- 7.Kondos GT, Hoff JA, Sevrukov A, Daviglus ML, Garside DB, Devries SS, Chomka EV, Liu K. Electron-beam tomography coronary artery calcium and cardiac events: a 37-month follow-up of 5635 initially asymptomatic low- to intermediate-risk adults. Circulation. 2003;107:2571–2576. doi: 10.1161/01.CIR.0000068341.61180.55. [DOI] [PubMed] [Google Scholar]
- 8.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 9.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 10.Reilly MP, Wolfe ML, Localio AR, Rader DJ. Coronary artery calcification and cardiovascular risk factors: impact of the analytic approach. Atherosclerosis. 2004;173:69–78. doi: 10.1016/j.atherosclerosis.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Anderson PD, Mehta NN, Wolfe ML, Hinkle CC, Pruscino L, Comiskey LL, Tabita-Martinez J, Sellers KF, Rickels MR, Ahima RS, Reilly MP. Innate immunity modulates adipokines in humans. J Clin Endocrinol Metab. 2007;92:2272–2279. doi: 10.1210/jc.2006-2545. [DOI] [PubMed] [Google Scholar]
- 12.Heijnen HF, van Donselaar E, Slot JW, Fries DM, Blachard-Fillion B, Hodara R, Lightfoot R, Polydoro M, Spielberg D, Thomson L, Regan EA, Crapo J, Ischiropoulos H. Subcellular localization of tyrosine-nitrated proteins is dictated by reactive oxygen species generating enzymes and by proximity to nitric oxide synthase. Free Radic Biol Med. 2006;40:1903–1913. doi: 10.1016/j.freeradbiomed.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Ischiropoulos H, Zhu L, Chen J, Tsai M, martin JC, Smith CD, Beckman JS. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch Biochem Biophys. 1992;298:431–437. doi: 10.1016/0003-9861(92)90431-u. [DOI] [PubMed] [Google Scholar]
- 14.Vadseth C, Souza JM, Thomson L, Seagraves A, Nagaswami C, Scheiner T, Torbet J, Vilaire G, Bennett JS, Murciano JC, Muzykantov V, Penn MS, Hazen SL, Weisel JW, Ischiropoulos H. Pro-thrombotic state induced by post-translational modification of fibrinogen by reactive nitrogen species. J Biol Chem. 2004;279:8820–8826. doi: 10.1074/jbc.M306101200. [DOI] [PubMed] [Google Scholar]
- 15.Pleiner J, Heere-Ress E, Langenberger H, Sieder AE, Bayerle-Eder M, Mittermayer F, Fuchsjager-Mayrl G, Bohm J, Jansen B, Wolzt M. Adrenoceptor hyporeactivity is responsible for Escherichia coli endotoxin-induced acute vascular dysfunction in humans. Arterioscler Thromb Vasc Biol. 2002;22:95–100. doi: 10.1161/hq0102.101818. [DOI] [PubMed] [Google Scholar]
- 16.Fibrinogen Studies Collaboration. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA. 2005;294:1799–1809. doi: 10.1001/jama.294.14.1799. [DOI] [PubMed] [Google Scholar]
- 17.Pignatelli B, Li CQ, Boffetta P, Chen Q, Ahrens W, Nyberg F, Mukeria A, Bruske-Hohlfeld I, Fortes C, Constantinescu V, Ischiropoulos H, Ohshima H. Nitrated and oxidized plasma proteins in smokers and lung cancer patients. Cancer Res. 2001;61:778–784. [PubMed] [Google Scholar]
- 18.Gole MD, Souza JM, Choi I, Hertkorn C, Malcolm S, Foust RF, 3rd, Finkel B, Lanken PN, Ischiropoulos H. Plasma proteins modified by tyrosine nitration in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2000;278:L961–L967. doi: 10.1152/ajplung.2000.278.5.L961. [DOI] [PubMed] [Google Scholar]
- 19.Dedon PC, Tannenbaum SR. Reactive nitrogen species in the chemical biology of inflammation. Arch Biochem Biophys. 2004;423:12–22. doi: 10.1016/j.abb.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 20.Gaut JP, Byun J, Tran HD, Lauber WM, Carroll JA, Hotchkiss RS, Belaaouaj A, Heinecke JW. Myeloperoxidase produces nitrating oxidants in vivo. J Clin Invest. 2002;109:1311–1319. doi: 10.1172/JCI15021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brennan ML, Wu W, Fu X, Shen Z, Song W, Frost H, Vadseth C, Narine L, Lenkiewicz E, Borchers MT, Lusis AJ, Lee JJ, Lee NA, Abu-Soud HM, Ischiropoulos H, Hazen SL. A tale of two controversies: defining both the role of peroxidases in nitrotyrosine formation in vivo using eosinophil peroxidase and myeloperoxidase-deficient mice, and the nature of peroxidase-generated reactive nitrogen species. J Biol Chem. 2002;277:17415–17427. doi: 10.1074/jbc.M112400200. [DOI] [PubMed] [Google Scholar]
- 22.Inada Y, Hessel B, Blombäck B. Photooxidation of fibrinogen in the presence of methylene blue and its effect on polymerization. Biochim Biophys Acta. 1978;532:161–170. doi: 10.1016/0005-2795(78)90459-2. [DOI] [PubMed] [Google Scholar]
- 23.Shacter E, Williams JA, Levine RL. Oxidative modification of fibrinogen inhibits thrombin-catalyzed clot formation. Free Radic Biol Med. 1995;18:815–821. doi: 10.1016/0891-5849(95)93872-4. [DOI] [PubMed] [Google Scholar]
- 24.Navab M, Anantharamaiah GM, Fogelman AM. The role of high-density lipoprotein in inflammation. Trends Cardiovasc Med. 2005;15:158–161. doi: 10.1016/j.tcm.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Baker PW, Rye KA, Gamble JR, Vadas MA, Barter PJ. Ability of reconstituted high density lipoproteins to inhibit cytokine-induced expression of vascular cell adhesion molecule-1 in human umbilical vein endothelial cells. J Lipid Res. 1990;40:345–353. [PubMed] [Google Scholar]
- 26.Rader DJ. Regulation of reverse cholesterol transport and clinical implications. Am J Cardiol. 2003;92:42J–49J. doi: 10.1016/s0002-9149(03)00615-5. [DOI] [PubMed] [Google Scholar]
- 27.Navab M, Anantharamaiah GM, Fogelman AM. The role of high-density lipoprotein in inflammation. Trends Cardiovasc Med. 2005;15:158–161. doi: 10.1016/j.tcm.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 28.Mineo C, Deguchi H, Griffin JH, Shaul PW. Endothelial and antithrombotic actions of HDL. Circ Res. 2006;98:1352–1364. doi: 10.1161/01.RES.0000225982.01988.93. [DOI] [PubMed] [Google Scholar]
- 29.Shishehbor MH, Aviles RJ, Brennan ML, Fu X, Goormastic M, Pearce GL, Gokce N, Keaney JF, Penn MS, Sprecher DL, Vita JA, Hazen SL. Association of nitrotyrosine levels with cardiovascular disease and modulation by statin therapy. JAMA. 2003;289:1675–1680. doi: 10.1001/jama.289.13.1675. [DOI] [PubMed] [Google Scholar]
- 30.Kumar V, Cotran RS, Robbins SL, editors. Robbins Basic Pathology. 7th. Vol. 2003 Saunders; Philadelphia: 2003. [Google Scholar]
- 31.Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA. 1998;279:1477–1482. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- 32.Folsom AR, Wu KK, Shahar E, Davis CE for the ARIC Study Investigation. Association of haemostatic variables with prevalent cardiovascular disease and asymptomatic carotid artery atherosclerosis. Arterioscler Thromb. 1993;13:1829–1836. doi: 10.1161/01.atv.13.12.1829. [DOI] [PubMed] [Google Scholar]
- 33.Tracy RP, Bovill EG, Yanez D, Psaty BM, Fried LP, Heiss G, Lee M, Polak JF, Savage PJ for the Cardiovascular Health Study. Fibrinogen and factor VIII, but not factor VII, are associated with measures of subclinical cardiovascular disease in the elderly: results from the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 1995;15:1269–1279. doi: 10.1161/01.atv.15.9.1269. [DOI] [PubMed] [Google Scholar]
- 34.Ebrahim S, Papacosta O, Whincup P, Wannamethee G, Walker M, Nicolaides AN, Dhanjil S, Griffin M, Belcaro G, Rumley A, Lowe GD. Carotid plaque, intimamedia thickness, cardiovascular risk factors, and prevalent cardiovascular disease in men and women: the British Regional Heart Study. Stroke. 1999;30:841–850. doi: 10.1161/01.str.30.4.841. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.