Abstract
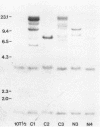
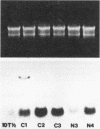
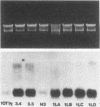
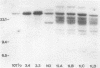
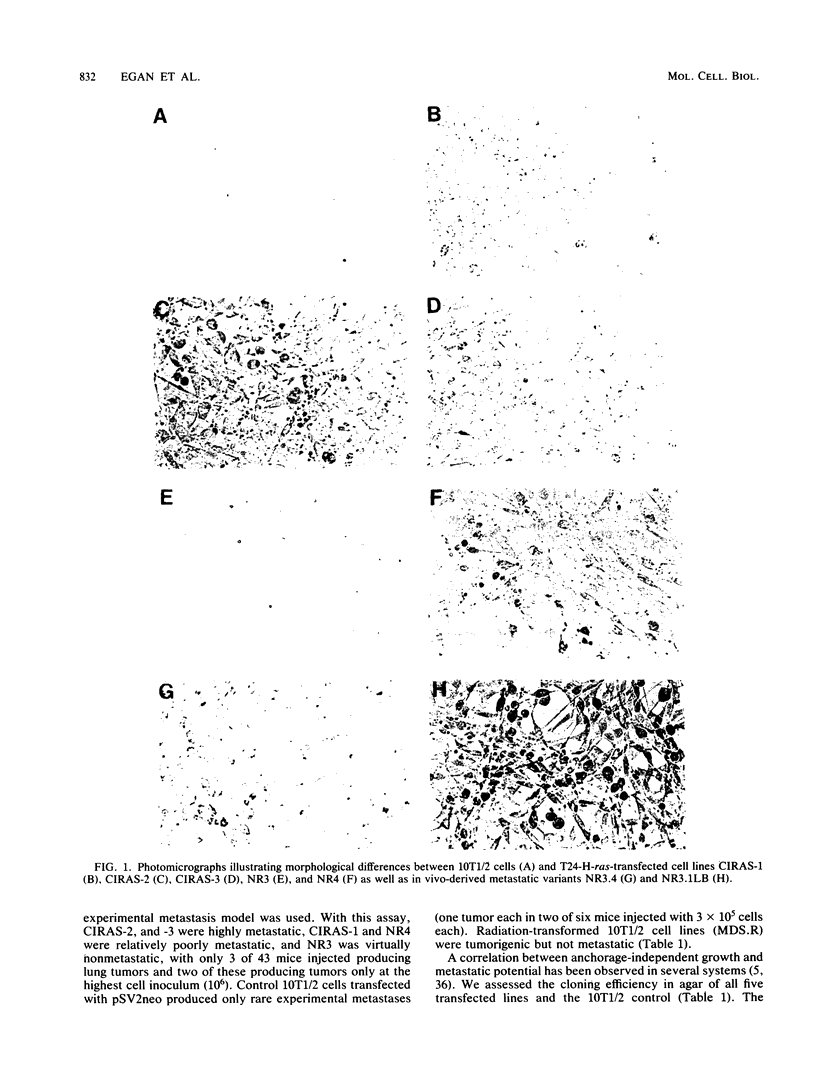
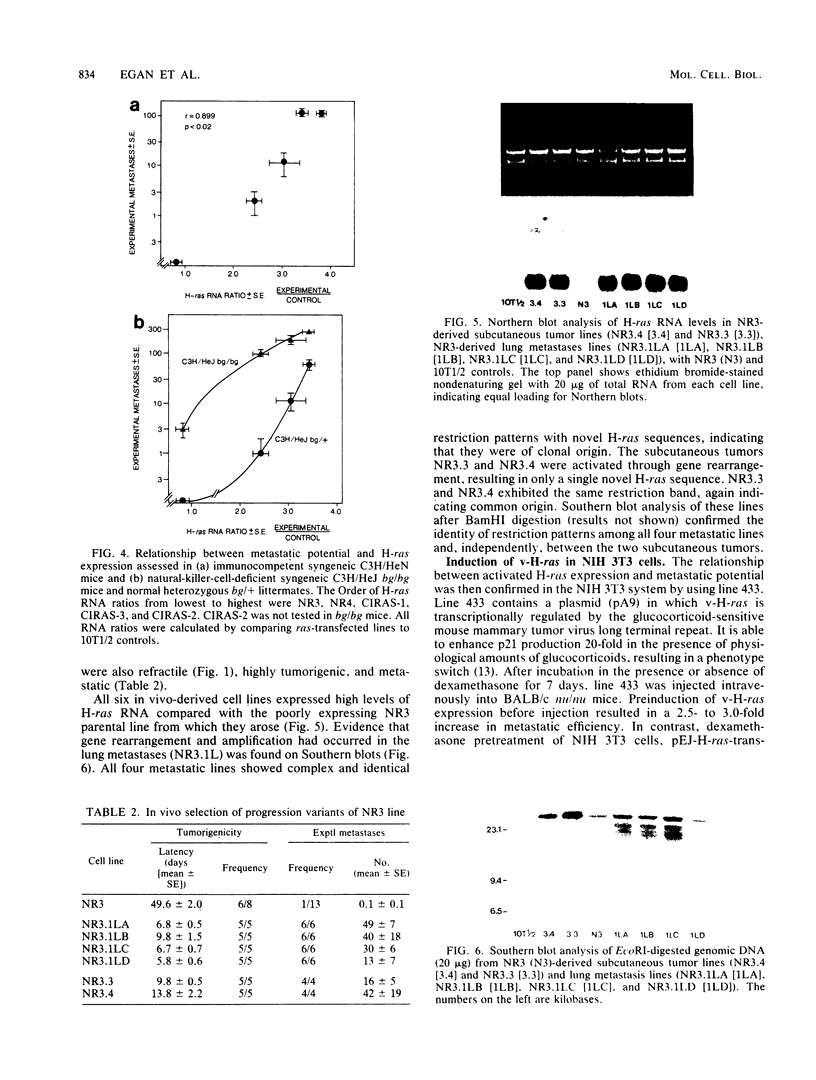
Using three independent approaches, we studied the effects of H-ras on metastasis formation. Analysis of five in vitro-ras-transfected 10T1/2 clones with either flat or refractile morphologies revealed a relationship between metastatic potential, H-ras expression, and anchorage-independent growth. Four metastatic variants derived from a poorly metastatic, low-H-ras-expressing line all expressed high levels of H-ras RNA and grew efficiently in soft agar. Activation of H-ras expression in the metastatic tumors had occurred through amplification and rearrangement of H-ras sequences. In addition, preinduction of p21 synthesis in NIH 3T3 line 433, which contains v-H-ras under transcriptional control of the glucocorticoid-sensitive mouse mammary tumor virus long terminal repeat, significantly increased metastatic efficiency. Glucocorticoid treatment of normal or pEJ-transformed NIH 3T3 cells did not affect metastatic potential. These data reveal a direct relationship between ras expression and metastasis formation and suggest that metastatic and transformed phenotypes may be coregulated in ras-transformed 10T1/2 and NIH 3T3 cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourne H. R. GTP-binding proteins. One molecular machine can transduce diverse signals. 1986 Jun 26-Jul 2Nature. 321(6073):814–816. doi: 10.1038/321814a0. [DOI] [PubMed] [Google Scholar]
- Bradley M. O., Kraynak A. R., Storer R. D., Gibbs J. B. Experimental metastasis in nude mice of NIH 3T3 cells containing various ras genes. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5277–5281. doi: 10.1073/pnas.83.14.5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. C., Trosko J. E., Kung H. J., Bombick D., Matsumura F. Potential role of the src gene product in inhibition of gap-junctional communication in NIH/3T3 cells. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5360–5364. doi: 10.1073/pnas.82.16.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cifone M. A., Fidler I. J. Correlation of patterns of anchorage-independent growth with in vivo behavior of cells from a murine fibrosarcoma. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1039–1043. doi: 10.1073/pnas.77.2.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. W., DeFeo D., Furth M. E., Scolnick E. M. Mouse cells contain two distinct ras gene mRNA species that can be translated into a p21 onc protein. Mol Cell Biol. 1982 Nov;2(11):1339–1345. doi: 10.1128/mcb.2.11.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Feramisco J. R., Clark R., Wong G., Arnheim N., Milley R., McCormick F. Transient reversion of ras oncogene-induced cell transformation by antibodies specific for amino acid 12 of ras protein. Nature. 1985 Apr 18;314(6012):639–642. doi: 10.1038/314639a0. [DOI] [PubMed] [Google Scholar]
- Fidler I. J., Kripke M. L. Metastasis results from preexisting variant cells within a malignant tumor. Science. 1977 Aug 26;197(4306):893–895. doi: 10.1126/science.887927. [DOI] [PubMed] [Google Scholar]
- Fleischman L. F., Chahwala S. B., Cantley L. ras-transformed cells: altered levels of phosphatidylinositol-4,5-bisphosphate and catabolites. Science. 1986 Jan 24;231(4736):407–410. doi: 10.1126/science.3001936. [DOI] [PubMed] [Google Scholar]
- Greig R. G., Koestler T. P., Trainer D. L., Corwin S. P., Miles L., Kline T., Sweet R., Yokoyama S., Poste G. Tumorigenic and metastatic properties of "normal" and ras-transfected NIH/3T3 cells. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3698–3701. doi: 10.1073/pnas.82.11.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. L., Ostrowski M. C., Berard D., Hager G. L. Glucocorticoid regulation of the Ha-MuSV p21 gene conferred by sequences from mouse mammary tumor virus. Cell. 1981 Dec;27(2 Pt 1):245–255. doi: 10.1016/0092-8674(81)90408-6. [DOI] [PubMed] [Google Scholar]
- Kripke M. L., Gruys E., Fidler I. J. Metastatic heterogeneity of cells from an ultraviolet light-induced murine fibrosarcoma of recent origin. Cancer Res. 1978 Sep;38(9):2962–2967. [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Cellular oncogenes and multistep carcinogenesis. Science. 1983 Nov 18;222(4625):771–778. doi: 10.1126/science.6356358. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Manoharan T. H., Burgess J. A., Ho D., Newell C. L., Fahl W. E. Integration of a mutant c-Ha-ras oncogene into C3H/10T1/2 cells and its relationship to tumorigenic transformation. Carcinogenesis. 1985 Sep;6(9):1295–1301. doi: 10.1093/carcin/6.9.1295. [DOI] [PubMed] [Google Scholar]
- Martinet Y., Bitterman P. B., Mornex J. F., Grotendorst G. R., Martin G. R., Crystal R. G. Activated human monocytes express the c-sis proto-oncogene and release a mediator showing PDGF-like activity. Nature. 1986 Jan 9;319(6049):158–160. doi: 10.1038/319158a0. [DOI] [PubMed] [Google Scholar]
- Mulcahy L. S., Smith M. R., Stacey D. W. Requirement for ras proto-oncogene function during serum-stimulated growth of NIH 3T3 cells. Nature. 1985 Jan 17;313(5999):241–243. doi: 10.1038/313241a0. [DOI] [PubMed] [Google Scholar]
- Muschel R. J., Williams J. E., Lowy D. R., Liotta L. A. Harvey ras induction of metastatic potential depends upon oncogene activation and the type of recipient cell. Am J Pathol. 1985 Oct;121(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L. Cancer metastasis. Organ colonization and the cell-surface properties of malignant cells. Biochim Biophys Acta. 1982 Dec 21;695(2):113–176. doi: 10.1016/0304-419x(82)90020-8. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Poste G. Tumor implantation and invasion at metastatic sites. Int Rev Exp Pathol. 1983;25:77–181. [PubMed] [Google Scholar]
- Noda M., Ko M., Ogura A., Liu D. G., Amano T., Takano T., Ikawa Y. Sarcoma viruses carrying ras oncogenes induce differentiation-associated properties in a neuronal cell line. Nature. 1985 Nov 7;318(6041):73–75. doi: 10.1038/318073a0. [DOI] [PubMed] [Google Scholar]
- Poste G., Fidler I. J. The pathogenesis of cancer metastasis. Nature. 1980 Jan 10;283(5743):139–146. doi: 10.1038/283139a0. [DOI] [PubMed] [Google Scholar]
- Pozzatti R., Muschel R., Williams J., Padmanabhan R., Howard B., Liotta L., Khoury G. Primary rat embryo cells transformed by one or two oncogenes show different metastatic potentials. Science. 1986 Apr 11;232(4747):223–227. doi: 10.1126/science.3456644. [DOI] [PubMed] [Google Scholar]
- Pulciani S., Santos E., Long L. K., Sorrentino V., Barbacid M. ras gene Amplification and malignant transformation. Mol Cell Biol. 1985 Oct;5(10):2836–2841. doi: 10.1128/mcb.5.10.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaphorst G. P., Vadasz J. A., Azzam E. I., Sargent M. D., Borsa J., Einspenner M. Comparison of heat and/or radiation sensitivity and membrane composition of seven X-ray-transformed C3H 10T1/2 cell lines and normal C3H 10T1/2 cells. Cancer Res. 1985 Nov;45(11 Pt 1):5452–5456. [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rohrschneider L., Reynolds S. Regulation of cellular morphology by the Rous sarcoma virus src gene: analysis of fusiform mutants. Mol Cell Biol. 1985 Nov;5(11):3097–3107. doi: 10.1128/mcb.5.11.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos E. Cellular adhesion, invasion and metastasis. Biochim Biophys Acta. 1984;738(4):263–284. doi: 10.1016/0304-419x(83)90008-2. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stackpole C. W., Fornabaio D. M., Alterman A. L. Phenotypic interconversion of B16 melanoma clonal cell populations: relationship between metastasis and tumor growth rate. Int J Cancer. 1985 May 15;35(5):667–674. doi: 10.1002/ijc.2910350516. [DOI] [PubMed] [Google Scholar]
- Thompson C. B., Challoner P. B., Neiman P. E., Groudine M. Expression of the c-myb proto-oncogene during cellular proliferation. 1986 Jan 30-Feb 5Nature. 319(6052):374–380. doi: 10.1038/319374a0. [DOI] [PubMed] [Google Scholar]
- Thorgeirsson U. P., Turpeenniemi-Hujanen T., Williams J. E., Westin E. H., Heilman C. A., Talmadge J. E., Liotta L. A. NIH/3T3 cells transfected with human tumor DNA containing activated ras oncogenes express the metastatic phenotype in nude mice. Mol Cell Biol. 1985 Jan;5(1):259–262. doi: 10.1128/mcb.5.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vousden K. H., Eccles S. A., Purvies H., Marshall C. J. Enhanced spontaneous metastasis of mouse carcinoma cells transfected with an activated c-Ha-ras-1 gene. Int J Cancer. 1986 Mar 15;37(3):425–433. doi: 10.1002/ijc.2910370315. [DOI] [PubMed] [Google Scholar]
- Weissman B., Aaronson S. A. Members of the src and ras oncogene families supplant the epidermal growth factor requirement of BALB/MK-2 keratinocytes and induce distinct alterations in their terminal differentiation program. Mol Cell Biol. 1985 Dec;5(12):3386–3396. doi: 10.1128/mcb.5.12.3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Winter E., Perucho M. Oncogene amplification during tumorigenesis of established rat fibroblasts reversibly transformed by activated human ras oncogenes. Mol Cell Biol. 1986 Jul;6(7):2562–2570. doi: 10.1128/mcb.6.7.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]