Abstract
Genetic evidence from patients with mutations of the thyroid hormone receptor α gene (THRA) indicates that the dominant negative activity of mutants underlies the pathological manifestations. However, the molecular mechanisms by which TRα1 mutants exert dominant negative activity in vivo are not clear. We tested the hypothesis that the severe hypothyroidism in patients with THRA mutations is due to an inability of TRα1 mutants to properly release the nuclear corepressors (NCORs), thereby inhibiting thyroid hormone-mediated transcription activity. We crossed Thra1PV mice, expressing a dominant negative TRα1 mutant (TRα1PV), with mice expressing a mutant Ncor1 allele (Ncor1ΔID mice) that cannot recruit the TR or PV mutant. TRα1PV shares the same C-terminal mutated sequences as those of patients with frameshift mutations of the THRA gene. Remarkably, NCOR1ΔID ameliorated abnormalities in the thyroid-pituitary axis of Thra1PV/+ mice. The severe retarded growth, infertility, and delayed bone development were partially reverted in Thra1PV/+ mice expressing NCOR1ΔID. The impaired adipogenesis was partially corrected by de-repression of peroxisome-proliferator activated receptor γ and CCAAT/enhancer-binding protein α gene, due to the inability of TRα1PV to recruit NCOR1ΔID to form a repressor complex. Thus, the aberrant recruitment of NCOR1 by TRα1 mutants could lead to clinical hypothyroidism in humans. Therefore, therapies aimed at the TRα1–NCOR1 interaction or its downstream actions could be tested as potential targets in treating TRα1 mutant-mediated hypothyroidism in patients.
Keywords: growth retardation, lipid metabolism, fertility defect
The thyroid hormone T3 regulates growth and development and maintains metabolic homeostasis in humans. The genomic signaling by T3 is via the thyroid hormone receptor (TR) isoforms α1, β1, and β2, which are encoded by the THRA and THRB genes located on two different chromosomes. These TR isoforms share extensive sequence homology in the DNA and T3 binding domains but differ in the amino terminal A/B domains (1). TR binds to the thyroid hormone response elements (TREs) and recruits nuclear coregulatory proteins to regulate gene transcription. In the absence of T3, TRs recruit the nuclear corepressors (NCOR1 and NCOR2) for transcriptional repression on the T3 positively regulated genes. In the presence of T3, the T3-bound TR undergoes structural changes, resulting in the release of corepressors, allowing recruitment of nuclear receptor coactivators (e.g., SRC-1) to facilitate transcription activation (2, 3).
The critical roles of TR in mediating biological functions of T3 are clearly evident, in that mutations of the THRB gene cause resistance to thyroid hormone (RTH) (3). The intriguing observation that no mutations of the THRA gene were ever found in RTH patients raised the possibilities that mutations of the THRA gene could be embryonic lethal or could confer different clinical manifestations. These possibilities were explored by using a powerful mouse genetic approach. Targeting a mutation identified in an RTH patient into the Thrb gene of a mouse (the ThrbPV-mouse) faithfully reproduces human RTH (4). PV mutation has a frameshift mutation in the C-terminal 14 amino acids, leading to a total loss of T3 binding activity and transcription capacity. It exerts powerful dominant negative activity in vitro and in vivo (5). Targeting the identical PV mutation in the Thra gene at the position to that in the TRα1 (the Thra1PV mouse) created a mutant mouse that does not show symptoms of RTH, but exhibits phenotypes distinct from those of the ThrbPV mouse, including severe growth retardation (dwarfism), decreased survival, and reduced fertility (6). Others have also shown that mutations of the Thra gene in mice lead to phenotypes distinct from those of mice with knockin mutations of the Thrb gene (7–9). However, these mouse genetic findings showing that mutations of the Thra gene are not embryonic lethal, but have phenotypes differ from mutations of the Thrb gene, were not verified in humans until recently when patients were found with mutations of the THRA gene (10, 11). Indeed, TRα1PV shares the same mutated C-terminal sequence (-TLPRGL) with truncated termination at amino acid L406 as those of two patients with frameshift mutations of the THRA gene (11).
Patients with mutations of the THRA gene display classic features of hypothyroidism, with growth and developmental retardation, skeletal dysplasia, and severe constipation, but with only borderline-abnormal thyroid hormone levels (10, 11). These patients are heterozygotes, indicating that TRα1 mutants act in a dominant negative manner to mediate the clinical manifestations of these patients. However, the molecular mechanisms by which these TRα1 mutants act in vivo in a dominant negative fashion are not known. In the present study, we tested the hypothesis that the severe hypothyroidism in patients with THRA mutations results from an inability of TRα1 mutants to properly release the nuclear receptor corepressors (NCORs), thereby inhibiting T3-mediated transcription activity. We therefore crossed the Thra1PV mouse with mice globally expressing an NCOR1 mutant protein (NCOR1∆ID) in which the receptor interaction domains have been modified, leading to the loss of the ability to interact with TR or with the PV mutant (Ncor1ΔID mice) (12, 13). Ncor1ΔID mice have increased sensitivity to thyroid hormones because, despite low T4 and T3 levels, these mice have a normal thyroid stimulating hormone (TSH), demonstrating the specificity of NCOR1 for regulating the thyroid axis in vivo (12, 14). Remarkably, expression of NCOR1∆ID protein in Thra1PV/+ mice ameliorated the abnormalities in the pituitary–thyroid axis and partially reverted infertility, growth retardation, impaired bone development, and lipid abnormalities. The results show that NCOR1 regulates the dominant negative actions of TRα1 mutants in vivo. Thus, strategies directed against NCOR1 recruitment or the complex it recruits could potentially serve as therapeutic targets for patients with mutations of the THRA gene.
Results
Deletion of Receptor Interaction Domains in NCOR1 Normalizes Dysregulation of the Pituitary–Thyroid Axis in Vivo.
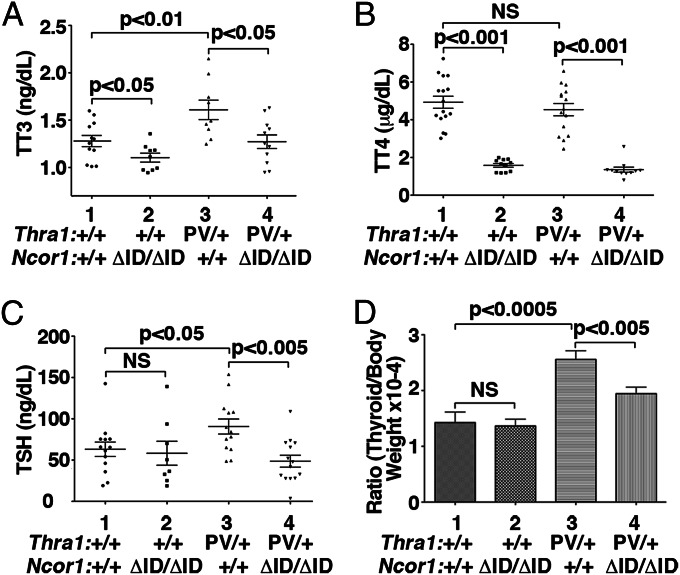
Knocking-in the PV mutation into the Thra gene severely impaired postnatal development such that homozygous Thra1PV/PV mice die shortly after birth (6). Therefore, the effect of the NCOR1ΔID mutant on TRα1PV actions can be studied only in heterozygous Thra1PV/+ mice. Fig. 1 compares the thyroid function tests in mice produced by the cross of Thra1PV/+ mice with Ncor1ΔID mice. Similar to the recently reported patients with mutations in the THRA gene (10, 11), Thra1PV/+ mice exhibit dysregulation of the pituitary–thyroid axis with elevated total T3 and mildly elevated TSH (6). Total T3 (TT3) was abnormally elevated in Thra1PV/+Ncor1+/+ mice (1.6 ± 0.1 ng/mL, n = 9; data group 3) compared with WT mice (1.3 ± 0.1 ng/mL, n = 12; data group 1, Fig. 1A). This abnormality was corrected to the level as that in WT mice by the expression of NCOR1ΔID in Thra1PV/+Ncor1ΔID/ΔID mice (1.3 ± 0.1 ng/mL, n = 11; data group 4 vs. group 1). TT3 was 15% lower in Thra1+/+Ncor1ΔID/ΔID mice than in WT mice, consistent with previous findings that indicate the importance of NCOR1 in establishing the set point of the thyroid axis (12).
Fig. 1.
Comparison of thyroid function tests and thyroid weights of Thra1PV/+ mice with or without mutant NCOR1ΔID. Serum levels of total T3 (A), total T4 (B), TSH (C), and thyroid weights (D) were determined in adult mice (3–5 mo old) as described in Materials and Methods. In D, ratios of thyroid weight vs. body weight were determined. The data are expressed as mean ± SEM with P value indicated (n = 8–17 animals per group).
Serum total T4 (TT4) was 68% lower in Thra1+/+Ncor1ΔID/ΔID mice (1.58 ± 0.09 μg/dL, n = 11; data group 2, Fig. 1B) than that in WT mice (4.9 ± 0.3 μg/dL, n = 16; data group 1, Fig. 1B). No differences in TT4 were observed between WT and Thra1PV/+Ncor1+/+ mice (4.5 ± 0.3 μg/dL, n = 15; data group 3). TT4 concentration was lower in Thra1PV/+Ncor1ΔID/ΔID mice (1.4 ± 0.1 μg/dL, n = 11; data group 4) than in Thra1PV/+Ncor1+/+ mice. No significant differences in TT4 between Thra1+/+Ncor1ΔID/ΔID mice and Thra1PV/+Ncor1ΔID/ΔID mice were found.
We also found that serum TSH levels were 1.44-fold higher in Thra1PV/+Ncor1+/+ than WT mice (90.7 ± 9.1, n = 13; data group 3 vs. 63.1 ± 8.7, n = 13; data group 1, Fig. 1C). Expression of NCOR1ΔID resulted in lower TSH serum levels for Thra1PV/+Ncor1ΔID/ΔID mice (48.6 ± 7.2, n = 14; data group 4). Expression of NCOR1ΔID in WT mice had no apparent effect on serum TSH levels (compare data group 1 and 2). Therefore, the expression of NCOR1ΔID abrogated both the elevated TSH and TT3 caused by the dominant negative action of PV.
We next examined the effect of the expression of NCOR1ΔID on thyroid growth. The expression of NCOR1ΔID in WT mice had no significant effect (compare bar 1 to bar 2, Fig. 1D). Consistent with elevated TSH levels in Thra1PV/+Ncor1+/+ mice (Fig. 1C), thyroid weights of Thra1PV/+Ncor1+/+ mice were enlarged ∼1.9-fold (Fig. 1D, bar 3 vs. bar 1). Consistent with the normal levels of TSH in Thra1PV/+Ncor1ΔID/ΔID mice, the expression of two alleles of the Ncor1ΔID gene decreased the thyroid weight by 20%, bringing it closer to that of Thra1+/+ mice (bar 4 vs. bar 1). Taken together, these results indicate that the expression of NCOR1ΔID ameliorated the defective regulation of the pituitary-thyroid axis of Thra1PV/+ mice.
Expression of NCOR1ΔID Lessens the Extent of Retarded Growth in Thra1PV/+ Mice.
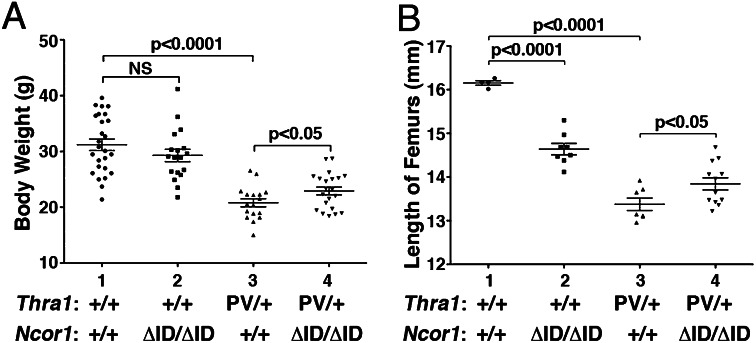
As juveniles, Thra1PV/+ mice are dwarfs with a body weight 40–50% less than their WT siblings, a size difference that persists into adulthood (6). Fig. 2A shows that, at 4 mo of age, the impaired weight gain of Thra1PV/+Ncor1+/+ mice (20.8 ± 0.7 g, n = 17; data group 3; 40% lower than Thra1+/+mice: 31.2 ± 1.0 g, n = 26; data group 1) was partially corrected with a significant 10% weight increase in mice expressing two alleles of the Ncor1ΔID gene (22.9 ± 0.7 g, n = 21; data group 4). Thus, the extent of the rescue in the retarded growth by the expression of NCOR1ΔID was 20.2% [(22.9 g − 20.8 g)/(31.2 g − 20.8 g)]. Because the expression of the Ncor1ΔID gene had no effect on the weights of WT mice (data groups 1, and 2), the reversal of the impaired weight gain in Thra1PV/+ mice was mediated via the lack of recruitment of NCOR1ΔID by TRα1PV.
Fig. 2.
Effects of NCOR1ΔID on growth of Thra1PV/+ mice. (A) Comparison of body weights among adult mice (3–5 mo old) with indicated genotypes. The difference in the body weight between Thra1PV/+Ncor1+/+ and Thra1PV/+Ncor1ΔID/ΔID mice (A) is significant (P < 0.05), (n = 17–26 animals per group). (B) Effects of NCOR1ΔID on bone growth of Thra1PV/+ mice. The data are expressed as mean ± SEM with P value indicated (n = 4–12 mice per group).
It is known that patients with mutations of the THRα gene have lower than normal serum insulin-like growth factor (IGF1) (10, 11). We therefore determined the serum IGF1 in WT mice and in Thra1+/+Ncor1ΔID/ΔID, Thra1PV/+Ncor1+/+, and Thra1PV/+Ncor1ΔID/ΔID mice. We also included ThrbPV/+Ncor1+/+ and ThrbPV/+Ncor1ΔID/ΔID mice for comparison. Consistent with the observations in patients, Fig. S1 shows that serum IGF1 levels were 13% lower in Thra1PV/+Ncor1+/+ mice than in WT mice. Although the expression of NCOR1ΔID in WT mice had no effect on the IGF1 level, the expression of NCOR1ΔID corrected the decreased IGF1 in Thra1PV/+Ncor1+/+ mice to the level of WT mice. In contrast, no changes in serum IGF1 levels in ThrbPV/+Ncor1+/+ mice and no significant effects on serum IGF1 levels of ThrbPV/+Ncor1+/+mice caused by the expression of NCOR1ΔID were detected. These results indicate that, consistent with the findings in patients with TRα1 mutations, lower serum IGF1 levels could contribute to the 40–50% decrease in body growth observed in Thra1PV/+Ncor1+/+ mice. Importantly, these data further show that the rescue in IGF1 level by NCOR1ΔID was specific to the TRα1 mutation, but not to the TRβ mutation.
In addition, we further evaluated the changes in the expression of the growth hormone (Gh) mRNA in the pituitaries of Thra1PV/+and Thra1PV/+mice with or without the expression of NCOR1ΔID (Fig. S2). Gh is a TR directly regulated gene in which the thyroid hormone response element is well defined (15). Fig. S2 shows that the expression of NCOR1ΔID had no effect on the expression of Gh mRNA in WT mice. However, consistent with the 40–50% reduction in the body weight of Thra1PV/+Ncor1+/+ mice, the expression of Gh mRNA in the pituitary of Thra1PV/+Ncor1+/+ mice was 48% lower than that of WT mice. Remarkably, this decrease was reversed to the level of WT mice by the expression of NCOR1ΔID in Thra1PV/+Ncor1ΔID/ΔID mice. In contrast, the expression of Gh mRNA was not affected in ThrbPV/+Ncor1+/+ mice or in ThrbPV/+Ncor1ΔID/ΔID mice. These data indicate that the rescue by NCOR1ΔID was specific to the TRα1 mutation, but not by TRβ mutation.
Besides reduced weight gain, Thra1PV/+ mice also display skeletal abnormalities, with severe and persistent postnatal linear growth impairment (6, 16). Fig. 2B shows the lengths of femurs of Thra1PV/+Ncor1+/+ mice (13.38 ± 0.14 mm, n = 7; data group 3) were ∼18% shorter than those of Thra1+/+ mice (16.15 ± 0.05 mm, n = 4; data group 1). The expression of NCOR1ΔID led to a nearly 20% reversal of the defect in retarded bone growth in Thra1PV/+Ncor1ΔID/ΔID mice (13.84 ± 0.13 mm, n = 12; data group 4). In contrast, expression of NCOR1ΔID led to the impairment of bone growth in Thra1+/+mice (14.64 ± 0.13 mm, n = 8, data group 2). Thus, these data indicate that the extent of the rescue in the impaired bone development by the expression of NCOR1ΔID was 16.6% [(13.84 mm − 13.38 mm/(16.15 mm − 13.38 mm)]. These results indicate that the lack of interaction of TRα1PV with NCOR1ΔID weakened the dominant negative action of TRα1PV in bone development.
Expression of NCOR1ΔID Rescues the Infertility of Thra1PV/+ Mice.
Since the creation of Thra1PV mice in 2001 (6), we have found that female Thra1PV/+ mice are infertile, as shown in Fig. 3A (bar 1). When female Thra1PV/+Ncor1+/+ mice were mated with male Thra1+/+Ncor1ΔID/ΔID, no improvement in the infertility of female Thra1PV/+Ncor1+/+ mice was detected (bar 2, Fig. 3A), indicating that the expression of NCOR1ΔID in male Thra1+/+ mice had no effect on pup production when they mated with female Thra1PV/+Ncor1+/+ mice. Remarkably, the expression of NCOR1ΔID had made it possible for female Thra1PV/+Ncor1ΔID/ΔID mice to produce pups when mated with male Thra1+/+Ncor1ΔID/+ mice, although with a small litter size (2–3 pups; bar 3). Bar 4 shows the normal litter size (12–13 pups per litter) when both parents are WT mice. The deleterious effects of mutations of the Thra gene on fertility were less severe in male Thra1PV/+ mice (bar 1, Fig. 3B) as pups were born when mated with female Thra1+/+Ncor1+/+ mice, although with a smaller than average litter size when both parents are WT mice (6–7 vs. 12–13 pups per litter, bar 4, Fig. 3B). The expression of both alleles of Ncor1ΔID corrected the defects in fertility of male Thra1PV/+ mice in that a nearly normal-size litter (∼12 pups per litter) was produced (bar 3, Fig. 3B). These findings show that the effects that mutations of the Thra gene have on fertility are sex-dependent and that sex dictates the sensitivity of TRα1PV to the effects of NCOR1.
Fig. 3.
Effects of NCOR1ΔID on the fertility of Thra1PV/+ mice. (A) Female Thra1PV/+ mice mated with male WT mice with or without the expression of NCOR1ΔID. (B) Male Thra1PV/+ mice mated with female WT mice with or without the expression of NCOR1ΔID. The litter size for the WT mice is indicated in bar 4.
Expression of NCOR1ΔID Improves Lipid Metabolism of Thra1PV/+ Mice.
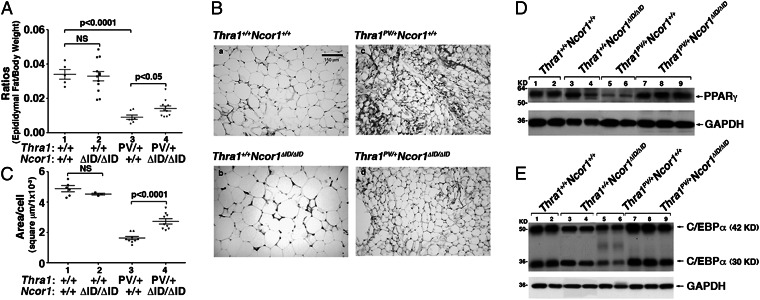
We have previously shown that Thra1PV/+ mice exhibit abnormalities in lipid metabolism (17). Consistent with our previous findings, the ratios of epididymal fat mass/body weight in Thra1PV/+Ncor1+/+ mice were 80% lower than that in WT mice (data group 3 vs. 1; Fig. 4A). This abnormality of Thra1PV/+ mice was partially corrected (60% lower than that of WT mice) by NCOR1ΔID in Thra1PV/+Ncor1ΔID/ΔID mice (compare data group 4 with group 3; Fig. 4A). By contrast, the expression of NCOR1ΔID had no significant effects on the epididymal fat of Thra1+/+mice (data group 2 vs. group 1). We further analyzed the size of cells in epididymal fat. Indeed, compared with the normal fat cells (Fig. 4 B, a), these fat cells of Thra1PV/+Ncor1+/+ mice were smaller (Fig. 4 B, c). These smaller cell sizes were partially corrected by the expression of NCOR1ΔID in the epididymal fat of Thra1PV/+Ncor1ΔID/ΔID mice (Fig. 4 B, d). Consistent with the fat pad weight shown in Fig. 4A, the expression of NCOR1ΔID had no effect on the fat cell size of WT mice (Fig. 4 B, b).
Fig. 4.
Effects of NCOR1ΔID on adipogenesis of epididymal fat of Thra1PV/+ mice. (A) Comparison of weights of epididymal fat mass in Thra1PV mice with or without NCOR1ΔID. The weights of epididymal fat mass in male mice with genotype indicated were measured (3–5 mo old; n = 5–12 mice per group). Ratios of fat mass vs. body weight were determined. Data are expressed as mean values ± SEM with P values shown. (B). Representative histological features of epididymal fat of Thra1+/+ and Thra1PV mice with or without NCOR1ΔID. All images are representative of n = 3 mice per group. (C) Fat cell size was measured for each genotype shown in B. Data are expressed as mean values ± SEM with P values shown. Effects of NCOR1ΔID on expression of PPARγ (D) and C/EBPα (E) proteins in the epididymal fat of Thra1PV/+ mice.
The extent of the correction by the expression of NCOR1ΔID in the epididymal fat of Thra1PV/+Ncor1ΔID/ΔID mice was demonstrated by measuring the area of cells in the four genotypes. The quantitative data are shown in Fig. 4C. The cell size in the epididymal fat of Thra1PV/+ mice was 1.45 ± 0.06 square microns × 10−3 (n = 6), 70% smaller than that of Thra1+/+ mice (4.9 ± 0.29 square microns × 10−3; n = 4). There was a 1.8-fold increase in cell size in Thra1PV/+Ncor1ΔID/ΔID mice (2.57 ± 0.19 square microns × 10−3, n = 6; data group 4 vs. group 3). No significant differences in cell size between WT and Thra1+/+Ncor1ΔID/ΔID mice (compare data group 1 and 2). Taken together, these data indicate that the expression of NCOR1ΔID partially reversed the impaired adipocyte size and mass present in Thra1PV/+ mice.
TRα1PV-Mediated Repression of Adipogenic Genes Is De-Repressed by the Expression of NCOR1ΔID in White Adipose Tissue of Thra1PV/+Ncor1ΔID/ΔID Mice.
Previously, we have shown that impaired adipocyte mass found in white adipose tissue (WAT) of Thra1PV/+ mice is potentially mediated by the repression of the adipogenic genes (17). To test the hypothesis that the expression of NCOR1ΔID could lead to de-repression of the adipogenic genes in the epididymal fat pad of Thra1PV/+Ncor1ΔID/ΔID mice, we first analyzed the expression of two pivotal regulators of adipogenesis: the peroxisome-proliferator activated receptor γ (Pparγ) and the CCAAT/enhancer-binding protein α gene (C/ebpα) (18). Consistent with previous findings (17), the protein abundance of PPARγ was lower in WAT of Thra1PV/+ mice than Thra1+/+mice (Fig. 4D, lanes 5 and 6 vs. lanes 1 and 2). In Thra1PV/+Ncor1ΔID/ΔID mice in which NCOR1ΔID was expressed, the lower PPARγ protein abundance caused by TRα1PV was abolished, as evidenced by elevated PPARγ protein (lanes 7–9 vs. 5–6) in the WAT of Thra1PV/+Ncor1ΔID/ΔID mice. In contrast, no effect of NCOR1ΔID on the expression of PPARγ protein abundance was observed in WT mice (lanes 3–4 vs. lanes 1–2). Similarly, we found that the low protein abundance of the two isoforms of C/EBPα (42 kDa and 30 kDa) in WAT of Thra1PV/+ mice (lanes 6 and 7: Fig. 4E) was higher by the expression of NCOR1ΔID in Thra1PV/+Ncor1ΔID/ΔID mice (lanes 7–9; Fig. 4E), but no effect of NCOR1ΔID on the expression of the two isoforms of C/EBPα was observed in WT mice (lanes 1–2 vs. 3–4). These data indicate that the TRα1PV-mediated repression of Pparγ and C/ebpα genes was reverted by an NCOR1ΔID protein that lacks the receptor interacting domain.
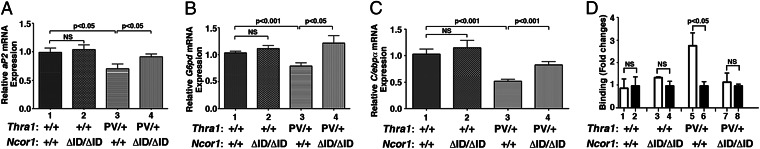
We further analyzed the effects of NCOR1ΔID on the expression of other lipogenic genes downstream of PPARγ. Consistent with the repression of the Pparγ gene shown in Fig. 4D, Fig. 5 shows that the expression of the fatty acid-binding protein gene (aP2) (Fig. 5A), glucose-6P-dehydrogenase gene (G6pd) (Fig. 5B) was repressed in the epididymal fat pad of Thra1PV mice (bars 3 vs. 1). Such gene expression was de-repressed by the expression of NCOR1ΔID in Thra1PV/+Ncor1ΔID/ΔID mice, rising to the similar level of that in Thra1+/+mice (bar 4). The elevated expression of these lipogenic genes led to the increased white fat pad of Thra1PV/+Ncor1ΔID/ΔID mice shown in Fig. 4.
Fig. 5.
Effects of NCOR1ΔID on TRα1PV-repressed lipogenic genes in the epididymal fat of Thra1PV/+ mice (A–C). The expression of mRNA was determined by real-time RT/PCR as described in Materials and Methods. The data are expressed as mean ± SEM (n = 5–7) and P values are indicated. (D) Lack of recruitment of NCOR1ΔID to the promoter of the C/ebpα gene abolishes the dominant negative effect of TRα1PV. ChIP assay was carried out using normal rabbit IgG (lanes 2, 4, 6, and 8) or anti-NCOR1 antibody (PHQQ) (lanes 1, 3, 5, and 7) as described in Materials and Methods. Data are expressed as mean ± SEM (n = 3). NS, Not significant.
The mouse C/ebpα gene is directly regulated by the TR as shown by the recent identification of a thyroid hormone response element (TRE) (19). This TRE is also conserved in the rat (20). We therefore further evaluated whether the expression of the C/ebpα mRNA was affected by NCOR1ΔID in the epididymal fat pad of Thra1PV/+Ncor1ΔID/ΔID mice. Fig. 5C shows that C/ebpα mRNA expression in the epididymal fat pad of Thra1PV/+Ncor1+/+ mice was 50% lower than that in Thra1+/+mice (bar 3 vs. bar 1, Fig. 5C). These findings are consistent with our earlier reports in that the expression of C/ebpα mRNA is repressed by TRα1PV in 3T3-L1 cells (21) and in the liver of Thra1PV/+ mice (19). Importantly, Fig. 5C shows that the TRα1PV-mediated repressed C/ebpα mRNA was abolished and that the mRNA expression level in the epididymal fat pad of Thra1PV/+Ncor1ΔID/ΔID mice rose to that of the level in Thra1+/+mice (bar 3 vs. bar 1). However, no effect on the expression of C/ebpα mRNA by NCOR1ΔID was observed in WT mice (bars 2 vs. 1, Fig. 5C). That the C/ebpα gene is a TR-direct target gene allowed us to elucidate whether the up-regulated C/ebpα mRNA expression in the epididymal fat pad of Thra1PV/+Ncor1ΔID/ΔID mice resulted from the lack of recruitment of NCOR1ΔID to TRE-bound TRα1PV. We previously showed that TRα1PV was bound to the TRE of the C/ebpα gene in 3T3-L1 cells stably expressing TRα1PV (21) and that TRE-bound TRα1PV recruited NCOR1 on the promoter of the C/ebpα gene in the hepatocytes (19). In this study, we found that NCOR1 was not recruited to the promoter of the C/ebpα gene in the epididymal fat pad of Thra1+/+Ncor1+/+ mice (bar 1, Fig. 5D), nor in that of Thra1+/+Ncor1ΔID/ΔID mice (bar 3, Fig. 5D), but was recruited to the promoter of the C/ebpα gene in the epididymal fat pad of Thra1PV/+Ncor1+/+ mice (bar 5). In contrast, no recruitment of NCOR1ΔID was detected in the promoter of Thra1PV/+Ncor1ΔID/ΔID mice (bar 7, Fig. 5D). These findings indicate that loss of interaction of TRα1PV with NCOR1ΔID led to the reversal in the expression of the C/ebpα gene in Thra1PV/+Ncor1ΔID/ΔID mice. Importantly, these results show that, in vivo, aberrant NCOR1 underlies the dominant negative actions of TRα1 mutants.
Discussion
Using two knockin mutant mice that harbor an identical mutation (PV) in either the Thrb gene (ThrbPV mice) or the Thra gene (Thra1PV mice), we have previously shown that mutations of these two genes lead to distinct abnormal phenotypes. ThrbPV mice faithfully reproduce human RTH (7). Thra1PV mice do not have RTH with reduced sensitivity to thyroid hormones in target tissues (3, 6), but display severe growth retardation (dwarfism), impaired bone development, abnormal lipid metabolism, reduced fertility, and decreased survival (6, 16). Moreover, the abnormal regulation patterns of T3 target genes are clearly distinct in the tissues of these two mutant mice (4, 6, 21–23). The findings from the extensive analyses of the phenotypes and molecular studies of these two mutant mice strongly suggested that mutations of the THRA gene could lead to human diseases with symptoms different from those of classical RTH. This prediction was confirmed by the recent discovery of patients with mutations of the THRA gene (10, 11). Although the number of patients with mutations of the THRA gene is currently small, this discovery definitively supports the conclusions reached from mouse models that the in vivo actions of TR mutants are isoform-dependent. The finding that TRα1PV shares the same mutated C-terminal sequence as that in patients reported by van Mullem et al. (11) has made the Thra1PV mouse a valid and highly relevant mouse model to understand the molecular basis underlying the pathogenesis of hypothyroidism in patients caused by mutations of the THRA gene.
Importantly, patients are heterozygous in the mutation of the THRA gene, indicating that the clinical manifestations are mediated via the dominant negative actions of the mutant receptors. In vitro studies of these human mutations further support the genetic evidence. Both the E403× TRα1 mutant described by Bochukova et al. (10) and the E397fs406× TRα1 mutant described by van Mullem et al. (11) act in a dominant negative fashion to interfere with the transcription activity of WT TRs. In vitro studies further show that the E403× TRα1 mutant is defective in its ability to release corepressors in the presence of T3 (10). We therefore crossed Thra1PV/+ mice with Ncor1ΔID mice that globally express a mutant NCOR1 (NCOR1ΔID) protein that cannot be recruited by TRs or by the PV mutant (12–14). The expression of NCOR1ΔID protein brought the mildly elevated TT3 and TSH levels in Thra1PV/+ mice down to the basal level of WT mice. Remarkably, the severe growth retardation and delayed long bone development were partially corrected in Thra1PV/+ mice by the expression of NCOR1ΔID. The abnormalities in lipid metabolism demonstrated by the marked loss of fat mass were also partially reverted in Thra1PV/+ mice expressing NCOR1ΔID. Although the phenotype of the abnormalities in lipid metabolism was not noted in the patients with mutations of the THRA gene, the expression of NCOR1ΔID totally or partially corrected the mild dysregulation in the pituitary–thyroid axis, the growth retardation, and impairment of bone development reported for patients. These findings support the idea that an aberrant recruitment of corepressors such as NCOR1 underlies the pathogenesis of the tissue-specific hypothyroidism caused by the mutant TRα1 in patients.
How NCOR1 regulates the in vivo actions of TRα1PV at the molecular level was explored in WAT. Consistent with our previous findings (17), the present studies showed impaired fat mass in the WAT of Thra1PV/+ mice. We have previously shown that this impaired fat mass is likely secondary to impaired adipogenesis. Indeed, TRα1PV mediates this defect by repressing the expression of PPARγ and C/EBPα, master regulators of adipogenesis, and their downstream adipogenic gene targets (Figs. 4 D and E and 5B). Strikingly, the expression of NCOR1ΔID de-represses all of these genes and whereas ChIP analysis shows strong recruitment of NCOR1 by TRα1PV to the promoter of the C/ebpα gene in WAT of Thra1PV/+ mice, no detectable recruitment of NCOR1ΔID was found in the promoter of the C/ebpα gene in WAT of Thra1PV/+Ncor1ΔID/ΔID mice (Fig. 5D). Thus, in the presence of NCOR1ΔID, the C/ebpα gene expression is activated and can then reactivate the repressed adipogenic program. These findings in WAT demonstrate that aberrant recruitment of corepressors underlies the dominant negative actions of TRα1 mutants in vivo.
However, it is of interest to note that, whereas the expression of NCOR1ΔID completely reversed the expression levels of PPARγ and C/EBPα in the WAT of Thra1PV/+Ncor1ΔID/ΔID mice (Fig. 4 D and E, respectively), the size and mass of the adipocytes were only partially corrected (Figs. 4 A, B, and C). Adipogenesis is a complicated process that requires coordinated regulation by the sequential activation of a battery of transcription factors. These factors include positive effectors such as AP-1 and Kruppel-like factor (KLFs 4 and 6) and negative regulators such as KLFs 2and 7 that are induced during clonal expansion. Other positive effectors such as PPARγ and C/EBPα and negative effectors such as Wnt-5a and Wnt-10b are induced during differentiation stage (24). We have previously shown that PPARγ and C/EBPα are TR-regulated genes that are repressed by TRα1PV in WAT of Thra1PV/+ mice (17, 19). In the present studies, we showed that the expression of NCOR1ΔID completely reverted the TRα1PV-mediated repression of these two genes. However, other transcription factors that are critical either for clonal expansion and/or differentiation of preadipocytes to mature adipoctyes, but are not direct TR-target genes, may indirectly contribute to the decreased size and mass of WAT. NCOR1ΔID may not affect the expression of these indirect TR target genes. Thus, we detected a lack of concordance in the effect of NCOR1ΔID on the complete reversal of the differentiation markers (PPARγ and C/EBPα) and incomplete phenotypic changes in the size and mass of adipocytes. Identification and characterization of the genes that contribute to size and mass of WAT would await further studies.
The present studies show that the extent of the correction of abnormalities in the Thra1PV/+ mouse caused by the expression of NCOR1ΔID varies across tissues. For example, the pituitary–thyroid axis and the mildly elevated TT3 and TSH levels were totally corrected to the basal levels of WT mice. However, only partial corrections in growth, bone length, and WAT mass were observed after the expression of NCOR1ΔID. These observations confirm the exquisite specificity of NCOR1 actions in the regulation of the pituitary–thyroid axis (12). However, in other target tissues, the redundancy of other nuclear corepressors such as NCOR2/silencing mediator of retinoic acid and thyroid hormone receptor (SMRT) could mediate the dominant negative action of TRα1 mutants. Moreover, complex modulation by a network of regulators could come into play to affect the phenotypic expression. Still, the finding that partial corrections were observed in these tissues clearly indicates that NCOR1 plays a prominent role in the regulation of the dominant negative actions of TRα1 mutants in vivo.
The interesting finding that the expression of NCOR1ΔID lessened the infertility of Thra1PV/+ mice in the present studies has important implications. The impact of TRα1PV on fertility is more severe in female than male mice. We have not been able to mate female Thra1PV/+ mice with WT males to produce pups, but there is a better chance, although at a markedly lower frequency than with WT mice, to mate male Thra1PV/+ mice with WT females to produce pups. Remarkably, the expression of NCOR1ΔID had made the female Thra1PV/+ mice fertile, as evidenced by the birth of a few pups (Fig. 3), and raised the low fertility of male Thra1PV/+ mice to a level similar to that of WT mice (10–12 pups per litter; Fig. 3B). Therefore, aberrant recruitment of NCOR1 by TRα1 mutants has severe deleterious effects beyond growth and postnatal development. It is not clear, at present, how and why mutations of the Thra gene lead to decreased fertility in Thra1PV/+ mice, with more severe effects in females than males. It would be virtually impossible to study the effects of THRA mutations on the fertility of patients, but the lessons learned from the Thra1PV/+ mouse model suggest that patients with mutations of the THRA gene would be rare because of decreased fertility. The rarity of such patients could be one of the reasons why the discovery of such patients lagged far behind that of RTH patients. However, with the availability of mouse models, it is now possible to dissect further the molecular basis of mutations of the THRA gene in disease, identify the potential molecular targets for treatment, and understand the pathology of mutations in other nuclear receptors. Finally, the results presented herein suggest that humans with THRA mutations could be treated with medications that prevent or abrogate the effects of corepressor recruitment or action (25).
Materials and Methods
Mouse Strains.
This animal study was carried out according to the protocol approved by the National Cancer Institute Animal Care and Use Committee. Mice harboring the mutant Thra1PV gene (Thra1PV mice) were prepared and genotyped as described earlier (6). Ncor1ΔID mice were prepared as described by Astapova et al. (12). Thra1PV mice were crossed with Ncor1ΔID mice to obtain different genotypes for studies. These mice were intercrossed several generations, and littermates with a similar genetic background were used in all experiments.
Hormone Assays.
The serum levels of total T4 and total T3 were determined using a Gamma Coat T4 and T3 assay RIA kit (Dia-Sorin). Serum TSH levels were measured as previously described (26).
RNA Isolation and Quantitative Real-Time RT-PCR.
Total RNA was extracted from pituitary by using TRIzol (Invitrogen), and fat tissues were extracted using an RNeasy lipid tissue mini kit (QIAGEN) according to the manufacturer’s instructions. Quantitative real-time RT-PCR was performed with a Quantitect SYBR Green RT-PCR kit (QIAGEN), according to the manufacturer's instructions and using a 7900HT Fast Real-Time PCR system (AB Applied Biosystems). Total RNA (200 ng) was used in RT-PCR determinations as described previously (27). The specific primer sequences used in RT/PCR are as follows: aP2 (access number: 11770) Forward 5′-CTGGACTTCAGAGGCTCATAGCA-3′; Reverse 5′-TACTCTCTGACCGGATGGTGACCAA-3′, G6PD (access number: 14381) Forward 5′-GAGGAGTTCTTTGCCCGTAAT-3′; Reverse 5′-CATCTCTTTGCCCAGGTAGTG-3′, C/ebpα (access number: 12606) Forward 5′-TTACAACAGGCCAGGTTTCC-3′; Reverse 5′-CTCTGGGATGGATCGATTGT-3′.
Western Blot Analysis.
Frozen epididymal fat pads were homogenized on ice in lysis buffer containing 50 mM Tris⋅HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 0.5% Triton X-100, 1× protease inhibitor mixture (Roche), and 0.2 μM okadaic acid, and Western blot analysis with the lysates was similarly carried out as described (19). The antibodies used were anti-C/EBPα (sc-61) and anti-PPARγ (sc-7196), which were purchased from Santa Cruz Biotechnology and used at a 1:200 dilution.
Chromatin Immunoprecipitation (ChIP) Assay.
ChIP assay with fat tissue was performed as described previously (28). Briefly, mouse epididymal fat tissues were dissected from mice with different genotypes (WT, Thra1PV/+, and Thra1PV/+Ncor1ΔID/ΔID). One gram of fat tissue was digested in 2 mg/mL collagenase (Worthington Biochemical Corp, Lakewood, NJ), fixed in 1% of formaldehyde for 10 min, and quenched by addition of glycine with a 0.125 M final concentration for 5 min. Subsequent steps in ChIP assays were carried out as described by Fozzatti, et al. (19).
Histological Analysis.
Epididymal fat was dissected, fixed in 10% (vol/vol) neutral buffered formalin (Sigma-Aldrich), and subsequently embedded in paraffin. Sections of 5-μm thickness were prepared and stained with hematoxylin and eosin (H&E). For each animal, single random sections through the epididymal fat were examined. For the determination of the area of white adipocytes, 200–400 cells per field were measured for the epididymal fat of Thra1+/+Ncor1+/+ mice, Thra1+/+ Ncor1ΔID/ΔID mice, Thra1PV/+Ncor1+/+ mice, and Thra1PV/+Ncor1ΔID/ΔID mice.
Statistical Analysis.
All data are expressed as mean ± SEM. Differences between groups were examined for statistical significance using Student t test with the use of GraphPad Prism 4.0a. P < 0.05 is considered statistically significant.
Supplementary Material
Acknowledgments
We thank Dr. O. Gavrilova (National Institute of Diabetes and Digestive and Kidney Diseases) for valuable advice and assistance during the course of the present work and for critical reading of the manuscript. The present research was supported by the Intramural Research Program at the Center for Cancer Research, National Cancer Institute, National Institutes of Health and by National Institutes of Health Extramural Grant DK056123 (to A.N.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1222334110/-/DCSupplemental.
References
- 1.Cheng SY. Multiple mechanisms for regulation of the transcriptional activity of thyroid hormone receptors. Rev Endocr Metab Disord. 2000;1(1-2):9–18. doi: 10.1023/a:1010052101214. [DOI] [PubMed] [Google Scholar]
- 2.Johnson AB, O’Malley BW. Steroid receptor coactivators 1, 2, and 3: Critical regulators of nuclear receptor activity and steroid receptor modulator (SRM)-based cancer therapy. Mol Cell Endocrinol. 2012;348(2):430–439. doi: 10.1016/j.mce.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Refetoff S, Weiss RE, Usala SJ. The syndromes of resistance to thyroid hormone. Endocr Rev. 1993;14(3):348–399. doi: 10.1210/edrv-14-3-348. [DOI] [PubMed] [Google Scholar]
- 4.Kaneshige M, et al. Mice with a targeted mutation in the thyroid hormone beta receptor gene exhibit impaired growth and resistance to thyroid hormone. Proc Natl Acad Sci USA. 2000;97(24):13209–13214. doi: 10.1073/pnas.230285997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng SY. 2004. New developments in thyroid hormone resistance. Hot Thyroidology No. 1.
- 6.Kaneshige M, et al. A targeted dominant negative mutation of the thyroid hormone alpha 1 receptor causes increased mortality, infertility, and dwarfism in mice. Proc Natl Acad Sci USA. 2001;98(26):15095–15100. doi: 10.1073/pnas.261565798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu YY, Schultz JJ, Brent GA. A thyroid hormone receptor alpha gene mutation (P398H) is associated with visceral adiposity and impaired catecholamine-stimulated lipolysis in mice. J Biol Chem. 2003;278(40):38913–38920. doi: 10.1074/jbc.M306120200. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto K, et al. An unliganded thyroid hormone receptor causes severe neurological dysfunction. Proc Natl Acad Sci USA. 2001;98(7):3998–4003. doi: 10.1073/pnas.051454698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tinnikov A, et al. Retardation of post-natal development caused by a negatively acting thyroid hormone receptor alpha1. EMBO J. 2002;21(19):5079–5087. doi: 10.1093/emboj/cdf523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bochukova E, et al. A mutation in the thyroid hormone receptor alpha gene. N Engl J Med. 2012;366(3):243–249. doi: 10.1056/NEJMoa1110296. [DOI] [PubMed] [Google Scholar]
- 11.van Mullem A, et al. Clinical phenotype and mutant TRα1. N Engl J Med. 2012;366(15):1451–1453. doi: 10.1056/NEJMc1113940. [DOI] [PubMed] [Google Scholar]
- 12.Astapova I, et al. The nuclear receptor corepressor (NCoR) controls thyroid hormone sensitivity and the set point of the hypothalamic-pituitary-thyroid axis. Mol Endocrinol. 2011;25(2):212–224. doi: 10.1210/me.2010-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Astapova I, et al. The nuclear corepressor, NCoR, regulates thyroid hormone action in vivo. Proc Natl Acad Sci USA. 2008;105(49):19544–19549. doi: 10.1073/pnas.0804604105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fozzatti L, et al. Resistance to thyroid hormone is modulated in vivo by the nuclear receptor corepressor (NCOR1) Proc Natl Acad Sci USA. 2011;108(42):17462–17467. doi: 10.1073/pnas.1107474108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Das P, Meyer L, Seyfert HM, Brockmann G, Schwerin M. Structure of the growth hormone-encoding gene and its promoter in mice. Gene. 1996;169(2):209–213. doi: 10.1016/0378-1119(95)00815-2. [DOI] [PubMed] [Google Scholar]
- 16.O’Shea PJ, Bassett JH, Cheng SY, Williams GR. Characterization of skeletal phenotypes of TRalpha1 and TRbeta mutant mice: Implications for tissue thyroid status and T3 target gene expression. Nucl Recept Signal. 2006;4:e011. doi: 10.1621/nrs.04011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ying H, Araki O, Furuya F, Kato Y, Cheng SY. Impaired adipogenesis caused by a mutated thyroid hormone alpha1 receptor. Mol Cell Biol. 2007;27(6):2359–2371. doi: 10.1128/MCB.02189-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henry SL, et al. White adipocytes: More than just fat depots. Int J Biochem Cell Biol. 2012;44(3):435–440. doi: 10.1016/j.biocel.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 19.Fozzatti L, Lu C, Kim DW, Cheng SY. Differential recruitment of nuclear coregulators directs the isoform-dependent action of mutant thyroid hormone receptors. Mol Endocrinol. 2011;25(6):908–921. doi: 10.1210/me.2010-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gnacińska M, Małgorzewicz S, Stojek M, Łysiak-Szydłowska W, Sworczak K. Role of adipokines in complications related to obesity: A review. Adv Med Sci. 2009;54(2):150–157. doi: 10.2478/v10039-009-0035-2. [DOI] [PubMed] [Google Scholar]
- 21.Mishra A, Zhu XG, Ge K, Cheng SY. Adipogenesis is differentially impaired by thyroid hormone receptor mutant isoforms. J Mol Endocrinol. 2010;44(4):247–255. doi: 10.1677/JME-09-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menéndez-Hurtado A, Santos A, Pérez-Castillo A. Characterization of the promoter region of the rat CCAAT/enhancer-binding protein alpha gene and regulation by thyroid hormone in rat immortalized brown adipocytes. Endocrinology. 2000;141(11):4164–4170. doi: 10.1210/endo.141.11.7756. [DOI] [PubMed] [Google Scholar]
- 23.Esaki T, et al. Functional activation of cerebral metabolism in mice with mutated thyroid hormone nuclear receptors. Endocrinology. 2003;144(9):4117–4122. doi: 10.1210/en.2003-0414. [DOI] [PubMed] [Google Scholar]
- 24.Sarjeant K, Stephens JM. Adipogenesis. Cold Spring Harb Perspect Biol. 2012;4(9):a008417. doi: 10.1101/cshperspect.a008417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lonard DM, O’Malley BW. Nuclear receptor coregulators: modulators of pathology and therapeutic targets. Nat Rev Endocrinol. 2012;8(10):598–604. doi: 10.1038/nrendo.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furumoto H, et al. An unliganded thyroid hormone beta receptor activates the cyclin D1/cyclin-dependent kinase/retinoblastoma/E2F pathway and induces pituitary tumorigenesis. Mol Cell Biol. 2005;25(1):124–135. doi: 10.1128/MCB.25.1.124-135.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ying H, et al. Alterations in genomic profiles during tumor progression in a mouse model of follicular thyroid carcinoma. Carcinogenesis. 2003;24(9):1467–1479. doi: 10.1093/carcin/bgg111. [DOI] [PubMed] [Google Scholar]
- 28.Pérez-Mancera PA, et al. Adipose tissue mass is modulated by SLUG (SNAI2) Hum Mol Genet. 2007;16(23):2972–2986. doi: 10.1093/hmg/ddm278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.