Abstract
Volunteers immunized under chloroquine chemoprophylaxis with Plasmodium falciparum sporozoites (CPS) develop complete, long-lasting protection against homologous sporozoite challenge. Chloroquine affects neither sporozoites nor liver-stages, but kills only asexual forms in erythrocytes once released from the liver into the circulation. Consequently, CPS immunization exposes the host to antigens from both preerythrocytic and blood stages, and induced immunity might target either of these stages. We therefore explored the life cycle stage specificity of CPS-induced protection. Twenty-five malaria-naïve volunteers were enrolled in a clinical trial, 15 of whom received CPS immunization. Five immunized subjects and five controls received a sporozoite challenge by mosquito bites, whereas nine immunized and five control subjects received an i.v. challenge with P. falciparum-infected erythrocytes. The latter approach completely bypasses preerythrocytic stages, enabling a direct comparison of protection against either life cycle stage. CPS-immunized subjects (13 of 14) developed anticircumsporozoite antibodies, whereas only one volunteer generated minimal titers against typical blood-stage antigens. IgG from CPS-immunized volunteers did not inhibit asexual blood-stage growth in vitro. All CPS-immunized subjects (5 of 5) were protected against sporozoite challenge. In contrast, nine of nine CPS-immunized subjects developed parasitemia after blood-stage challenge, with identical prepatent periods and blood-stage multiplication rates compared with controls. Intravenously challenged CPS-immunized subjects showed earlier fever and increased plasma concentrations of inflammatory markers D-dimer, IFN-γ, and monokine induced by IFN-γ than i.v. challenged controls. The complete lack of protection against blood-stage challenge indicates that CPS-induced protection is mediated by immunity against preerythrocytic stages. However, evidence is presented for immune recognition of P. falciparum-infected erythrocytes, suggesting memory responses unable to generate functional immunity.
Malaria remains one of the most common and severe infectious diseases, with an estimated 216 million cases and 655,000 deaths annually (1). The malaria parasite Plasmodium falciparum is responsible for most of these cases, particularly in sub-Saharan Africa. P. falciparum sporozoites are transmitted to humans by the bites of infected Anopheles mosquitoes. Sporozoites migrate from the skin to the liver, where they invade hepatocytes, develop, and multiply. Approximately 6 d after invasion, hepatocytes rupture and merozoites are released into the bloodstream, where they multiply in 48-h cycles of erythrocyte invasion, replication, erythrocyte rupture, and release of infectious merozoites. These asexual blood-stage parasites cause the clinical symptoms of malaria. To fight malaria, an effective vaccine is urgently needed. Development of vaccines generally has been stage-oriented, specifically targeting preerythrocytic or asexual blood stages of the parasite (2).
In the controlled human malaria infection model, we previously showed that immunization of healthy malaria-naïve volunteers while they are taking chloroquine prophylaxis with P. falciparum sporozoites via infected mosquito bites [chemoprophylaxis and sporozoite (CPS) immunization] induces long-lasting sterile protection against a homologous challenge infection (3, 4). The unprecedented efficacy of the CPS immunization model is represented by the low dose sufficient to induce protection, i.e., three times 12–15 infected mosquito bites, compared with 1,000 bites required in the irradiated sporozoite approach (5).
Chloroquine kills only developing blood stages of P. falciparum, without affecting sporozoites or liver stages (6). This results in transient low-level blood-stage parasitemia during CPS immunization (3). Consequently, the host’s immune system will be exposed to a relatively broad repertoire of antigens, including sporozoite, liver-stage, and early blood-stage antigens. Humoral and cellular immune responses are induced against both sporozoites and blood stages (3, 7). In addition, many antigens are shared between these stages (8), leaving open the possibility that the observed protection may be mediated by immune responses against either of these parasite life cycle stages or a combination thereof (9). The absence of parasitemia after challenge infection and the predominant induction of preerythrocytic antibodies suggest that preerythrocytic immunity primarily is responsible for protection, although a possible requirement for immune responses against asexual stages cannot be ruled out (3). Indeed, previously it was shown that exposure to very low densities of blood stages may induce protection in the controlled human malaria infection model (10). In this study, protected subjects displayed strong parasite-specific T-cell proliferation and IFN-γ production (10). Moreover, CPS-immunized volunteers also exhibited strong IFN-γ responses upon in vitro restimulation with infected erythrocytes (7).
To explore the possible role of immunity against the preerythrocytic and/or blood stage in protection, CPS-immunized volunteers were subjected to either a P. falciparum sporozoite or an asexual blood-stage challenge. Because the latter approach completely bypasses the liver stages, any protection seen would indicate that blood-stage immunity may contribute to CPS-induced protection.
Results
Twenty-five of 42 screened subjects (median age 21 y; range 19–32 y) were included in the study (Fig. S1). Fifteen volunteers were immunized according to the CPS protocol as described previously (3). Briefly, while taking chloroquine prophylaxis, volunteers (groups 1 and 2) were exposed to bites of 15 P. falciparum-infected mosquitoes (8 mosquitoes with the NF54 strain and 7 mosquitoes with the 3D7 clone) at monthly intervals for a period of 3 mo. Control volunteers (groups 3 and 4) received chloroquine prophylaxis only. One subject in group 1 withdrew consent after the third immunization for reasons unrelated to the trial.
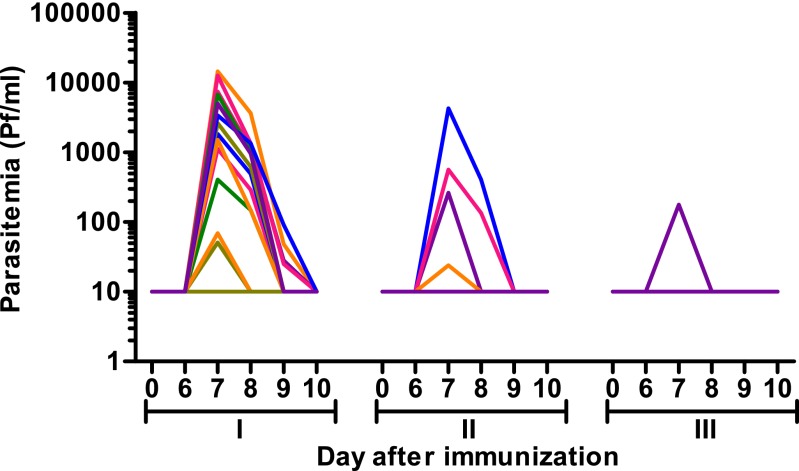
After the first immunization, 14 of 15 subjects (groups 1 and 2) developed transient low blood-stage parasitemia, as retrospectively detected by quantitative real time PCR (qPCR). The geometric mean of peak parasitemia was 1,378 parasites per milliliter [95% confidence interval (CI), 456–4,165 parasites per milliliter; Fig. 1). Thick smears remained negative, except in two subjects (one each in groups 1 and 2) who developed a positive thick smear on day 7. Their peak parasitemia was 14,454 and 6,761 P. falciparum per milliliter. Both the severity and frequency of adverse events (AEs) were similar to those in the other subjects, and chloroquine plasma concentrations were within the prophylactic range (53 and 56 µg/L). These two subjects were treated promptly with atovaquone/proguanil and continued study participation according to protocol. All subjects in groups 1 and 2 reported solicited AEs (mean duration, 1.0 ± 0.11 d) after the first immunization. The most common AEs were headache (13/15 subjects), and fever and nausea (both in 8/15 subjects). Four subjects experienced a grade 3 AE (headache n = 2, malaise n = 2; mean duration 1.8 ± 0.6 d), which all occurred between days 7 and 10 after the first immunization and were considered probably related to the immunization.
Fig. 1.
Blood-stage parasitemia during CPS immunization. Blood-stage parasitemia was measured from day 6 until day 10 after the first (I), second (II), and third (III) immunization by qPCR. Each line represents an individual subject (n = 15); values shown as 10 on the logarithmic scale were negative.
After the second immunization, four subjects developed parasitemia by qPCR (geometric mean peak parasitemia, 351 parasites per milliliter; 95% CI, 43–2,857; Fig. 1), whereas thick smears remained negative. Two subjects experienced mild or moderate AEs. After the third immunization, only one subject showed blood-stage parasitemia (178 parasites per milliliter; Fig. 1) and three subjects experienced mild AEs. No serious AEs occurred during the trial.
Antibody levels against the circumsporozoite protein (CSP), apical membrane antigen 1 (AMA-1), and glutamate-rich protein (GLURP) were measured before CPS immunization and before challenge. CPS-immunized subjects (13/14) showed induction of anti-CSP antibodies (at least a twofold increase in antibody titer), whereas only a single subject (group 1) showed a minimal increase in AMA-1 and GLURP antibody titers (Table 1). IgG was isolated from plasma of all immunized subjects at baseline and before challenge infection. In vitro blood-stage growth inhibition assay (GIA) did not show an inhibitory effect of purified IgG on blood-stage parasite growth in any of the subjects (Table 1).
Table 1.
Antibody titers and in vitro growth inhibition
| Test | I-7 | C-1 | Δ[(C-1) − (I-7)] | P value |
| Antibody titer, AU | ||||
| CSP | 0.91 (0; 2.15) | 24.3 (15.2; 48.5) | 24.3 (10.8; 47.3) | 0.006 |
| AMA-1 | 0.12 (0.08; 0.17) | 0.11 (0.09; 0.17) | 0.00 (−0.03; 0.01) | 0.35 |
| GLURP | 1.23 (1.01; 2.39) | 1.06 (0.90; 3.39) | −0.13 (−0.35; 0.14) | 0.46 |
| Growth inhibition, % | −3.3 (−7.1; −1.1) | −8.6 (−10.0; −5.46) | −4.4 (−6.0; 1.8) | 0.03 |
Antibody titers against CSP, AMA-1, and GLURP and in vitro growth inhibitory activity of isolated IgG in all CPS-immunized subjects, before immunization (I-7) and on the day before challenge (C-1). Data are expressed as median (25; 75 percentile). Differences between time points were tested using a paired t test. AU, arbitrary units.
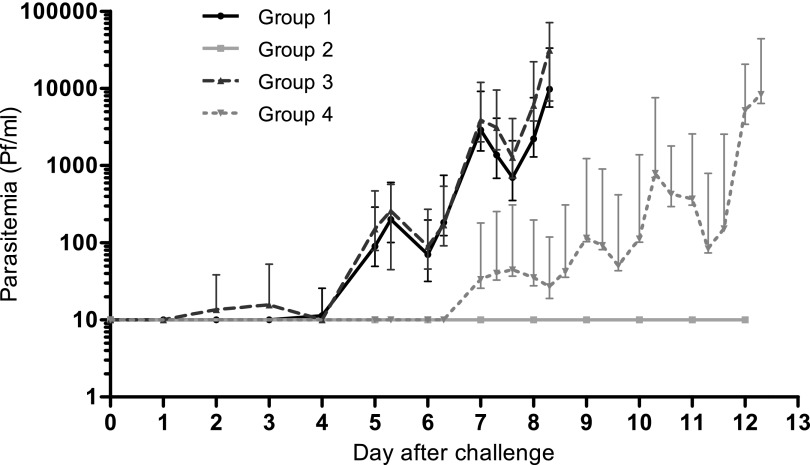
The minimum therapeutic plasma chloroquine concentration is 30 μg/L (11), and its reported half-life varies from 5 to 58 d (11, 12). To ensure sufficient clearance of chloroquine in view of the very low blood-stage challenge dose, the challenge infection was conducted 17 wk after the last chloroquine dose, corresponding to 21 wk after the last immunization. Group 1 (n = 9) and group 3 (n = 5) received a blood-stage challenge by i.v. administration of 3D7 asexual parasites. Group 2 (n = 5) and group 4 (n = 5) were subjected to a sporozoite challenge using five mosquitoes infected with 3D7 sporozoites (13). There was no difference in parasitemia between CPS-immunized group 1 and control group 3; both groups became thick smear positive, with a median prepatent period of 8.0 d (range 7.0–8.3 d and 8.0–8.3 d, respectively; P = 0.83). Likewise, the prepatent period by qPCR was similar in both groups (median 5.0 d; range 3.0–5.3 d and 2.0–6.3 d, respectively; P = 0.41; Table 2). Furthermore, there was no statistically significant difference in multiplication rates of blood-stage parasites between the CPS-immunized subjects and naïve controls [median 8 (range 6–18) and 14 (range 7–24), respectively; P = 0.19; Table 2 and Fig. 2).
Table 2.
Protection against blood-stage versus sporozoite challenge by CPS immunization
| Prepatent period, d, median (range) |
||||||
| Challenge | Protected/total no. of volunteers | Protection, % | Thick smear | PCR | Blood-stage parasite multiplication rate, median (range) | |
| Immunized | Sporozoite | 5/5 | 100 | N/A | N/A* | N/A |
| Blood stage | 0/9 | 0 | 8.0 (7.0–8.3) | 5.0 (3.0–5.3) | 8 (6-18) | |
| Control | Sporozoite | 0/5 | 0 | 12.3 (9.3–12.3) | 9.0 (7.0–10.0) | 10 (5-23) |
| Blood stage | 0/5 | 0 | 8.0 (8.0–8.3) | 5.0 (2.0–6.3) | 14 (7-24) | |
One subject became PCR positive on day 21 after challenge. N/A, not applicable.
Fig. 2.
Parasitemia after challenge as assessed by qPCR. Geometric mean parasite density ±95% CI from day of inoculation until the day of treatment after blood-stage challenge [black line, group 1, CPS-immunized (n = 9); dashed dark gray line, group 3, controls (n = 5)] or sporozoite challenge [light gray line, group 2, CPS-immunized (n = 5); dashed light gray line, group 4, controls (n = 5)]. Values shown as 10 on the logarithmic scale were negative.
In group 2, challenged with sporozoites, four of five CPS-immunized subjects remained negative throughout the follow-up period by both thick smear and qPCR. One CPS-immunized subject, however, showed a positive qPCR at day 21 post challenge (457 parasites per milliliter, determined retrospectively). Controls in group 4 all became thick smear positive, with a median prepatent period of 12.3 d (range 9.3–12.3; multiplication rate 10, range 5–23). Parasitemia in group 4 (sporozoite-challenged controls) developed approximately 4 d later compared with group 3 (blood stage-challenged controls; day 5 vs. 9 by qPCR, P = 0.01, and day 8 vs. 12.3 by thick smear, P = 0.01; Table 2). The range in prepatent periods was significantly smaller in group 3 (8.0–8.3 d) than in group 4 (9.3–12.3 d). The parasite multiplication rate in the blood of control subjects was similar in those receiving either a blood-stage or sporozoite challenge [14 (7–24) vs. 10 (5–23), respectively; P = 0.57].
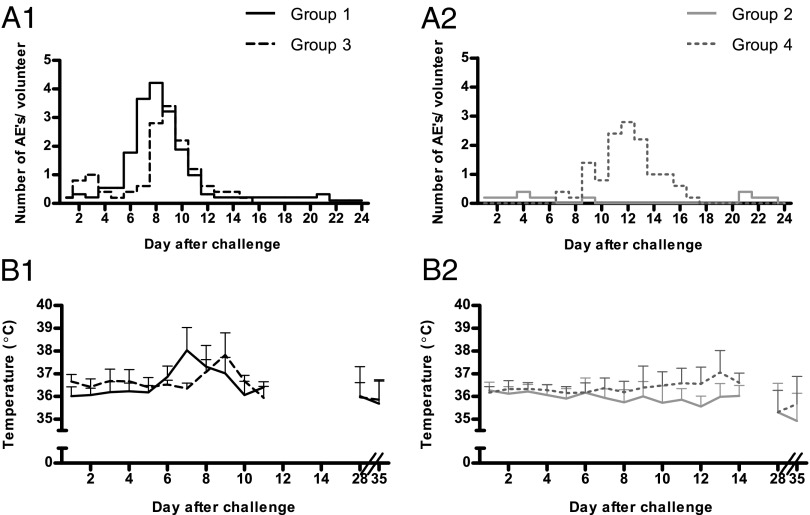
All 19 unprotected volunteers reported solicited AEs considered possibly or probably related to the challenge (mean number of AEs per subject, 6.4; mean duration, 1.4 ± 0.1 d), including headache, fever, and nausea as the most common symptoms (Table S1). The peak of AEs occurred later in subjects who received a sporozoite challenge, concordant with the later onset of parasitemia, but there was no difference in accumulative duration of AEs compared with blood stage-challenged controls (Fig. 3 A1 and A2; P = 0.24). In contrast, protected subjects showed significantly fewer AEs: three of five experienced mild or moderate AEs (mean number/subject, 1.4; mean duration, 0.3 ± 0.1 d; P = 0.002 compared with unprotected subjects; Fig. 3A2).
Fig. 3.
AEs and temperature after challenge. AEs and body temperature were recorded daily after challenge. Blood-stage challenge (A1) and sporozoite challenge (A2): mean number of possibly or probably related solicited AEs per subject. Blood-stage challenge (B1) and sporozoite challenge (B2): temperature (mean +SEM). Black line, group 1 (CPS-immunized, blood-stage challenge; n = 9); dashed dark gray line, group 3 (controls, blood-stage challenge; n = 5); light gray line, group 2 (CPS-immunized, sporozoite challenge; n = 5); dashed light gray line, group 4 (controls, sporozoite challenge; n = 5).
Lymphocyte counts decreased after challenge in all unprotected subjects (Fig. 4A), as did platelet counts (Fig. 4B), with the exception of one volunteer. Platelet counts declined below the lower limit of normal (150 × 109/L) in 9 of 19 unprotected subjects (mean lowest value 132 ± 10 × 109/L). D-dimer concentrations were elevated in all thick smear-positive subjects (n = 19; mean peak concentration 3,908 ± 650 ng/mL; Fig. 4C). All abnormal laboratory values normalized without complications; bleeding or thrombotic complications were not detected in any of the subjects.
Fig. 4.
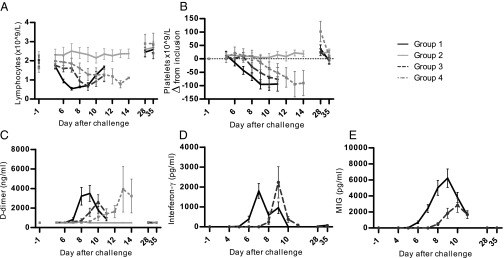
Hematological and inflammatory markers after challenge. (A) Peripheral lymphocyte counts (×109/L). (B) Platelet counts (difference from value at inclusion, ×109/L). (C) D-dimer (nanograms per milliliter). (D) IFN-γ (picograms per milliliter). (E) MIG (picograms per milliliter). Black line, group 1 (CPS-immunized, blood-stage challenge; n = 9); dashed dark gray line, group 3 (controls, blood-stage challenge; n = 5); light gray line, group 2 (CPS-immunized, sporozoite challenge; n = 5); dashed light gray line: Group 4 (controls, sporozoite challenge; n = 5). Data are shown as mean ± SEM.
There was a remarkable difference in occurrence of fever and AEs between blood stage-challenged groups 1 and 3, although curves of developing parasitemia were identical (Figs. 2 and 3). CPS-immunized subjects in group 1 developed fever at a significantly earlier time point than controls in group 3 (mean first day of temperature ≥37.5 °C, day 7.25 vs. day 8.5; P = 0.002; Fig. 3B1), concordant with an earlier mean decline in lymphocytes (Fig. 4A; P < 0.01) and increase in D-dimer concentrations (mean first day of D-dimer >1,000 ng/mL, day 8.0 vs. day 9.0; P = 0.05; Fig. 4C).
We next investigated plasma concentrations of IFN-γ, a key mediator of cellular immunity in malaria (14), and monokine induced by IFN-γ (MIG), a downstream mediator in the IFN-γ pathway (15). Fig. 4 D and E shows distinct increases in both IFN-γ and MIG plasma concentrations upon blood-stage challenge at 2–3 d earlier in group 1 compared with group 3 (P < 0.001).
Discussion
This study shows that sporozoite immunization by P. falciparum-infected mosquito bites of human subjects while taking chloroquine chemoprophylaxis (CPS immunization) does not protect against an i.v. administered blood-stage challenge infection. The presence of transient low-level parasitemia during CPS immunization is sufficient to induce immune recognition of asexual forms, as indicated by an earlier increase of IFN-γ and MIG after blood-stage challenge. However, these responses apparently are insufficient to confer any functional blood-stage immunity. In contrast, complete protection is obtained against a sporozoite challenge by mosquito bites as described before (3, 4).
The previously observed absence of detectable parasitemia in CPS-immunized subjects after mosquito challenge suggested predominance of preerythrocytic immunity, but asexual stage immunity might have contributed to protection (3). In the present study, however, the complete lack of any sign of clinical and/or parasitological protection against even an unphysiologically low blood-stage infection [<2,000 ring forms, i.e., 20-fold lower than an estimated average of 40,000 merozoites released from a single infected hepatocyte (16)], suggests the complete absence of any functional blood-stage immunity. This is supported by the lack of antibodies against blood-stage antigens after CPS immunization in all but one volunteer, and the absence of in vitro growth-inhibitory activity of IgG isolated from CPS-immunized subjects. The single volunteer who developed detectable, although very low, AMA-1 and GLURP antibody levels was the only subject who experienced qPCR-detectable blood-stage parasitemia after all three immunizations. One immunized subject developed parasitemia on day 21 after sporozoite challenge, as retrospectively detected by qPCR. The blood-stage parasite multiplication rate after controlled human malaria infection in malaria-naïve subjects is 10.9 on average, but may be as low as 2 (17). With a multiplication rate of 2, a load of 457 parasites per milliliter on day 21 would be the result of an estimated 9,000 merozoites released from the liver (i.e., one infected hepatocyte). Given the total lack of in vivo protection from blood-stage challenge and in vitro growth-inhibitory activity of IgG in all immunized subjects, the delayed prepatency in this volunteer most likely was caused by either a profound reduction in liver-stage burden or a prolonged liver stage and therefore delayed release of merozoites into the blood.
Although clinical immunity and control of blood-stage parasitemia are acquired with repeated parasite exposure in endemic populations, the occurrence of sterile protection mediated by sporozoite/liver stages alone has not been confirmed (18). Hence, this study is a unique and unambiguous demonstration of induction of sterile preerythrocytic immunity generated against nonattenuated wild-type P. falciparum sporozoites. Sterile protection induced by immunization with irradiated sporozoites that arrest early after liver cell invasion also most likely is based on preerythrocytic immunity (5). In this situation, asexual forms never occur, and the apparent lack of blood-stage immunity was shown in the 1970s in a single irradiated sporozoite-immunized volunteer challenged with blood-stage parasites (19).
Blood stage-challenged subjects in our study showed neither a delay in the prepatent period nor a reduction in asexual multiplication rate compared with naïve controls. This is remarkable because several studies have shown that protective immunity to P. falciparum blood stages can be induced readily after very few infections (10, 20): (i) Adult patients treated with prolonged P. falciparum infections for neurosyphilis in the 1920s and 1930s showed clear evidence of clinical and parasitological immunity during a second asexual blood-stage infection by a decrease in frequency of fever and parasitemia (20). (ii) Repeated administration of ±30 P. falciparum-infected erythrocytes followed by early treatment with atovaquone/proguanil induced protection against blood-stage challenge (10) in three of four subjects, although a potential effect of residual atovaquone blood levels could not be ruled out (21). A plausible explanation for the absence of blood-stage protection might be the short duration and low grade of parasitemia as a result of the use of chloroquine and hence insufficient exposure to blood-stage antigens. Induction of protective immunity against blood stages requires several cycles of parasite replication and sufficient duration of parasitemia (20, 22). Even in the trial by Pombo et al. (10), in which subjects were immunized with unphysiologically low numbers of blood-stage parasites, treatment was initiated only after 8 or 14 d, allowing at least four replication cycles and therefore sufficiently long exposure to blood-stage antigens. This stands in contrast to the CPS immunization protocol, in which prophylactic levels of chloroquine constantly are present, preventing a full blood-stage replication cycle of parasites. Thus, although the occurrence of low parasitemia during CPS immunization might benefit the induction of preerythrocytic immunity as a result of the expression of cross-stage antigens, it clearly is insufficient to induce a functional protective immune response against blood stages.
Notwithstanding the absence of protection against blood-stage challenge, we do find evidence for immune recognition of blood stages. Previously, we showed that P. falciparum-infected erythrocytes elicit release of IFN-γ, mainly from innate cells including natural killer and γδT cells (7, 23, 24). This innate response may be enhanced through and supplemented by adaptive memory T cells producing cytokines (25). In addition, effector memory T cells produce IL-2 and IFN-γ upon in vitro restimulation with P. falciparum-infected erythrocytes (7). In the present study, CPS-immunized subjects, while unprotected against a blood-stage challenge, showed an earlier in vivo peak of plasma IFN-γ in the course of blood-stage infection than naïve controls, despite identical kinetics of developing parasitemia. Early recognition of blood stages by memory cells in these immunized subjects apparently led to an accelerated and enhanced production of IFN-γ and further downstream mediators, including the chemokine MIG (15). MIG may have contributed to the observed earlier lymphocyte recruitment out of the peripheral circulation. The clinical and laboratory signs/symptoms in the unprotected CPS-immunized and blood stage-challenged volunteers (group 1) most likely represent a shift of inflammatory responses common to malaria (26) to earlier time points compared with the challenged controls. Thus, immune recognition represented by these markers took place at an earlier time point in CPS-immunized individuals compared with naïve volunteers, suggesting the presence of memory responses to asexual blood stages despite the absence of protection.
Mechanisms and target antigens for protective immunity induced by CPS immunization remain to be unraveled. Although mainly antibodies are important in controlling blood-stage parasitemia (27), rodent and primate studies indicate that CD8+ T-cell responses against parasite liver stages are critically involved in preerythrocytic immunity (28–30). Therefore, detailed analysis of T-cell responses will be the subject of future studies.
Furthermore, this efficient immunization model will enable studies of antigen specificity of cellular and humoral immune responses for identification of potential new antigens or combinations thereof for subunit vaccine candidates. Malaria vaccine development to date has been stage oriented, aimed at targeting either the preerythrocytic or asexual blood stage of the parasite. Vaccines against asexual blood-stage antigens likely will not prevent infection, but instead may reduce parasite densities and provide protection against clinical disease. Preerythrocytic immunization strategies such as CPS immunization, however, induce sterile protection, thereby preventing blood-stage infection (31).
In conclusion, sporozoite immunization by the CPS protocol may induce sterile protection entirely mediated by immune responses against the preerythrocytic stages of P. falciparum. These findings support a continued focus on vaccine development toward preerythrocytic stages, particularly whole-sporozoite approaches.
Materials and Methods
Study Design.
We conducted this single-center, open-label study at the Radboud University Nijmegen Medical Centre (Nijmegen, The Netherlands) from April 2011 until March 2012 following approval by the Central Committee for Research Involving Human Subjects of The Netherlands (CCMO NL34273.091.10). The study team complied with the Declaration of Helsinki and Good Clinical Practice, including monitoring of data. The trial is registered at ClinicalTrials.gov (NCT01236612). Written informed consent of all volunteers was obtained before screening.
Twenty-five healthy subjects (age 18–35 y) without a history of malaria or residence in a malaria-endemic area in the 6 mo before study entry were included (SI Materials and Methods, Screening of Study Subjects) and randomly assigned to four groups (groups 1, 2, 3, and 4; Fig. S1). Fifteen subjects received CPS immunization (groups 1 and 2) as described in detail in SI Materials and Methods. Ten controls (groups 3 and 4) received only chloroquine chemoprophylaxis.
Seventeen weeks after discontinuation of chloroquine prophylaxis, corresponding to 21 wk after the last immunization, all subjects received a challenge infection. Group 1 (n = 9; 1 lost to follow-up) and control group 3 (n = 5) were challenged by i.v. administration of 1,962 viable 3D7 P. falciparum-infected erythrocytes (blood-stage challenge), which were derived from a stock produced at the Queensland Institute of Medical Research as described previously (32) and used in numerous studies (10, 32–36). Group 2 (n = 5) and group 4 (n = 5) were exposed to the bites of five 3D7 P. falciparum-infected Anopheles stephensi mosquitoes (sporozoite challenge). Subjects and investigators were aware of the study group, whereas primary outcome assessors were kept blinded to the allocation. All volunteers were treated with a curative regimen of antimalarial drugs at the time of thick smear positivity, or presumptively on day 21 after challenge if thick smears remained negative.
Study Outcomes.
The primary study outcome was time to parasitemia after challenge, as assessed by microscopy (SI Materials and Methods). The prepatent period was defined as the period between challenge and the first positive thick smear. Volunteers were defined as protected from challenge if they remained thick smear negative until day 21. Additionally, parasitemia was measured retrospectively by real-time qPCR (37). Blood-stage parasite multiplication rate was calculated as described previously (17). Assessment of in vitro growth inhibition and measurements of antibodies, hematological parameters, MIG, and IFN-γ are described in detail in SI Materials and Methods.
Statistical Methods.
Statistical analysis was performed using GraphPad Prism 5. The difference in AEs among groups was calculated by unpaired Student t test on the accumulative duration of AEs. Differences among groups on the first day of fever (≥37.5 °C), first day of D-dimer increase above two times the upper limit of normal (≥1,000 ng/mL), and first day of detectable IFN-γ and MIG were tested by unpaired Student t test. Differences among groups in prepatent periods by thick smear and qPCR and blood-stage parasite multiplication rates were tested by the Mann–Whitney test. Differences in antibody levels and in vitro growth inhibition between time points were tested by paired t test.
Analysis of lymphocyte kinetics after challenge was performed with SPSS version 18 and based on data obtained at days 5, 6, and 7 (pretreatment). Two regression-type models were fitted to the data. The dependent variable was lymphocyte number, and independent variables were time, treatment, the interaction between time and treatment, and the baseline observation of the dependent variable. The longitudinal character of the data was accommodated using general least-squares estimation; a heterogeneous, unstructured covariance matrix was assumed.
Supplementary Material
Acknowledgments
We thank all the trial volunteers, the staff from the Clinical Research Centre Nijmegen, and the staff from the Radboud University Nijmegen Medical Centre Pharmacy who made this study possible; P. Beckers, W. Arts, N. Huiberts, C. Siebes, M. Kooreman, P. Daemen, and E. Driessen for reading many thick smears; and G. Pop for his cardiac monitoring of the trial volunteers. We also thank J. Klaassen, L. Pelser-Posthumus, J. Kuhnen, and A. Pouwelsen for assistance in generating infected mosquitoes and in immunizing and challenging the volunteers; J. McCarthy (Queensland Institute of Medical Research) for providing the blood-stage inoculum and his advice on the blood-stage challenge; A. Hill, S. Draper, and their team at Oxford University for providing protocols for preparation of the blood-stage inoculum; P. C. van Krimpen (Sanquin) for her excellent advice and guidance; the members of the Safety Monitoring Committee, M. Good, M. Serlie, and A. Rennings for participation, guidance, and safety recommendations throughout the trial; R. Donders for advice and help with the statistical analysis; M. Schoneveld for performance of the troponin T measurements; P. Houze for the chloroquine measurements; E. Brienen (Leiden University Medical Center) for performing DNA isolation and qPCR; Nicole van der Werff for performing the GIA; and CromSource for clinical monitoring. This study was supported by a grant from the European Sixth Framework Programme integrated project European Malaria Vaccine Development Association (EMVDA; Contract LSHP-CT-2007-037506). A.C.T. was funded by the EMVDA. A.S. received a European Molecular Biology Organization long-term fellowship.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1220360110/-/DCSupplemental.
References
- 1.World Health Organization . World Malaria Report 2011. Geneva: World Health Organization; 2011. [Google Scholar]
- 2.Sauerwein RW. Clinical malaria vaccine development. Immunol Lett. 2009;122(2):115–117. doi: 10.1016/j.imlet.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Roestenberg M, et al. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med. 2009;361(5):468–477. doi: 10.1056/NEJMoa0805832. [DOI] [PubMed] [Google Scholar]
- 4.Roestenberg M, et al. Long-term protection against malaria after experimental sporozoite inoculation: An open-label follow-up study. Lancet. 2011;377(9779):1770–1776. doi: 10.1016/S0140-6736(11)60360-7. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman SL, et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002;185(8):1155–1164. doi: 10.1086/339409. [DOI] [PubMed] [Google Scholar]
- 6.Yayon A, Vande Waa JA, Yayon M, Geary TG, Jensen JB. Stage-dependent effects of chloroquine on Plasmodium falciparum in vitro. J Protozool. 1983;30(4):642–647. doi: 10.1111/j.1550-7408.1983.tb05336.x. [DOI] [PubMed] [Google Scholar]
- 7.Teirlinck AC, et al. Longevity and composition of cellular immune responses following experimental Plasmodium falciparum malaria infection in humans. PLoS Pathog. 2011;7(12):e1002389. doi: 10.1001371/journal.ppat.1002389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tarun AS, et al. A combined transcriptome and proteome survey of malaria parasite liver stages. Proc Natl Acad Sci USA. 2008;105(1):305–310. doi: 10.1073/pnas.0710780104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butler NS, Vaughan AM, Harty JT, Kappe SH. Whole parasite vaccination approaches for prevention of malaria infection. Trends Immunol. 2012;33(5):247–254. doi: 10.1016/j.it.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Pombo DJ, et al. Immunity to malaria after administration of ultra-low doses of red cells infected with Plasmodium falciparum. Lancet. 2002;360(9333):610–617. doi: 10.1016/S0140-6736(02)09784-2. [DOI] [PubMed] [Google Scholar]
- 11.Rombo L, Bergqvist Y, Hellgren U. Chloroquine and desethylchloroquine concentrations during regular long-term malaria prophylaxis. Bull World Health Organ. 1987;65(6):879–883. [PMC free article] [PubMed] [Google Scholar]
- 12.Frisk-Holmberg M, Bergqvist Y, Termond E, Domeij-Nyberg B. The single dose kinetics of chloroquine and its major metabolite desethylchloroquine in healthy subjects. Eur J Clin Pharmacol. 1984;26(4):521–530. doi: 10.1007/BF00542151. [DOI] [PubMed] [Google Scholar]
- 13.Sauerwein RW, Roestenberg M, Moorthy VS. Experimental human challenge infections can accelerate clinical malaria vaccine development. Nat Rev Immunol. 2011;11(1):57–64. doi: 10.1038/nri2902. [DOI] [PubMed] [Google Scholar]
- 14.McCall MB, Sauerwein RW. Interferon-γ—central mediator of protective immune responses against the pre-erythrocytic and blood stage of malaria. J Leukoc Biol. 2010;88(6):1131–1143. doi: 10.1189/jlb.0310137. [DOI] [PubMed] [Google Scholar]
- 15.Farber JM. A macrophage mRNA selectively induced by gamma-interferon encodes a member of the platelet factor 4 family of cytokines. Proc Natl Acad Sci USA. 1990;87(14):5238–5242. doi: 10.1073/pnas.87.14.5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shortt HE, Fairley NH, Covell G, Shute PG, Garnham PC. The pre-erythrocytic stage of Plasmodium falciparum. Trans R Soc Trop Med Hyg. 1951;44(4):405–419. doi: 10.1016/s0035-9203(51)80019-1. [DOI] [PubMed] [Google Scholar]
- 17.Roestenberg M, de Vlas SJ, Nieman A-E, Sauerwein RW, Hermsen CC. Efficacy of preerythrocytic and blood-stage malaria vaccines can be assessed in small sporozoite challenge trials in human volunteers. J Infect Dis. 2012;206(3):319–323. doi: 10.1093/infdis/jis355. [DOI] [PubMed] [Google Scholar]
- 18.Doolan DL, Dobaño C, Baird JK. Acquired immunity to malaria. Clin Microbiol Rev. 2009;22(1):13–36. doi: 10.1128/CMR.00025-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clyde DF. Immunity to falciparum and vivax malaria induced by irradiated sporozoites: A review of the University of Maryland studies, 1971-75. Bull World Health Organ. 1990;68(Suppl):9–12. [PMC free article] [PubMed] [Google Scholar]
- 20.Collins WE, Jeffery GM. A retrospective examination of secondary sporozoite- and trophozoite-induced infections with Plasmodium falciparum: Development of parasitologic and clinical immunity following secondary infection. Am J Trop Med Hyg. 1999;61(1) Suppl:20–35. doi: 10.4269/tropmed.1999.61-020. [DOI] [PubMed] [Google Scholar]
- 21.Edstein MD, et al. Lengthy antimalarial activity of atovaquone in human plasma following atovaquone-proguanil administration. Antimicrob Agents Chemother. 2005;49(10):4421–4422. doi: 10.1128/AAC.49.10.4421-4422.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baird JK, et al. Onset of clinical immunity to Plasmodium falciparum among Javanese migrants to Indonesian Papua. Ann Trop Med Parasitol. 2003;97(6):557–564. doi: 10.1179/000349803225001472. [DOI] [PubMed] [Google Scholar]
- 23.Artavanis-Tsakonas K, et al. Activation of a subset of human NK cells upon contact with Plasmodium falciparum-infected erythrocytes. J Immunol. 2003;171(10):5396–5405. doi: 10.4049/jimmunol.171.10.5396. [DOI] [PubMed] [Google Scholar]
- 24.Hermsen CC, et al. Circulating concentrations of soluble granzyme A and B increase during natural and experimental Plasmodium falciparum infections. Clin Exp Immunol. 2003;132(3):467–472. doi: 10.1046/j.1365-2249.2003.02160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCall MB, et al. Memory-like IFN-γ response by NK cells following malaria infection reveals the crucial role of T cells in NK cell activation by P. falciparum. Eur J Immunol. 2010;40(12):3472–3477. doi: 10.1002/eji.201040587. [DOI] [PubMed] [Google Scholar]
- 26.Schofield L, Grau GE. Immunological processes in malaria pathogenesis. Nat Rev Immunol. 2005;5(9):722–735. doi: 10.1038/nri1686. [DOI] [PubMed] [Google Scholar]
- 27.Cohen S, McGregor IA, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 28.Belnoue E, et al. Protective T cell immunity against malaria liver stage after vaccination with live sporozoites under chloroquine treatment. J Immunol. 2004;172(4):2487–2495. doi: 10.4049/jimmunol.172.4.2487. [DOI] [PubMed] [Google Scholar]
- 29.Schofield L, et al. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987;330(6149):664–666. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- 30.Weiss WR, Sedegah M, Beaudoin RL, Miller LH, Good MF. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc Natl Acad Sci USA. 1988;85(2):573–576. doi: 10.1073/pnas.85.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodman AL, Draper SJ. Blood-stage malaria vaccines—recent progress and future challenges. Ann Trop Med Parasitol. 2010;104(3):189–211. doi: 10.1179/136485910X12647085215534. [DOI] [PubMed] [Google Scholar]
- 32.Cheng Q, et al. Measurement of Plasmodium falciparum growth rates in vivo: A test of malaria vaccines. Am J Trop Med Hyg. 1997;57(4):495–500. doi: 10.4269/ajtmh.1997.57.495. [DOI] [PubMed] [Google Scholar]
- 33.Duncan CJ, et al. Impact on malaria parasite multiplication rates in infected volunteers of the protein-in-adjuvant vaccine AMA1-C1/Alhydrogel+CPG 7909. PLoS One. 2011;6(7):e22271. doi: 10.1371/journal.pone.0022271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawrence G, et al. Effect of vaccination with 3 recombinant asexual-stage malaria antigens on initial growth rates of Plasmodium falciparum in non-immune volunteers. Vaccine. 2000;18(18):1925–1931. doi: 10.1016/s0264-410x(99)00444-2. [DOI] [PubMed] [Google Scholar]
- 35.McCarthy JS, et al. A pilot randomised trial of induced blood-stage Plasmodium falciparum infections in healthy volunteers for testing efficacy of new antimalarial drugs. PLoS One. 2011;6(8):e21914. doi: 10.1371/journal.pone.0021914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanderson F, et al. Blood-stage challenge for malaria vaccine efficacy trials: A pilot study with discussion of safety and potential value. Am J Trop Med Hyg. 2008;78(6):878–883. [PubMed] [Google Scholar]
- 37.Adegnika AA, et al. Microscopic and sub-microscopic Plasmodium falciparum infection, but not inflammation caused by infection, is associated with low birth weight. Am J Trop Med Hyg. 2006;75(5):798–803. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.