Remarkably, many bacteria live and thrive in the earth’s subsurface by respiring extracellular insoluble minerals. Okamato et al. (1) in PNAS report how this process may be accelerated by the presence of flavins that bind as cofactors to electron transport proteins on the cell surface that are key to extracellular mineral respiration.
Humans obtain the energy needed for life by respiring oxygen. This process involves using electrons extracted from food to reduce oxygen into water in our mitochondria. Free energy is released in this process, and we use this to make ATP, which is the universal energy currency of life. Our dependency on oxygen makes us obligate aerobes—take away the oxygen and we die. Thus, humans are confined to living on the surface of planet Earth where oxygen is freely available. However, the vast proportion of the earth’s habitable environments is not exploited by humans, but by a diversity of microorganisms, including bacteria, that can live in the absence of oxygen through a process called anaerobic respiration.
Some of the best studied anaerobic respiratory electron acceptors are water soluble, such as nitrate or sulfate, and these anions can be transported relatively easily into bacterial cells where their respiratory reduction occurs. However, insoluble minerals, particularly minerals of iron and manganese, represent some of the most abundant respiratory substrates in the earth's subsurface environments. In fact “mineral iron respiration” is among the most widespread respiratory processes in anoxic zones and so has wide environmental significance (2–4). For example, it directly impacts on the balance of several biogeochemical cycles such as the nitrogen, sulfur, and carbon cycles, and in turn influence the release of potent greenhouse gases, such as nitrous oxide.
In some aspects, the way bacteria respire mineral iron (or manganese) is similar to the way in which our own mitochondria respire oxygen. Electrons generated from organic carbon metabolism are used to “reduce” the respiratory substrate. Thus, electrons generated by metabolism inside the bacterial cell are passed to the mineral iron, which is reduced from the ferric to ferrous state. However, because the ferric iron mineral is an insoluble particle, it cannot freely diffuse into bacterial cells. Consequently, if a bacterium is to be able to use a ferric mineral as a respiratory electron acceptor, it must have a molecular answer to the challenge of moving electrons generated by intracellular metabolism to minerals located outside of the cell (5).
The mechanism by which electrons are transferred to, and across, the microbe–mineral interface is not fully resolved and represents a major question in the study of the biochemistry of an environmentally abundant group of bacteria. Answers will provide new insights into bacterial energetics. They are also likely to have important biotechnological impacts because there is potential for harnessing mineral-respiring bacteria in bioremediation processes for the clean-up of environments contaminated with toxic organic or inorganic pollutants. For example, reduction of soluble uranium (VI) to insoluble uranium (IV) can reduce the levels of uranium in the groundwater of contaminated sites.
Mineral-respiring bacteria also have applications in microbial fuel cells and microbial electrosynthesis, both of which rely on electron exchange between microbes and solid extracellular substrates in the form of electrodes (6). It is such electron exchange, between bacteria and electrodes, that has been studied by Okamato et al. (1) using a technique called differential pulse voltammetry. Monolayer biofilms of the mineral iron-reducing bacterium Shewanella oneidensis were grown on indium tin oxide (ITO) electrodes, and the flow of current due to interfacial electron exchange was measured in the absence and presence of added flavins.
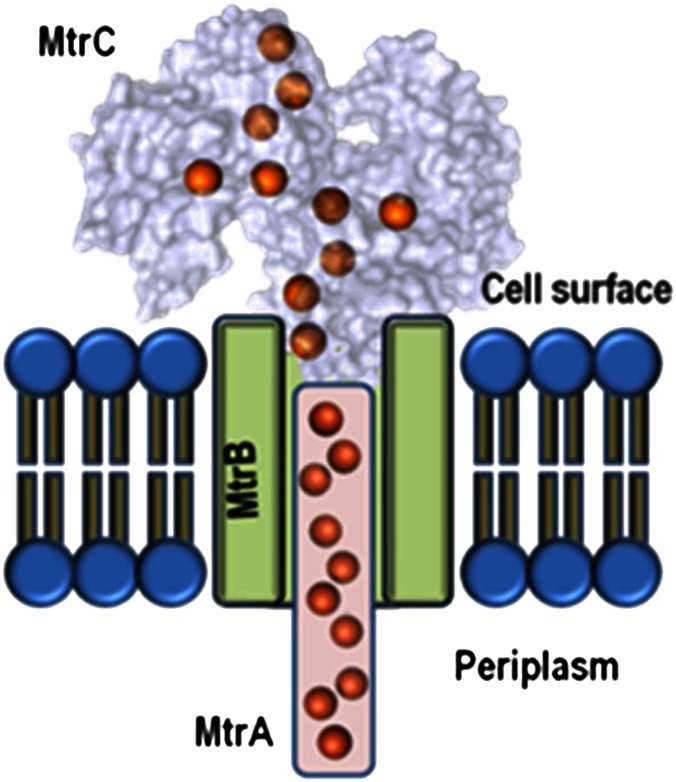
Bacteria from the genus Shewanella respire on extracellular substrates by transporting electrons to the cell surface via porin–cytochrome complexes in the outer membrane (7). These complexes facilitate electron transport by allowing periplasmic cytochromes to transfer electrons to cell-surface cytochromes through a transmembrane porin (Fig. 1). The best characterized of these porin–cytochrome complexes is the metal reduction (MtrCAB) complex from S. oneidensis, which binds a total of 20 electron-carrying heme cofactors that form an electron transfer conduit of some 20 nm.
Fig. 1.
A diagram of the transmembrane 20-heme cytochrome–porin MtrCAB complex. The staggered heme cross indicated in MtrC is expected to be a feature of both MtrC and OmcA studied by Okamoto et al. (1), who have proposed that flavin binds as a cofactor to these proteins and that this binding modulates the redox properties of the flavin, so enhancing the rate of electron transfer from MtrC and OmcA to insoluble extracellular substrates. The red spheres depict heme cofactors.
The Shewanella family of outer membrane multiheme cytochromes (OMMCs) form four major clades—outer membrane cytochrome A (OmcA), metal reducing cytochrome C (MtrC), undecyl cytochrome A (UndA), and metal reducing cytochrome F (MtrF). Recent work (8, 9), provided the first structures of OMMCs from Shewanella and revealed that ten hemes were bound as a “staggered cross” on the cell surface (Fig. 1). Of the Shewanella OMMCs, the S. oneidensis MtrC and OmcA cytochromes that are the subject of the Okamato et al. paper (1) have been the most widely studied. They are exported to the extracellular environment by the type II secretion system (10), and omcA mtrC mutants are severely compromised for respiratory mineral Fe(III) reduction and electron transfer to anodes in microbial fuel cells (11, 12). The OmcA protein may form a 2:1 complex with MtrCAB, resulting in a system with 40 hemes that is referred to as the “OmcA–MtrCAB” complex in the paper of Okamoto et al. (1). Protein film voltammetry measurements have shown that S. oneidensis OmcA and MtrC can transfer electrons rapidly and directly to graphite and hematite electrodes (13–16). Most recently, the MtrCAB complex was reconstituted in proteoliposomes and shown to move electrons rapidly from an intravesicular electron pool to extravesicular nanoparticle suspensions of goethite, hematite, and lepidocrocite (17).
By studying the electron transfer properties of biofilms prepared from wild-type and mutant strains of S. oneidensis, deficient in MtrC or OmcA, Okamato et al. (1) show that flavin molecules secreted by S. oneidensis MR-1 enhance the ability of its OMMCs to transport electrons to ITO electrodes. The reduction potential of flavin added to the biofilm was raised by around 120 mV compared with that of flavin free in solution. Okamato et al. (1) conclude that the flavin is serving as an integral redox cofactor of the bacterium, rather than as part of a freely diffusible flavin pool. They argue that this observation is due to interactions between flavins and OMMCs that stabilize the one-electron reduced semiquinone (SQ) form at the expense of the two-electron reduced hydroquinone form. Thus, whereas free flavin undergoes a reversible two-electron reduction at −260 mV, the flavin bound to OMMCs is proposed to cycle reversibly between the oxidized and SQ states in a one-electron process at −140 mV. The SQ was detected directly using electron paramagnetic resonance. Its presence was dependent on the organic substrate lactate, which is the electron source for reduction of the extracellular electron acceptor (the ITO electrode in this case). These results suggest that the flavin–OMMC interaction allows the rate of extracellular electron transport to be coupled to the levels of intracellular catabolic activity.
Extracellular redox-active flavin in the forms of flavin mononucleotide (FMN) or riboflavin (RF) has been hypothesized to play a major role in the delivery of electrons from cells to extracellular insoluble respiratory substrates. By diffusing between the OMMCs and the solid substrate, the flavins can shuttle two electrons from the microbe to the solid (11, 18). Flavin reductase activity has been observed for the purified OMMCs (8, 19), and mutants deficient in extracellular FMN
The study of Okamoto et al. brings into focus many questions regarding the role of flavins in the respiratory reduction of solid substrates.
or RF production have a severely compromised ability to reduce solid extracellular substrates (20). Okamato et al. (1) argue that the positive shift in flavin reduction potential of ∼120 mV on binding to OMMCs in the biofilm overcomes an otherwise strongly endergonic electron transfer from OMMC to flavin. The result is electron transfer from OMMC to the bound flavin cofactor that is 103- to 105-fold faster than to free flavin. Recent studies on MtrCAB-containing proteoliposomes showed that the MtrCAB porin–cytochrome complex represents the minimal requirement for rapid electron transfer to the microbe–mineral interface (17). However, this work does not exclude a role for flavins in enhancing the rates of electron transfer under certain conditions. Indeed, it is compelling that the OMMCs have some structural features that suggest the possibility of flavin-binding domains that are within electron transfer distance of heme termini in the proteins (8, 9, 21). Here, it is of note that Okamoto et al. (1) find that the specificity of OMMCs for binding flavin species may differ, with OmcA preferentially forming an SQ species with RF and MtrC forming an SQ species with FMN.
The study of Okamoto et al. (1) brings into focus many questions regarding the role of flavins in the respiratory reduction of solid substrates. For example, what is the role for the free extracellular flavin pool for which there is good evidence under certain growth conditions in Shewanella species? How do flavins bind to the OMMCs in a manner that leads to the modulation of their redox properties? Is there indeed selectivity by different OMMCs for different flavin species, and, if so, what is the molecular basis for this discrimination? What role, if any, has the extracellular polymeric matrix secreted by Shewanella species (22) in altering the environment for both OMMC and flavin? The emerging molecular structures for OMMCs should allow for detailed studies of the interaction between flavins and OMMCs using purified proteins and reconstituted proteoliposome systems that will shed light on these questions.
Footnotes
The authors declare no conflict of interest.
See companion article on page 7856.
References
- 1.Okamoto A, Hashimoto K, Nealson KH, Nakamura R. Rate enhancement of bacterial extracellular electron transport involves bound flavin semiquinones. Proc Natl Acad Sci USA. 2013;110:7856–7861. doi: 10.1073/pnas.1220823110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nealson KH, Myers CR. Microbial reduction of manganese and iron: New approaches to carbon cycling. Appl Environ Microbiol. 1992;58(2):439–443. doi: 10.1128/aem.58.2.439-443.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fredrickson JK, Gorby YA. Environmental processes mediated by iron-reducing bacteria. Curr Opin Biotechnol. 1996;7(3):287–294. doi: 10.1016/s0958-1669(96)80032-2. [DOI] [PubMed] [Google Scholar]
- 4.Lovley DR, Holmes DE, Nevin KP. Dissimilatory Fe(III) and Mn(IV) reduction. Adv Microb Physiol. 2004;49:219–286. doi: 10.1016/S0065-2911(04)49005-5. [DOI] [PubMed] [Google Scholar]
- 5.Shi L, et al. Molecular underpinnings of Fe(III) oxide reduction by Shewanella oneidensis MR-1. Front Microbiol. 2012;3:50. doi: 10.3389/fmicb.2012.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ross DE, Flynn JM, Baron DB, Gralnick JA, Bond DR. Towards electrosynthesis in Shewanella: Energetics of reversing the Mtr pathway for reductive metabolism. PLoS ONE. 2011;6(2):e16649. doi: 10.1371/journal.pone.0016649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartshorne RS, et al. Characterization of an electron conduit between bacteria and the extracellular environment. Proc Natl Acad Sci USA. 2009;106(52):22169–22174. doi: 10.1073/pnas.0900086106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke TA, et al. Structure of a bacterial cell surface decaheme electron conduit. Proc Natl Acad Sci USA. 2011;108(23):9384–9389. doi: 10.1073/pnas.1017200108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edwards MJ, Fredrickson JK, Zachara JM, Richardson DJ, Clarke TA. Analysis of structural MtrC models based on homology with the crytsal structure of MtrF. Biochem Soc Trans. 2012;40(6):1181–1185. doi: 10.1042/BST20120132. [DOI] [PubMed] [Google Scholar]
- 10.Shi L, et al. Direct involvement of type II secretion system in extracellular translocation of Shewanella oneidensis outer membrane cytochromes MtrC and OmcA. J Bacteriol. 2008;190(15):5512–5516. doi: 10.1128/JB.00514-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coursolle D, Baron DB, Bond DR, Gralnick JA. The Mtr respiratory pathway is essential for reducing flavins and electrodes in Shewanella oneidensis. J Bacteriol. 2010;192(2):467–474. doi: 10.1128/JB.00925-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reardon CL, et al. Role of outer-membrane cytochromes MtrC and OmcA in the biomineralization of ferrihydrite by Shewanella oneidensis MR-1. Geobiology. 2010;8(1):56–68. doi: 10.1111/j.1472-4669.2009.00226.x. [DOI] [PubMed] [Google Scholar]
- 13.Hartshorne RS, et al. Characterization of Shewanella oneidensis MtrC: A cell-surface decaheme cytochrome involved in respiratory electron transport to extracellular electron acceptors. J Biol Inorg Chem. 2007;12(7):1083–1094. doi: 10.1007/s00775-007-0278-y. [DOI] [PubMed] [Google Scholar]
- 14.Firer-Sherwood M, Pulcu GS, Elliott SJ. Electrochemical interrogations of the Mtr cytochromes from Shewanella: Opening a potential window. J Biol Inorg Chem. 2008;13(6):849–854. doi: 10.1007/s00775-008-0398-z. [DOI] [PubMed] [Google Scholar]
- 15.Eggleston CM, et al. Binding and direct electrochemistry of OmcA, an outer-membrane cytochrome from an iron reducing bacterium, with oxide electrodes: A candidate biofuel cell system. Inorg Chim Acta. 2008;361:769–777. [Google Scholar]
- 16.Meitl LA, et al. Electrochemical interaction of Shewanella oneidensis MR-1 and its outer membrane cytochromes OmcA and MtrC with hematite electrodes. Geochim Cosmochim Acta. 2009;73:5292–5307. [Google Scholar]
- 17.White GF, et al. Rapid electron exchange between surface-exposed bacterial cytochromes and Fe(III) minerals. Proc Natl Acad Sci USA. 2013;110(16):6346–6351. doi: 10.1073/pnas.1220074110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marsili E, et al. Shewanella secretes flavins that mediate extracellular electron transfer. Proc Natl Acad Sci USA. 2008;105(10):3968–3973. doi: 10.1073/pnas.0710525105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ross DE, Brantley SL, Tien M. Kinetic characterization of OmcA and MtrC, terminal reductases involved in respiratory electron transfer for dissimilatory iron reduction in Shewanella oneidensis MR-1. Appl Environ Microbiol. 2009;75(16):5218–5226. doi: 10.1128/AEM.00544-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotloski NJ, Gralnick JA. Flavin electron shuttles dominante extracellular electron transfer by Shewanella oneidensis. MBio. 2013;4(1):e00553-12. doi: 10.1128/mBio.00553-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edwards MJ, et al. The crystal structure of the extracellular 11-heme cytochrome UndA reveals a conserved 10-heme motif and defined binding site for soluble iron chelates. Structure. 2012;20(7):1275–1284. doi: 10.1016/j.str.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 22.Cao B, et al. Extracellular polymeric substances from Shewanella sp. HRCR-1 biofilms: Characterization by infrared spectroscopy and proteomics. Environ Microbiol. 2011;13(4):1018–1031. doi: 10.1111/j.1462-2920.2010.02407.x. [DOI] [PubMed] [Google Scholar]