Abstract
Receptive fields (RFs) of neurons in primary visual cortex have traditionally been subdivided into two major classes: “simple” and “complex” cells. Simple cells were originally defined by the existence of segregated subregions within their RF that respond to either the on- or offset of a light bar and by spatial summation within each of these regions, whereas complex cells had ON and OFF regions that were coextensive in space [Hubel DH, et al. (1962) J Physiol 160:106–154]. Although other definitions based on the linearity of response modulation have been proposed later [Movshon JA, et al. (1978) J Physiol 283:53–77; Skottun BC, et al. (1991) Vision Res 31(7-8):1079–1086], the segregation of ON and OFF subregions has remained an important criterion for the distinction between simple and complex cells. Here we report that response profiles of neurons in primary auditory cortex of monkeys show a similar distinction: one group of cells has segregated ON and OFF subregions in frequency space; and another group shows ON and OFF responses within largely overlapping response profiles. This observation is intriguing for two reasons: (i) spectrotemporal dissociation in the auditory domain provides a basic neural mechanism for the segregation of sounds, a fundamental prerequisite for auditory figure-ground discrimination; and (ii) the existence of similar types of RF organization in visual and auditory cortex would support the existence of a common canonical processing algorithm within cortical columns.
Keywords: cortical microarchitecture, canonical circuit, single-unit recording, boundary detection, sound segmentation
In primary visual cortex, Hubel and Wiesel found two major categories of cells with distinct receptive field (RF) types (1): Simple cells have discrete subareas of a particular orientation that respond either to the onset or the offset of a small spot of light; complex cells, by contrast, respond with mixed ON and OFF responses throughout their RF. In addition, simple cells show spatial summation within each of their subregions. In both auditory and somatosensory cortex, inhibitory surrounds or sidebands next to excitatory RFs have been described, which have been compared with the OFF sidebands of ON-center simple cells (2–5). This analogy, however, misses the point that visual simple cells are really characterized by the existence of adjacent excitatory bands that respond with a firing-rate increase to a light stimulus being turned on or off, rather than by the existence or absence of inhibitory sidebands. Complex cells, by contrast, have OFF responses but no inhibitory sidebands. It would be of great interest to determine whether a similar distinction between segregated and nonsegregated ON and OFF responses exists in other sensory areas, such as auditory cortex. If so, this could provide more generalized insight into the microarchitecture and processing algorithms of cortical columns, a debate that is still ongoing 50 y after Hubel and Wiesel’s initial discovery (6–9).
To look for the existence of similar RF organization in primary auditory cortex (A1), we tested excitatory responses, i.e., increases in firing rate, after switching a pure tone (PT) or band-passed noise (BPN) burst on or off while varying its (center) frequency in 1/3-octave steps. This resulted in two “response profiles,” or “frequency-rate curves,” showing firing rate as a function of stimulus frequency: one profile for the ON response and one for the OFF response. Two-dimensional (2D) space and sound frequency may be considered equivalent in vision and audition, respectively, based on the nature of the peripheral receptor surface in retina and basilar membrane (10, 11). Therefore, we hypothesized that auditory cortical response profiles might exist with ON and OFF responses segregated in frequency. Such an organization would result in distinct frequency tuning for ON and OFF responses in the same neuron. If such neurons exist, they could play an important role in the detection and enhancement of auditory event boundaries, just as simple-cell orientation detectors have been assigned a function in visual contour segregation.
Auditory physiology has mostly reported excitatory responses to the onset of an auditory stimulus, such as a tone burst. OFF responses have been described and analyzed less frequently (12–14). The relative lack of OFF responses in the published literature may in part be due to the more common use of anesthetized animals in the past, in which transient ON responses dominate and, possibly, to the predominant use of tonal stimuli (15). In the present study, all recordings were performed in awake rhesus monkeys that were trained to attend to an occasionally occurring oddball sound. We recorded single-unit activity from primary auditory cortex in two awake rhesus monkeys and measured excitatory responses after the onset and offset of PT and BPN bursts of varying bandwidth and center frequency. These tests were meant to provide initial insight into whether a distinction analogous to simple and complex cells can be made in auditory cortex, to be followed up with testing of linearity and response modulation analogous to visual studies in the future.
Results
A total of 149 neurons were collected from the left hemisphere in the auditory cortex of two awake behaving monkeys. Of these, 144 were responsive to sound and 129 were located in A1. The remaining 15 cells were located in cortical areas neighboring A1 in the rostral direction (rostral field, R, and anterolateral field, AL, as defined on the basis of tonotopic map reversal at the border between A1 and AL/R). Further analysis was restricted to A1 cells.
Raw data from two neurons are shown in Fig. 1. In the first example (Fig. 1A), the neuron is stimulated with a BPN burst (bandwidth: 1/3 octave; center frequency varying in steps of 1/3 octave) at a sound intensity well above threshold (55 dBA, as was most commonly used). Within its overall tuning range, the cell responded with an excitatory ON response at frequencies from 3.2 to 6.3 kHz. Between 8 and 12.5 kHz, the ON response was much reduced, and a firing increase (an excitatory OFF response) appeared instead when the stimulus was turned off. At 16 kHz, the ON response reappeared with a sizeable peak, whereas the OFF response was largely gone.
Fig. 1.
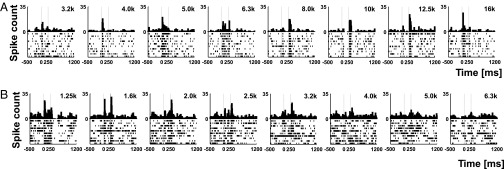
Responses of single neurons in primary auditory cortex (A1) of rhesus monkeys to band-passed noise (BPN) bursts centered at particular frequencies. (A and B) Peristimulus time histograms (PSTHs) and raster displays for two different A1 neurons. Center frequency of the BPN burst increases in each row from left to right in steps of 1/3 octave, as displayed above each PSTH. The bandwidth of the BPN burst was also 1/3 octave, and its duration was 250 ms. Two vertical dashed lines indicate onset and offset times of the BPN burst at t = 0 and t = 250 ms, following a pretrial interval starting at −500 ms. ON and OFF responses were quantified by measuring peak firing rate (minus baseline rate during the pretrial interval) within a 40-ms sliding window after stimulus onset or offset, respectively. Statistical significance of the responses was determined with the Wilcoxon Signed Rank test. Spike times were recorded at a resolution of 1 ms but displayed at a binwidth of 10 ms. Two different response types were noted, and typical examples for each type are displayed here. (A) Type-S (simple-like) cell responds either to the onset or the offset of the BPN burst depending on center frequency. Only occasionally both significant ON and OFF responses are seen. (B) Type-C (complex-like) cell regularly responds to both onset and offset of BPN burst (or not at all) regardless of center frequency.
A different type of neuron is shown in Fig. 1B: In this case, the cell responded to sound bursts with both ON and OFF responses throughout most of its tuning range. We will refer to the first type of cell (with discrete ON/OFF-response areas) as “type S” or “simple-like” and to the second type of cell (with mixed ON/OFF-response areas) as “type C” or “complex-like.”
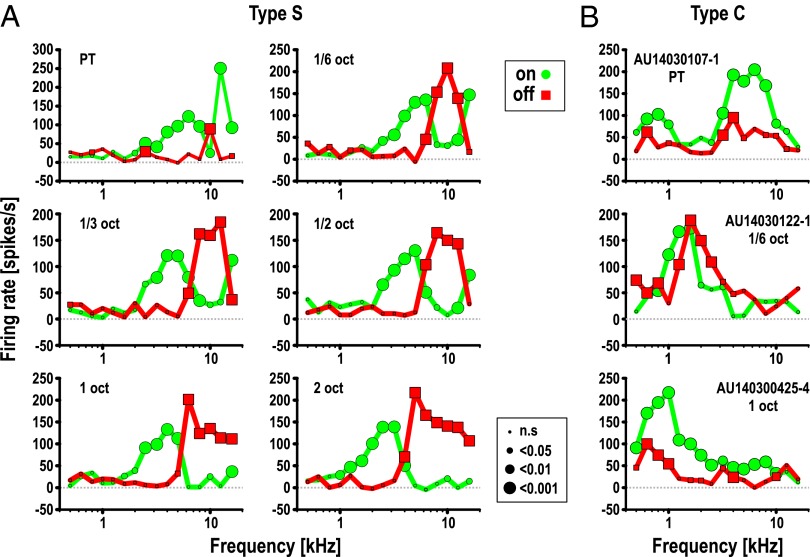
The response profiles (frequency-rate curves) of a typical type-S neuron are displayed for different bandwidths in Fig. 2A, whereas Fig. 2B shows three examples of type-C cells. For type-C cells, the OFF-response region was usually contained within the ON region, whereas the response profiles of type-S cells consisted of largely segregated subregions that responded either to switching the stimulus on or off. It should be noted that, when stimulated with PTs, the OFF response of type-S cells often became almost negligible. However, when stimulated with BPN bursts, an OFF response in the high-frequency response area was clearly apparent, suggesting summation within these bandwidths.
Fig. 2.
Excitatory response profiles of A1 neurons determined from peak firing rates in response to the onset and offset of a BPN burst with varying frequency. Peak firing rates were extracted from PSTHs as in Fig. 1. Green circles connected by green lines show ON responses. Red squares and lines show OFF responses. Size of the symbols indicates significance level of the response in Wilcoxon Signed Rank test. (A) Response profiles of a typical type-S (simple-like) cell. In addition to center frequency, bandwidth of the BPN stimuli was also varied, as indicated within each panel. ON- and OFF-response profiles appear largely separated into discrete frequency ranges. The effect was consistent for different bandwidths, although OFF responses were often markedly diminished for PT stimuli (Upper Left). (B) Three examples of type-C (complex-like) cells. In all cases, the response profiles of ON and OFF responses are similar or show significant overlap, although OFF responses are considerably weaker in two of the examples (Upper and Lower).
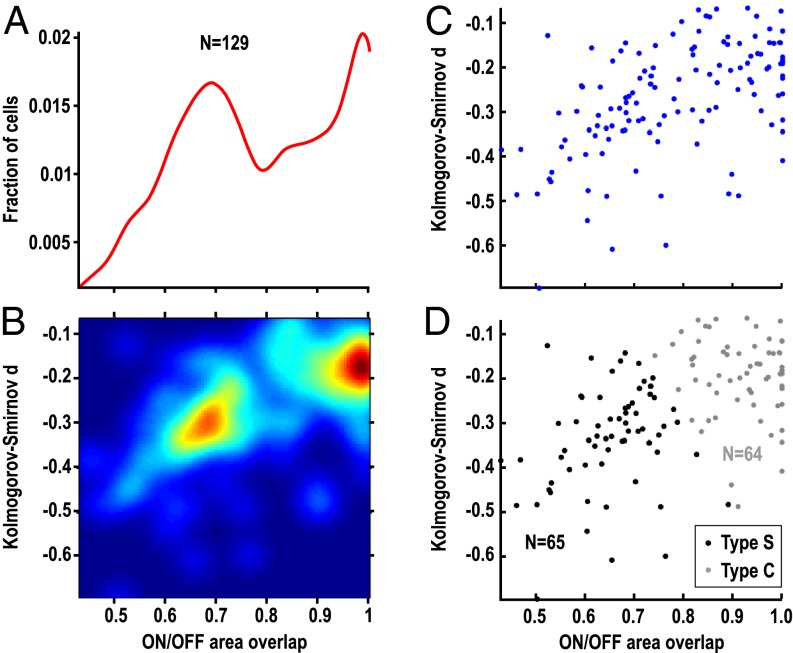
The amount of overlap between ON- and OFF-response profiles was determined quantitatively in all neurons from the area under the frequency-rate curve (Fig. 3A). The distribution of ON/OFF-area overlap was bimodal with a peak near 0.7, a trough at 0.79, and a second peak near 1. To classify the cells into different types, we performed a bivariate analysis by combining ON/OFF-area overlap with other measures of similarity between ON and OFF response profiles (Methods). One combination that proved particularly effective was that of ON/OFF-area overlap with Kolmogorov–Smirnov (K–S) distance (multiplied by −1; Methods). Alternative approaches using other pairs of response profile similarity measures, or using principal components derived from multiple measures, are shown for comparison in SI Results and Figs. S1–S3. There was good agreement between classifications based on all these approaches (79.8–88.4%, Table S1).
Fig. 3.
Classification of A1 neurons into type-S and type-C classes. (A) ON/OFF-area overlap plotted as 100-bin histogram smoothed with a Gaussian kernel (σ = 5 bins). The histogram demonstrates bimodal distribution of ON/OFF-area overlap. (B) ON/OFF-area overlap plotted against K–S distance (the latter multiplied by −1 for presentation) as a 100 × 100 bivariate histogram smoothed with a 2D kernel (σ = 5 bins). Distinct clusters are apparent in the density plot. (C) Scatterplot of ON/OFF-area overlap against K–S distance. Each dot represents one neuron. (D) Class assignments resulting from k-means clustering are shown in black (type S) and gray (type C) in the plot of ON/OFF-area overlap against K–S distance (compare with C).
The 2D distribution of ON/OFF-area overlap and K–S distance revealed two distinct clusters (Fig. 3B): One cluster is centered at ON/OFF-area overlap values close to 1 and at a K–S distance around −0.15 and presumably corresponds to type-C cells. The second cluster at smaller ON/OFF-area overlap values (peak 0.6–0.7) and a larger K–S distance (around −0.3) is assumed to correspond to type-S cells. This 2D bimodal distribution was formally divided into two classes with unsupervised k-means clustering (Methods); the resulting classes accurately matched what was inferred from visual inspection of the distribution (compare Fig. 3 D with C). The number of clusters was confirmed using an “evaluation function” proposed by Pham et al. (ref. 16, SI Results, and Fig. S4). With this classification method, about one half (n = 65) of 129 neurons located in A1 were classified as type-S cells and the other half (n = 64) as type-C cells.
A small number of neurons (n = 11) were tested at more than one sound level. Ten neurons were tested at two, and one neuron at three levels. Although response amplitude varied with sound level (sometimes in a nonmonotonic fashion), as one would expect, ON and OFF responses changed in similar proportions, and no systematic differences in the relationship of ON- and OFF-tuning curves were apparent (see Fig. S5 for two examples).
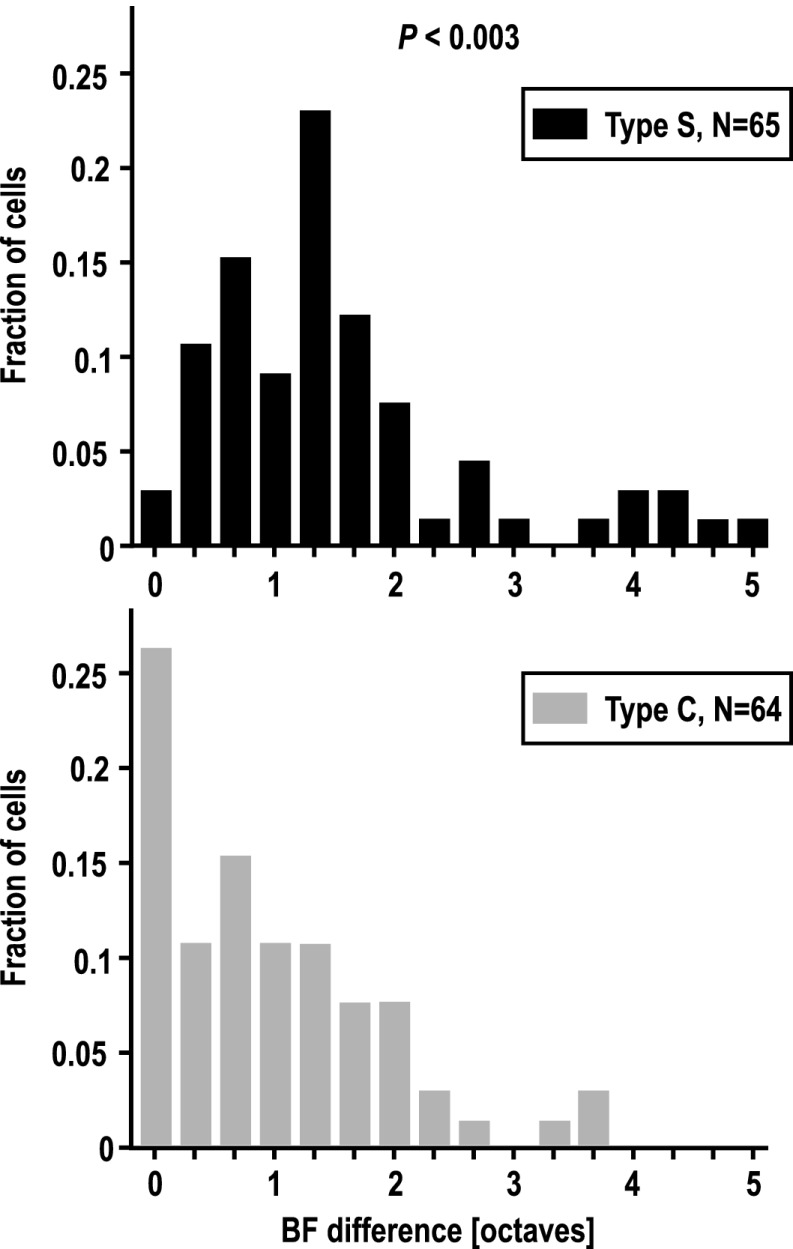
Cells assigned to the two clusters were analyzed further to test the hypothesis that they constituted two classes analogous to simple and complex cells in visual cortex. First, we determined best frequency (BF) quantitatively from response profiles, separately for ON and OFF responses, and the absolute difference between BF–ON and BF–OFF was then calculated. Our hypothesis would predict that type-S cells with little ON/OFF overlap should display a relatively large BF difference, whereas cells with high ON/OFF overlap values should show similar BFs for ON and OFF, i.e., a near-zero BF difference. As Fig. 4 demonstrates, most type-S (simple-like) cells indeed showed relatively large differences between BF–ON and BF–OFF, with a peak at 11/3 octaves (Fig. 4, Upper). Type-C (complex-like) cells, by contrast, showed a distribution that peaked at zero and tapered down rather quickly (Fig. 4, Lower). The difference between the absolute BF difference of type-S and type-C neurons was significant (P < 0.003, Mann–Whitney test).
Fig. 4.
Comparison of best frequencies (BFs) of OFF vs. ON responses. BF was determined separately for ON and OFF responses from response profiles of the type shown in Fig. 2. Distributions of absolute difference between BF–OFF and BF–ON are plotted in 1/3-octave bins. The distribution of type-S neurons (Upper) was dominated by moderate values of difference between BF–ON and BF–OFF, with a peak at 11/3 octaves. Notably, virtually no cells (n = 2, 3%) had a difference close to zero. The distribution for type-C neurons (Lower) had its peak at zero, indicating that BF–OFF and BF–ON typically do not differ by much. In 64% of type-C neurons, BF–OFF and BF–ON were within ±1 octave of each other, compared with 38% of type-S neurons.
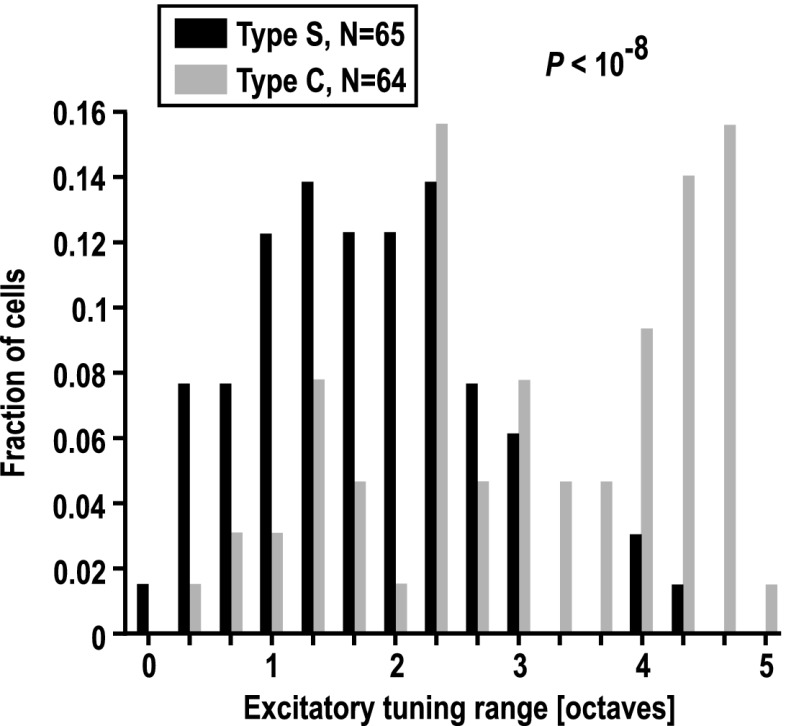
Although the response profiles in type-C cells may appear, at first sight, more plain than those of type-S cells, two additional measures point to the fact that type-C cells (like complex cells in the visual cortex) are actually at a hierarchically higher processing level. One of these measures is excitatory tuning range (ETR) of the cells. Fig. 5 demonstrates that type-C cells were more broadly tuned than type-S (simple-like) cells. The difference was highly significant (P < 10−8, Mann–Whitney test). This difference is mainly due to the existence of response profiles with very broad ETR (>3 octaves), which hardly exist among type-S neurons. Conversely, neurons with a tuning range of less than 1 octave rarely exist among type-C neurons.
Fig. 5.
Excitatory tuning range of type-S and type-C cells in primary auditory cortex. The distributions of frequency tuning range of excitatory ON responses in type-S (Upper) and type-C neurons (Lower) are shown in steps of 1/3 octave. Type-C (complex-like) neurons have a significantly broader excitatory tuning range than type-S (simple-like) neurons. This difference is mainly due to the existence of response profiles with a very large range (more than 3 octaves, n = 32, 50%), which hardly exist among type-S neurons (n = 3, 5%). Conversely, neurons with a range of 1 octave or less rarely exist among type-C neurons (n = 5, 8%) and are more common in type-S cells (n = 19, 29%).
A second measure to determine the relative processing stage is response latency. Here again, type-C cells on average had longer latencies than type-S cells (mean values: 67.9 vs. 47.2 ms, P < 0.03, t test; median values: 38 vs. 33 ms, P < 0.15, Mann–Whitney test). Alternative approaches to cell classification resulted in even clearer latency differences (SI Results).
A strong argument for the hierarchy of simple and complex cells in the visual cortex is their laminar distribution, with simple cells found mostly in layers 4 and 6, and complex cells found mostly in layers 2, 3, and 5 (17, 18). However, the present recordings were performed in awake monkeys using single-contact electrodes and a vertical approach with long electrode travel distances, rendering determination of laminar distribution virtually impossible. This will remain an important facet of future study, possibly involving multicontact linear probes or high-resolution structural MRI.
Discussion
Our results demonstrate first of all that OFF responses in neurons of the auditory cortex have generally been underreported (with some notable exceptions, see refs. 12, 14, and 19–21). Although ON responses in our sample were usually stronger than OFF responses (P < 0.001, df = 121, paired t test), over 90% of A1 neurons did show an excitatory OFF response at moderate sound intensities for at least one frequency and bandwidth. The more frequent occurrence of OFF responses in our sample may be due to the use of BPN stimuli in comparison with tones of a single frequency. As our results demonstrate, summation occurs within the OFF subregion, which is more likely to occur with BPN bursts (11). Even bandwidths as narrow as 1/6 of an octave led to significant summation effects compared with PTs (Fig. 2A). Equivalently, spatial summation is seen in simple-cell RFs of visual cortex, where stimuli with optimal size lead to larger responses than small spots or narrow slits of light (1, 22). In fact, linear spatial summation within the RF has become part of the definition of simple cells. Use of BPN stimuli in our study also enhances OFF responses in type-C cells, because preferred stimuli, as everywhere, evoke higher firing rates in neurons of the auditory cortex (15, 23). Similarly, edges (roughly equivalent to BPN stimuli with a bandwidth of 2 octaves or more) evoke strong responses in visual cortical cells (1).
The fact that auditory cortical neurons can have distinct, complementary response profiles depending on the response type (ON or OFF) was at first quite unexpected. Inhibitory sidebands, as described previously, would not necessarily predict the tuning of excitatory OFF responses. Although different best frequencies for ON and OFF have been found (24), they have been interpreted as inconsistencies or gradual phase shifts. The existence of cells with discrete, nonoverlapping ON and OFF subregions has not been reported to our knowledge. The finding may have been missed in other studies because OFF responses were simply not measured or distinguished, or their frequency-tuning range was not directly compared with that of ON responses, the assumption being that frequency tuning of excitatory ON and OFF responses would be the same. The latter is largely what we found in type-C cells. They had overlapping ON/OFF regions, and both frequency-tuning range and BF were often similar for ON and OFF responses. Thus, these cells are comparable to complex cells in the visual cortex, in which mixed ON/OFF responses are also found throughout the RF (1). However, in another type of auditory cortex neuron, which we termed type-S cells, response profiles of excitatory ON and OFF responses were clearly segregated with little overlap. This RF organization is highly reminiscent of simple cells in the visual cortex, which also have segregated ON/OFF subregions (1).
The distinction between simple and complex cells in primary visual cortex has also been confirmed on the basis of the linearity of extracellular response modulation by drifting sine-wave gratings (22, 25, 26), although other reports have found a more continuous distribution when activity is recorded intracellularly (7, 27). Simple cells, which are thought to add their inputs linearly, produce a response modulation ratio (between the amplitude of the first harmonic and the mean of the response, F1/F0) >1; complex cells, with nonlinear integration, have F1/F0 ratios <1. In auditory cortex, dynamic spectral ripple stimuli, which are considered the equivalent of drifting sine-wave gratings (28), have been used to search for linear and nonlinear units (29). Both types of cells were found, but their distribution was unimodal along a continuum with average F1/F0 ratios near 1 (30). Thus, simple and complex cells in visual cortex as well as their possible equivalents in audition are not reliably identified as distinct groups on the basis of response linearity alone, as most cells seem to incorporate both linear and nonlinear mechanisms. Combining both approaches in the same study may help to illuminate some of the differences. In particular, presenting alternating ON- and OFF-BPN bursts in the spectral region of the ON response in simple-like cells might produce discharge modulated at the frequency of alternation up to the highest temporal frequencies at which they respond. By contrast, the same stimuli should produce responses in complex-like cells at two times the frequency of alternation until the frequency becomes too high, at which point they would switch to a sustained elevation of discharge. Such a result would constitute a very strong parallel to simple and complex cells in the visual cortex.
Whether type-S cells (simple-like cells) in auditory cortex are made up of convergent excitatory input from thalamic units or involve intracortical mechanisms also deserves to be studied in more detail. According to the “input alignment model” of Hubel and Wiesel (1), ON and OFF responses of visual cortical units originate from segregated ON and OFF channels in thalamic cells, which provide excitatory input to cortical simple cells. Thus, the ON subregions of simple cells are made up from excitatory convergence of ON-center cells in the lateral geniculate nucleus (LGN), whereas OFF subregions are made up from excitatory convergence of OFF-center cells. This model has received strong support from subsequent studies, in which segregated ON and OFF channels were found in the LGN and in the afferents to visual cortex (31, 32) and activity of LGN cells and simple cells was recorded simultaneously (33). Other accounts have emphasized the importance of intracortical mechanisms for setting up different RF types in the cortex (8, 34), or an interaction of subcortical and intracortical input (35, 36). In the auditory system, segregated ON and OFF channels also exist at the thalamic level, in the medial geniculate nucleus (MGN) (37). On the other hand, the existence of multipeaked frequency tuning curves in auditory cortex has been taken as evidence for intracortical inhibitory mechanisms capable of shaping auditory cortical response profiles (38, 39). In such a scheme, OFF responses could conceivably be generated as “rebound from inhibition” rather than by feedforward mechanisms. To decide between these two alternatives, the precise alignment of OFF responses relative to inhibitory regions of the receptive field (and vice versa) will have to be established.
At the next hierarchical level within visual cortex, complex cells are thought to originate from a convergence of simple cells (1). This convergence could lead to greater feature specificity and has been referred to in the auditory cortex as combination-sensitivity (11, 40). By virtue of this convergence in complex cells the segregated subregions are lost and a “generalization” of the stimulus over a larger part of the visual field takes place (25, 41). Type-C cells in auditory cortex may similarly participate in forming invariances across stimuli in one category and participate in the coding of complex sounds, such as environmental sounds, animal communication sounds or (in humans) words in speech (42). If confirmed, both findings would provide another link toward the argument that cortical columns are made up of the same circuitry across different regions and carry out similar computational operations, but apply them to different input signals (8, 35, 43). Other comparisons have drawn similar analogies between visual direction and auditory FM selectivity (44) and between visual size and auditory bandwidth tuning (45).
Simple cells in vision are thought to detect the contours outlining the shape of a visual object, whereas complex cells may participate in the integration of visual information across space (46). By analogy, type-S cells in auditory cortex could be involved in sharpening the contrast between two successive sounds with different spectra, thus detecting the boundaries between events occurring in the environment or facilitating the segmentation of auditory scenes (47). Such mechanisms may have also enabled the ultimate emergence of feature detectors apt for the segmentation of speech in humans. It is reasonable to assume that such a process of boundary detection and event segmentation would occur relatively early in the cortical hierarchy. Indeed, evidence for perceptual stream segregation at the neural level has previously been reported for rhesus monkey primary auditory cortex (48, 49). In a subsequent step, temporal integration of acoustic information is necessary to accomplish the encoding of sound sequences, e.g., in the form of musical melodies (50, 51) or auditory “objects” (52–54). Type-C cells in the auditory cortex may form the beginning of this integration.
Methods
Most of the electrophysiological recording techniques used here have been described in detail (14, 49).
Acoustic Stimulation.
Acoustic stimuli were presented in a double-walled acoustic chamber. They comprised PTs and BPN bursts (duration: 250 ms) produced digitally with the SIGNAL software (Engineering Design). The stimulus bandwidths were 0 octaves (PT) and 1/6, 1/3, 1/2, 1, and 2 octaves (BPN), and (center) frequencies covered 0.5–16 kHz in 1/3-octave steps. Stimulus amplitude was calibrated and computer-controlled from SIGNAL in 5-dB steps until the response was maximal and well above threshold. Sound pressure level of the stimuli was 45–75 dBA (as measured at the monkey's head measured with a Bruel & Kjaer 2235 Sound Level Meter), which was well within the linear range of the sound delivery system.
Chronic Techniques.
Behavioral training.
Recordings were performed in two awake rhesus monkeys (Macaca mulatta) weighing between 7 and 8 kg. The animals were involved in a simple auditory discrimination task: namely, to release a bar only to a specified stimulus (S+) to receive a water reward, and to hold the bar to all other (S−) sounds, where no water reward was given. The S+ sound was a short melody consisting of four PT notes (CEGC with C at 512 Hz on a tempered scale), each of 100-ms duration; PTs and BPNs were treated as S−. After a training period of several weeks, the monkeys were able to perform the task with a correct-response rate at >90%, indicating that they were attending to the stimuli. Monkeys under training and recording were given controlled access to water following National Institutes of Health guidelines. All studies were approved by the Georgetown University Animal Care and Use Committee.
Implant surgery.
When initial training was near completion, the animal's head was scanned by MRI at 1.5 T to localize target brain structures. A recording chamber and a post for holding the head were surgically fastened to the skull under isoflurane anesthesia based on the MRI scan, followed by performing a small craniotomy in the chamber, though the dura was left intact. After complete recovery from surgery, the animals' training was resumed with the head fixed. This final stage of training again lasted several weeks before recordings commenced.
Electrophysiological Recording and Analysis.
Lacquer-insulated tungsten microelectrodes (<10 MOhm) were inserted into cortex with a hydraulic microdrive. Because the dura remained intact, a guide tube was inserted into predetermined positions of a grid within the recording chamber (Crist Instruments). Neuronal spike activity was recorded from single neurons on the supratemporal plane in and around primary auditory cortex (A1), as determined by functional criteria (tonotopic gradient, frequency tuning, response latency, etc.). Neurons were sampled at an average distance of 250 µm along the track. In all animals, the left hemisphere was studied. Recording sessions typically lasted 2–6 h per day, 5–7 d per week.
Action potentials were filtered, detected with a window discriminator, and time stamps were collected at 1-ms resolution by a personal computer using the CORTEX program. Raster displays and peristimulus time histograms (PSTHs) were generated and evaluated in response to various forms of acoustic stimulation. Response profiles (rate-tuning curves) were determined for PT and each bandwidth of BPN at a 1/3-octave resolution. Stimulus presentation was repeated 10–50 times in a randomized fashion. A pretrial interval of 500 ms was used to record spontaneous activity (baseline). For off-line analysis, Matlab (Mathworks) routines were used. The maximum number of spikes (minus baseline) within a 40-ms sliding window (shifted in 1-ms steps) was determined from the PSTH and divided by the same time interval (40 ms) to receive a peak firing rate (FR). This procedure was applied separately for both ON and OFF periods, i.e., within 250 ms after the onset and offset of the stimulus, respectively, so peak FRs were obtained for ON and OFF responses. Latency of ON and OFF responses was determined from the time position relative to stimulus onset or offset of the sliding window yielding the peak FR. Some responses (n = 11) were measured at two or more intensity levels to test for level independence.
Best frequency (BF; for BPN: best center frequency) was determined by fitting a quadratic function to the response profile at the frequency that elicited the peak FR and the two neighboring frequencies, one on either side (14). The location of the maximum of this quadratic function was then defined as the BF of the neuron.
The significance level of the neural response was determined by the Wilcoxon Signed Rank test for paired data. An ON response was defined as a significant increase (compared with baseline) in FR within the 40-ms sliding window as applied during the stimulus-ON period; an OFF response was defined as a significant increase within the same duration after stimulus offset. Excitatory tuning range (ETR) was assessed as the number of stimulus frequencies that elicited a significant ON response.
Classification of Neurons.
Neurons were classified into type-S and type-C classes based on measures of ON- and OFF-response profile similarity. The principal measure was ON/OFF-area overlap. Before calculating this measure, any negative values of ON and OFF response profiles resulting from baseline subtraction were replaced with zeros. Areas under the ON and OFF profiles were calculated as the sum of FR values for each profile, and the overlap area was calculated by summing the smaller of the ON and OFF responses (FRs) over all tested frequencies. ON/OFF-area overlap values were computed separately for ON and OFF responses by dividing the overlap area by the area under the respective profile. The larger of the two area overlap values was taken as the final overlap measure. This step allowed handling cases like the one shown in Fig. 2B, Upper: here, the overlap value was 0.43 for the ON profile and 1 for the OFF profile. The latter value was chosen to indicate complete coverage of one profile by the other.
Another measure of ON/OFF-profile similarity was K–S distance. It was calculated by replacing negative values of ON- and OFF-response profiles with zeros, followed by computing cumulative values of the profiles and finding the largest difference between them. The procedure is identical to calculating the d statistics in the two-sample K–S test. Unlike ON/OFF-area overlap (and most other measures that were tested for comparison purposes; SI Methods) whose values increase with increasing similarity of ON- and OFF-response profiles, K–S distance measures dissimilarity, so it decreases with increasing profile similarity. Thus, for consistency of presentation, K–S distance values were multiplied by −1 before plotting or use in subsequent analyses.
All measures of ON/OFF-profile similarity were calculated separately for each of six stimulus bandwidths. Of these, the measure obtained with the bandwidth that elicited the highest FR was selected for further analysis (see also SI Methods for an alternative approach).
ON/OFF-area overlap values were binned into 100 bins, smoothed with a Gaussian kernel (σ = 5 bins) and plotted (Fig. 3A). A bimodal distribution with a clear trough was apparent. Eventual classification was based on a bivariate distribution of ON/OFF-area overlap and K–S distance (Fig. 3C). The same classification approach was also applied to other pairs of measures, or to the first two principal components derived from the full set of seven similarity measures (SI Methods). The bivariate distribution was displayed as a density plot by constructing a 100 bin × 100 bin histogram smoothed with a 2D Gaussian kernel (σ = 5 bins, Fig. 3B), which allowed to identify clustering of cells visually. Cells were then divided into two classes using k-means clustering (Fig. 3D). The number of clusters was selected based on visual inspection of the 2D density plot, and confirmed with a formal measure (“evaluation function”) (ref. 16, SI Methods).
Absolute difference between ON response BF and OFF response BF, excitatory tuning range (ETR), and latency were compared between the resulting classes using the Mann–Whitney test.
Supplementary Material
Acknowledgments
This work was funded by National Institutes of Health Grants R01-NS052494 and R01DC003489 (to J.P.R.) and National Science Foundation Grant PIRE OISE-0730255.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1221062110/-/DCSupplemental.
References
- 1.Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J Physiol. 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suga N, Tsuzuki K. Inhibition and level-tolerant frequency tuning in the auditory cortex of the mustached bat. J Neurophysiol. 1985;53(4):1109–1145. doi: 10.1152/jn.1985.53.4.1109. [DOI] [PubMed] [Google Scholar]
- 3.Miller KD, Pinto DJ, Simons DJ. Processing in layer 4 of the neocortical circuit: New insights from visual and somatosensory cortex. Curr Opin Neurobiol. 2001;11(4):488–497. doi: 10.1016/s0959-4388(00)00239-7. [DOI] [PubMed] [Google Scholar]
- 4.Linden JF, Schreiner CE. Columnar transformations in auditory cortex? A comparison to visual and somatosensory cortices. Cereb Cortex. 2003;13(1):83–89. doi: 10.1093/cercor/13.1.83. [DOI] [PubMed] [Google Scholar]
- 5.Winer JA, Miller LM, Lee CC, Schreiner CE. Auditory thalamocortical transformation: Structure and function. Trends Neurosci. 2005;28(5):255–263. doi: 10.1016/j.tins.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Abbott LF, Chance FS. Rethinking the taxonomy of visual neurons. Nat Neurosci. 2002;5(5):391–392. doi: 10.1038/nn0502-391. [DOI] [PubMed] [Google Scholar]
- 7.Mechler F, Ringach DL. On the classification of simple and complex cells. Vision Res. 2002;42(8):1017–1033. doi: 10.1016/s0042-6989(02)00025-1. [DOI] [PubMed] [Google Scholar]
- 8.Douglas RJ, Martin KA. Neuronal circuits of the neocortex. Annu Rev Neurosci. 2004;27:419–451. doi: 10.1146/annurev.neuro.27.070203.144152. [DOI] [PubMed] [Google Scholar]
- 9.Atencio CA, Sharpee TO, Schreiner CE. Hierarchical computation in the canonical auditory cortical circuit. Proc Natl Acad Sci USA. 2009;106(51):21894–21899. doi: 10.1073/pnas.0908383106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendelson JR, Cynader MS. Sensitivity of cat primary auditory cortex (AI) neurons to the direction and rate of frequency modulation. Brain Res. 1985;327(1-2):331–335. doi: 10.1016/0006-8993(85)91530-6. [DOI] [PubMed] [Google Scholar]
- 11.Rauschecker JP, Tian B, Hauser M. Processing of complex sounds in the macaque nonprimary auditory cortex. Science. 1995;268(5207):111–114. doi: 10.1126/science.7701330. [DOI] [PubMed] [Google Scholar]
- 12.Recanzone GH. Response profiles of auditory cortical neurons to tones and noise in behaving macaque monkeys. Hear Res. 2000;150(1-2):104–118. doi: 10.1016/s0378-5955(00)00194-5. [DOI] [PubMed] [Google Scholar]
- 13.He J. OFF responses in the auditory thalamus of the guinea pig. J Neurophysiol. 2002;88(5):2377–2386. doi: 10.1152/jn.00083.2002. [DOI] [PubMed] [Google Scholar]
- 14.Kusmierek P, Rauschecker JP. Functional specialization of medial auditory belt cortex in the alert rhesus monkey. J Neurophysiol. 2009;102(3):1606–1622. doi: 10.1152/jn.00167.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Lu T, Snider RK, Liang L. Sustained firing in auditory cortex evoked by preferred stimuli. Nature. 2005;435(7040):341–346. doi: 10.1038/nature03565. [DOI] [PubMed] [Google Scholar]
- 16.Pham DT, Dimov SS, Nguyen CD. Selection of K in K-means clustering. Proc IME C J Mech Eng Sci. 2005;219:103–119. [Google Scholar]
- 17.Gilbert CD. Laminar differences in receptive field properties of cells in cat primary visual cortex. J Physiol. 1977;268(2):391–421. doi: 10.1113/jphysiol.1977.sp011863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez LM, et al. Receptive field structure varies with layer in the primary visual cortex. Nat Neurosci. 2005;8(3):372–379. doi: 10.1038/nn1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He J, Hashikawa T, Ojima H, Kinouchi Y. Temporal integration and duration tuning in the dorsal zone of cat auditory cortex. J Neurosci. 1997;17(7):2615–2625. doi: 10.1523/JNEUROSCI.17-07-02615.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heil P, Langner G, Scheich H. Processing of frequency-modulated stimuli in the chick auditory cortex analogue: Evidence for topographic representations and possible mechanisms of rate and directional sensitivity. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1992;171(5):583–600. doi: 10.1007/BF00194107. [DOI] [PubMed] [Google Scholar]
- 21.Durif C, Jouffrais C, Rouiller EM. Single-unit responses in the auditory cortex of monkeys performing a conditional acousticomotor task. Exp Brain Res. 2003;153(4):614–627. doi: 10.1007/s00221-003-1613-3. [DOI] [PubMed] [Google Scholar]
- 22.Movshon JA, Thompson ID, Tolhurst DJ. Spatial summation in the receptive fields of simple cells in the cat’s striate cortex. J Physiol. 1978;283:53–77. doi: 10.1113/jphysiol.1978.sp012488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King AJ. Hearing. Asking the auditory cortex the right question. Curr Biol. 1995;5(10):1110–1113. doi: 10.1016/s0960-9822(95)00223-5. [DOI] [PubMed] [Google Scholar]
- 24.Qin L, Chimoto S, Sakai M, Wang J, Sato Y. Comparison between offset and onset responses of primary auditory cortex ON-OFF neurons in awake cats. J Neurophysiol. 2007;97(5):3421–3431. doi: 10.1152/jn.00184.2007. [DOI] [PubMed] [Google Scholar]
- 25.Movshon JA, Thompson ID, Tolhurst DJ. Receptive field organization of complex cells in the cat’s striate cortex. J Physiol. 1978;283:79–99. doi: 10.1113/jphysiol.1978.sp012489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skottun BC, et al. Classifying simple and complex cells on the basis of response modulation. Vision Res. 1991;31(7-8):1079–1086. doi: 10.1016/0042-6989(91)90033-2. [DOI] [PubMed] [Google Scholar]
- 27.Priebe NJ, Mechler F, Carandini M, Ferster D. The contribution of spike threshold to the dichotomy of cortical simple and complex cells. Nat Neurosci. 2004;7(10):1113–1122. doi: 10.1038/nn1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kowalski N, Depireux DA, Shamma SA. Analysis of dynamic spectra in ferret primary auditory cortex. I. Characteristics of single-unit responses to moving ripple spectra. J Neurophysiol. 1996;76(5):3503–3523. doi: 10.1152/jn.1996.76.5.3503. [DOI] [PubMed] [Google Scholar]
- 29.Ahmed B, Garcia-Lazaro JA, Schnupp JW. Response linearity in primary auditory cortex of the ferret. J Physiol. 2006;572(Pt 3):763–773. doi: 10.1113/jphysiol.2005.104380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atencio CA, Sharpee TO, Schreiner CE. Cooperative nonlinearities in auditory cortical neurons. Neuron. 2008;58(6):956–966. doi: 10.1016/j.neuron.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stryker MP, Zahs KR. On and off sublaminae in the lateral geniculate nucleus of the ferret. J Neurosci. 1983;3(10):1943–1951. doi: 10.1523/JNEUROSCI.03-10-01943.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McConnell SK, LeVay S. Segregation of on- and off-center afferents in mink visual cortex. Proc Natl Acad Sci USA. 1984;81(5):1590–1593. doi: 10.1073/pnas.81.5.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reid RC, Alonso JM, Usrey WM. 2002. Integration of thalamic inputs by cat primary visual cortex. The Cat Primary Visual Cortex, Cerebral Cortex, eds Payne BR, Peters A (Academic Press, New York), Vol 8, pp 319–337.
- 34.Hirsch JA, Martinez LM. Circuits that build visual cortical receptive fields. Trends Neurosci. 2006;29(1):30–39. doi: 10.1016/j.tins.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Roe AW, Pallas SL, Kwon YH, Sur M. Visual projections routed to the auditory pathway in ferrets: Receptive fields of visual neurons in primary auditory cortex. J Neurosci. 1992;12(9):3651–3664. doi: 10.1523/JNEUROSCI.12-09-03651.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferster D, Miller KD. Neural mechanisms of orientation selectivity in the visual cortex. Annu Rev Neurosci. 2000;23:441–471. doi: 10.1146/annurev.neuro.23.1.441. [DOI] [PubMed] [Google Scholar]
- 37.He J. On and off pathways segregated at the auditory thalamus of the guinea pig. J Neurosci. 2001;21(21):8672–8679. doi: 10.1523/JNEUROSCI.21-21-08672.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kadia SC, Wang X. Spectral integration in A1 of awake primates: Neurons with single- and multipeaked tuning characteristics. J Neurophysiol. 2003;89(3):1603–1622. doi: 10.1152/jn.00271.2001. [DOI] [PubMed] [Google Scholar]
- 39.Sutter ML, Schreiner CE, McLean M, O’connor KN, Loftus WC. Organization of inhibitory frequency receptive fields in cat primary auditory cortex. J Neurophysiol. 1999;82(5):2358–2371. doi: 10.1152/jn.1999.82.5.2358. [DOI] [PubMed] [Google Scholar]
- 40.Suga N, O’Neill WE, Manabe T. Cortical neurons sensitive to combinations of information-bearing elements of biosonar signals in the mustache bat. Science. 1978;200(4343):778–781. doi: 10.1126/science.644320. [DOI] [PubMed] [Google Scholar]
- 41.Gilbert CD. Microcircuitry of the visual cortex. Annu Rev Neurosci. 1983;6:217–247. doi: 10.1146/annurev.ne.06.030183.001245. [DOI] [PubMed] [Google Scholar]
- 42.DeWitt I, Rauschecker JP. Phoneme and word recognition in the auditory ventral stream. Proc Natl Acad Sci USA. 2012;109(8):E505–E514. doi: 10.1073/pnas.1113427109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Braitenberg V, Schuez A. Cortex: Statistics and Geometry of Neuronal Connectivity. 2nd Ed. Berlin: Springer; 1998. [Google Scholar]
- 44.Tian B, Rauschecker JP. Processing of frequency-modulated sounds in the lateral auditory belt cortex of the rhesus monkey. J Neurophysiol. 2004;92(5):2993–3013. doi: 10.1152/jn.00472.2003. [DOI] [PubMed] [Google Scholar]
- 45.Rauschecker JP, Tian B. Processing of band-passed noise in the lateral auditory belt cortex of the rhesus monkey. J Neurophysiol. 2004;91(6):2578–2589. doi: 10.1152/jn.00834.2003. [DOI] [PubMed] [Google Scholar]
- 46.Riesenhuber M, Poggio T. Hierarchical models of object recognition in cortex. Nat Neurosci. 1999;2(11):1019–1025. doi: 10.1038/14819. [DOI] [PubMed] [Google Scholar]
- 47.Bregman AS. Auditory Scene Analysis: The Perceptual Organization of Sound. Cambridge, MA: MIT Press; 1994. [Google Scholar]
- 48.Fishman YI, Reser DH, Arezzo JC, Steinschneider M. Neural correlates of auditory stream segregation in primary auditory cortex of the awake monkey. Hear Res. 2001;151(1-2):167–187. doi: 10.1016/s0378-5955(00)00224-0. [DOI] [PubMed] [Google Scholar]
- 49.Micheyl C, Tian B, Carlyon RP, Rauschecker JP. Perceptual organization of tone sequences in the auditory cortex of awake macaques. Neuron. 2005;48(1):139–148. doi: 10.1016/j.neuron.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 50.Leaver AM, Van Lare J, Zielinski B, Halpern AR, Rauschecker JP. Brain activation during anticipation of sound sequences. J Neurosci. 2009;29(8):2477–2485. doi: 10.1523/JNEUROSCI.4921-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sridharan D, Levitin DJ, Chafe CH, Berger J, Menon V. Neural dynamics of event segmentation in music: Converging evidence for dissociable ventral and dorsal networks. Neuron. 2007;55(3):521–532. doi: 10.1016/j.neuron.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 52.Tian B, Reser D, Durham A, Kustov A, Rauschecker JP. Functional specialization in rhesus monkey auditory cortex. Science. 2001;292(5515):290–293. doi: 10.1126/science.1058911. [DOI] [PubMed] [Google Scholar]
- 53.Zatorre RJ, Bouffard M, Belin P. Sensitivity to auditory object features in human temporal neocortex. J Neurosci. 2004;24(14):3637–3642. doi: 10.1523/JNEUROSCI.5458-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leaver AM, Rauschecker JP. Cortical representation of natural complex sounds: Effects of acoustic features and auditory object category. J Neurosci. 2010;30(22):7604–7612. doi: 10.1523/JNEUROSCI.0296-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.