Abstract
Few mammalian organs vary as dramatically among species as the placenta. This variation is remarkable considering that the placenta’s primary function—transfer of nutrients and waste between mother and offspring—does not differ among species. Evolutionary changes in placental morphology remain poorly understood, with suggestions that parent–offspring conflict or evolutionary changes in life history might drive placental evolution. Here we demonstrate that life history differences among eutherian mammals are associated with major transitions in maternofetal interdigitation and placental invasiveness. We show that the repeated evolution of villous interdigitation is associated with reduced offspring production early in life and an increased lifespan. Further changes in placental morphology that reestablish a larger surface area are also associated with a change back to greater offspring production. After controlling for these differences in interdigitation, we also show that the least invasive placental type is associated with a fast pace of life. We predict that selection for a faster pace of life intensifies parent–offspring conflict, and that the repeated evolution of less-invasive placental structures might have allowed mothers to wrest back control of gestation from the fetus and alter their relative allocation to offspring production across life.
Keywords: aging, senescence, reproduction, placentation
The mammalian placenta varies dramatically between species in a variety of morphological components, most notably in the invasiveness of fetal tissues and their access to maternal blood flow (1, 2). Hemochorial placentation is most invasive and allows fetal tissues to be bathed directly in the maternal blood. In partially invasive endotheliochorial placentas, only the endothelial wall of the maternal blood vessels separates the fetus from the maternal blood (3). Epitheliochorial placentas, the least invasive, have three layers of maternal tissue separating the fetus from maternal blood. Classically, invasive hemochorial placentation was thought to have evolved from noninvasive epitheliochorial placentation (4), and this assumption has been central to theories of placental evolution (5). However, molecular phylogenies have predicted that the noninvasive epitheliochorial placenta is the most recently derived placental morphology and has evolved at least three times independently over mammalian evolution (6). Thus, it is the repeated evolution of less-invasive placentation that requires explanation (7).
Mammal species also differ in the degree of interdigitation between maternal and fetal tissues, a trait that greatly affects the surface area available for exchange between mother and fetus (3). In species with simple villous interdigitation, fetal tissues branch to form villi that are either bathed in maternal blood or covered by maternal tissue. In labyrinthine placentas, fetal capillaries and maternal blood form a complex trophoblastic meshwork, providing a dramatically larger surface area for exchange compared with placentas with villous interdigitation (8). Species with trabecular placentation express an intermediate level of interdigitation between villous and labyrinthine forms (3). Molecular phylogenies indicate that the ancestral mammalian placenta had labyrinthine maternofetal interdigitation, and that villous placentas have evolved at least three times independently (6).
Studies of the independent origins of matrotrophy (i.e., transfer of nutrients from mother to embryo) in nonmammalian taxa, notably fishes, have provided considerable insight into the evolutionary forces that might have shaped mammalian placentation. Recent modeling (9) and empirical research (10, 11) suggest that, in live-bearing fish at least, matrotrophy likely evolves from lecithotrophy (i.e., embryos nourished by yolk from an egg) where food is abundant and predictable, and that placentation facilitates the evolution of life history traits that depend on heavy maternal allocation to reproduction. In the fish genus Poeciliopsis, increased placentation is associated with an increased rate of offspring production early in life, supporting this life history facilitation hypothesis (10).
In contrast, Haig (5) proposed that mother–offspring interactions during pregnancy, as well as aspects of placental physiology, have been shaped by conflicts between parents and their offspring over resource transfer. It has been further argued that invasive placentation evolved from noninvasive placentation in situations of heightened parent–offspring conflict, allowing the fetus greater control of resource transfer (5, 12). Alternatively, we propose that noninvasive placentation could have evolved in response to heightened parent–offspring conflict, giving the mother greater control over resource transfer. Such a transition may have allowed mothers to withhold resources from individual offspring and increase their inclusive fitness through a higher rate of offspring production, when environmental conditions select for such a life history.
In the present study, we examined whether morphology of the mammalian placenta could have been shaped by selection for the optimum life history in a given environment. Such differences in life history also likely influence the intensity of mother–offspring conflict. Previous comparative studies have highlighted that aspects of placental invasiveness explain some of the variation in relative brain size (13), and that placental interdigitation is linked to differences in duration of gestation (7), but whether placental morphology is linked to general life history and pace of life in mammals remains unclear. We gathered data across 60 mammal species on placentation and age-specific mortality, required for robust demographic life history analyses (14). Where more species were available, we increased our sample size (n = 110–155) when examining the relationship between placentation and individual life history traits (Table 1). To test for associations between placental morphology and life history traits, we used phylogenetic generalized least squares (PGLS) models with a variance-covariance matrix extracted from the phylogenetic tree. This method estimates an index of phylogenetic correlation, λ, that is introduced into the analysis to control for phylogenetic inertia (15). We included body mass as a covariate to control for the strong allometric constraints on life history traits and to assess the influence of the pace of life independent of body size (Materials and Methods).
Table 1.
Comparative analyses for effects of placental invasiveness and interdigitation on life history traits
| Difference between intercepts |
||||||||||||
| Test for fit |
Epitheliochorial vs. hemochorial and endothelochorial |
Trabecular and villous vs. labyrinthine |
||||||||||
| n | LRT | df | P | Estimate ± SE | t | Pace of life | Estimate ± SE | t | Pace of life | λ | r2 | |
| Offspring per year | 131 | 5.39 | 1 | 0.020 | — | — | — | −0.11 ± 0.05 | −2.34* | ↓ | 0.94 | 0.37 |
| Gestation period | 155 | 14.96 | 1 | <0.001 | — | — | — | 0.69 ± 0.17 | 4.01*** | ↓ | 0.81 | 0.56 |
| Age at first reproduction | 110 | 8.85 | 2 | 0.012 | −0.86 ± 0.32 | −2.68** | ↑ | 0.93 ± 0.28 | 3.27*** | ↓ | 0.52 | 0.57 |
| Maximum lifespan | 54 | 8.39 | 2 | 0.015 | −0.82 ± 0.26 | −3.20** | ↑ | 0.72 ± 0.24 | 2.96* | ↓ | 0.64 | 0.66 |
| Rate of senescence | 54 | 11.09 | 2 | 0.004 | 2.56 ± 0.48 | 5.27*** | ↑ | −2.52 ± 0.48 | −5.23*** | ↓ | < 0.01 | 0.41 |
| Onset of senescence | 54 | 6.73 | 1 | 0.010 | −0.72 ± 0.25 | 2.91** | ↑ | — | — | — | < 0.01 | 0.21 |
| Generation time | 61 | 4.93 | 2 | 0.085 | −0.71 ± 0.45 | −1.56 | — | 0.95 ± 0.42 | 2.25* | ↓ | 0.86 | 0.41 |
The final PGLS ANCOVA model for each life history trait is shown. Separate placenta variables are included only when they increase the fit of the data from a simple allometric model of the life history trait regressed against body mass. No final model included a contrast between either endotheliochorial or hemochorial placentas and villous and trabecular placentas; thus, these types are always combined as a single variable. Where a cell is blank, that particular placenta variable is not included in the final model because it did not improve the fit of the data. LRT represents the log-likelihood score for the final placentation model compared with a simple allometric model. The significance of this difference is tested with a χ2 distribution, with df corresponding to the number of placental parameters added to the model. Arrows indicate the change in pace of life associated with the transition to epitheliochorial invasiveness and to trabecular or villous interdigitation. λ is the lambda value (the index of phylogenetic correlation), and the r2 is the adjusted value.
P < 0.05; **P < 0.01; ***P < 0.005.
Results
Transitions.
Major transitions in placental morphology have occurred only a few times (6). In support of this, we found that departures from labyrinthine interdigitation might have occurred only four times within the taxa sampled (Fig. 1 and Fig. S1), although additional transitions between trabecular and villous interdigitation have occurred within the primates and the Xenarthra (of which we included two armadillos and one anteater). Hemochorial invasiveness is the expected ancestral placenta morphology (6, 16). Thus, transitions from hemochorial to endotheliochorial invasiveness might have occurred only five times, with one loss (hyenas), and transitions from hemochorial to epitheliochorial invasiveness might have occurred only three times within the taxa sampled.
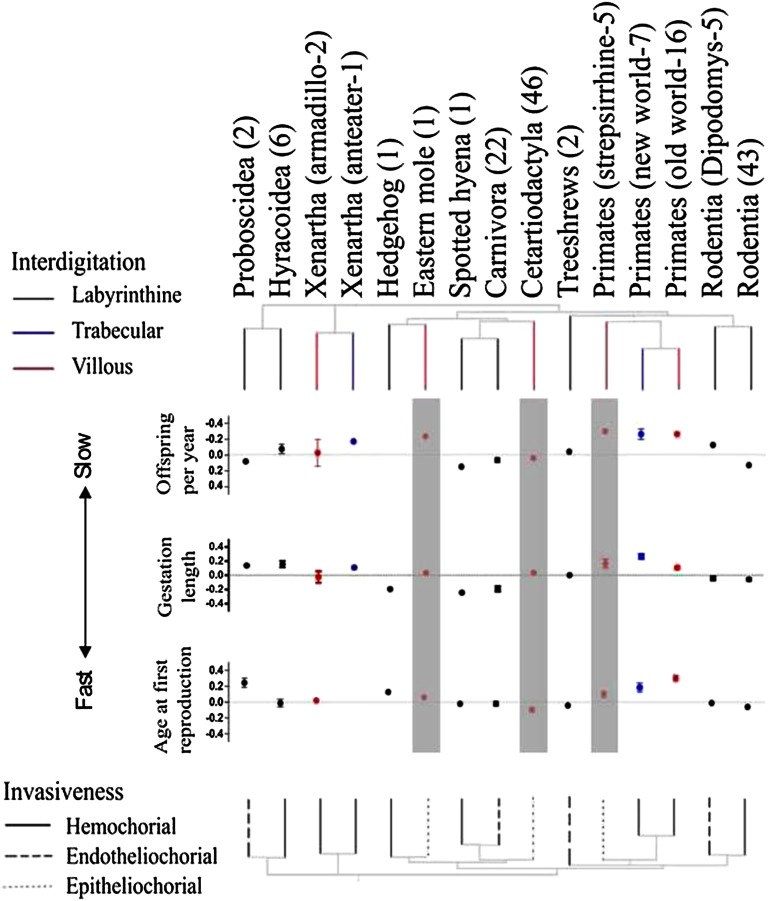
Fig. 1.
Relationships between placental morphology and residual life history traits across 160 eutherian mammals. Residuals are extracted from a least squares regression against body mass (on a log scale). This reduced tree allows a different branch in which an apparent placental transition has occurred within the species in our dataset (sample sizes in brackets). The colored tree above highlights changes in interdigitation, and the gray-scale tree below presents changes in invasiveness. Error bars are colored to highlight the difference between species of each branch in interdigitation. Gray shading denotes those species with epitheliochorial invasiveness. A higher value for each life history trait refers to a slower pace of life (e.g., longer generation time involving fewer offspring per year, longer gestation period, later age at first reproduction). Note that the y- axis has been inverted for offspring per year, so that a reduction in offspring per year appears as a higher error bar.
Life History Changes.
After controlling for body mass, allocation to all seven life history traits (offspring per year, gestation length, age at first reproduction, maximum species lifespan, onset of senescence, rate of senescence, and generation time) varied among species with different types of placental interdigitation, invasiveness, or both. Adding variables distinguishing species on the basis of one or both of these placental traits to simple allometric models of each life history trait increased the fit of the data, as demonstrated by log-likelihood test results (Table 1).
In general, the evolutionary transitions from the ancestral labyrinthine interdigitation (which provides the largest surface area for exchange between mother and fetus) to less-interdigitated trabecular and villous interdigitation (which provide smaller surface areas for maternofetal exchange) are associated with transitions to slower life histories, as shown by the differences in intercept for interdigitation in Table 1. Specifically, these transitions are associated with bearing fewer offspring per year, longer gestation periods, and older age at first reproduction (Fig. 1)—changes that reduce the overall production of offspring early in life. In the meantime, analysis of demographic parameters in these animals revealed that these changes are coupled with longer lifespans and a slower rate of senescence (Fig. 2), although interdigitation is not associated with a detectable change in age at onset of senescence. These combined effects indicate a slower pace of life in species with villous interdigitation.
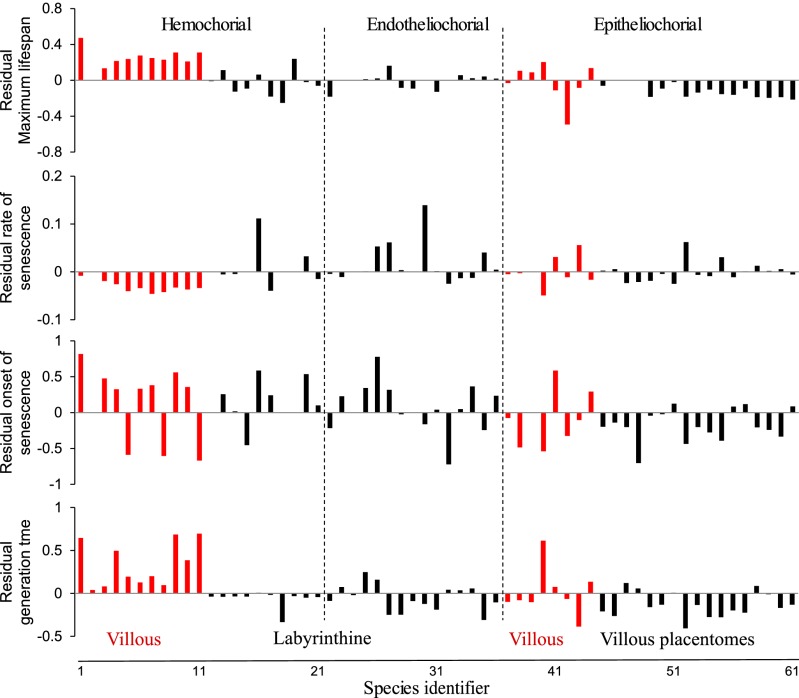
Fig. 2.
Residuals for different demographic life history traits extracted from a least squares regression against body mass (on a log scale). Positive residuals refer to longer maximum lifespans, faster rates of senescence, later onset of senescence, and longer generation times. Red bars indicate species with villous or trabecular interdigitation (referred to as villous), which provide a relatively small surface area for exchange. Species with a larger surface area (labyrinthine or villous with placentomes) are denoted with black bars. Species with different types of invasiveness are also distinguished between dashed lines. The names of the species and their locations on the x-axis are provided in Table S3. In species with an epitheliochorial placenta, species with placentomes also have shorter lifespans than those without this morphology (increase in fit for lifespan in epitheliochorial species: LRT2 = 14.59, P = 0.006).
The relationship between interdigitition and life history is also apparent when considering only species with a hemochorial placenta, within which all three types of placenta interdigitation are seen. The transition from labyrinthine to villous/trabecular interdigitation is clearly associated with the production of fewer offspring per year (Fig. 3A) and later age at first reproduction (Fig. 3B).
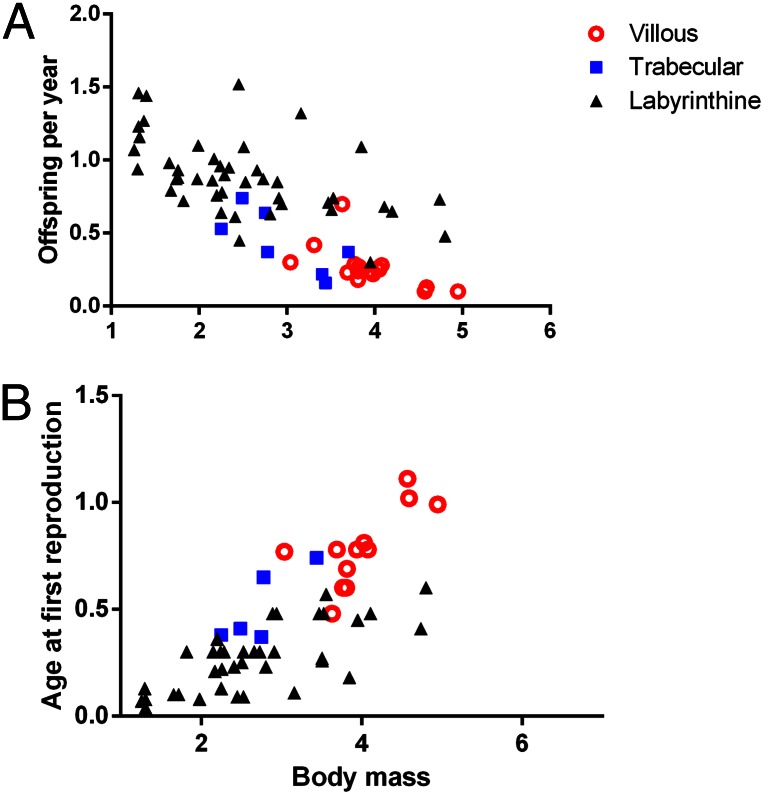
Fig. 3.
The relationships between annual fecundity (A), age at first reproduction (B), body mass and placenta surface area in species with a hemochorial placenta. Allowing for separate intercepts for each type of interdigitation improves the fit of the data from simple allometric models for each life history trait regressed against body mass (increase in fit for offspring per year: LRT2 = 5.19, P = 0.023; age at first reproduction: LRT2 = 4.53, P = 0.033). Values for age at first reproduction and body mass have been log-transformed while offspring per year was transformed using a cubed root transformation.
The most common transition in placental invasiveness, from the highly invasive ancestral hemochorial placentas to the less invasive endotheliochorial placentas, is not associated with changes in any life history trait. The transition from invasive placentation (hemochorial and endotheliochorial) to the less invasive epitheliochorial placenta, however, is associated with younger first reproduction (Fig. 1) and a corresponding decrease in lifespan, steeper rate of senescence, and earlier onset of senescence (Table 1 and Fig. 2). Gestation period, number of offspring per year, and generation time had no significant association with changes in placental invasiveness.
Previous analyses have demonstrated correlated evolution of epitheliochorial placentation and villous interdigitation (7, 17). In our dataset, all epitheliochorial placentas had villous interdigitation. Thus, the younger first reproduction and associated reduction in lifespan in taxa with epitheliochorial invasiveness (e.g., Cetartiodactyla, strepsirrhine primates, American mole) appear to represent a “speeding up” of the pace of life, after controlling for the slowdown associated with the evolution of villous interdigitation. This slowdown can still be seen in the two groups (Xenarthra and anthropoid primates) with villous interdigitation that retained hemochorial invasiveness (Fig. 1).
Among groups that share the highly derived villous, epitheliochorial placental type, the Cetartiodactyla are notable for their fast life history relative to body size (Fig. 1). Within the Cetartiodactyla, this appears to be less true for the Cetacea (whales and dolphins) compared with the Suinamorpha (peccaries and pigs) and Ruminantiamorpha (ruminants other than camels and lamas). Intriguingly, whereas the Cetacea retain a classic villous interdigitation, similar to other epitheliochorial species, the Suinamorpha and Ruminantiamorpha have independently evolved structures that greatly influence the surface exchange area. Within Ruminantia, some species have evolved placentomes, which serve to increase the surface area for exchange between mother and fetus by as much as 5- to 10-fold compared with epitheliochorial species with a more simple, diffuse villous placenta (18). Species with placentomes start reproducing at younger ages, produce more offspring per year (Fig. 4), and live shorter lives (Fig. 2) compared with those epitheliochorial species with simple diffuse placentas.
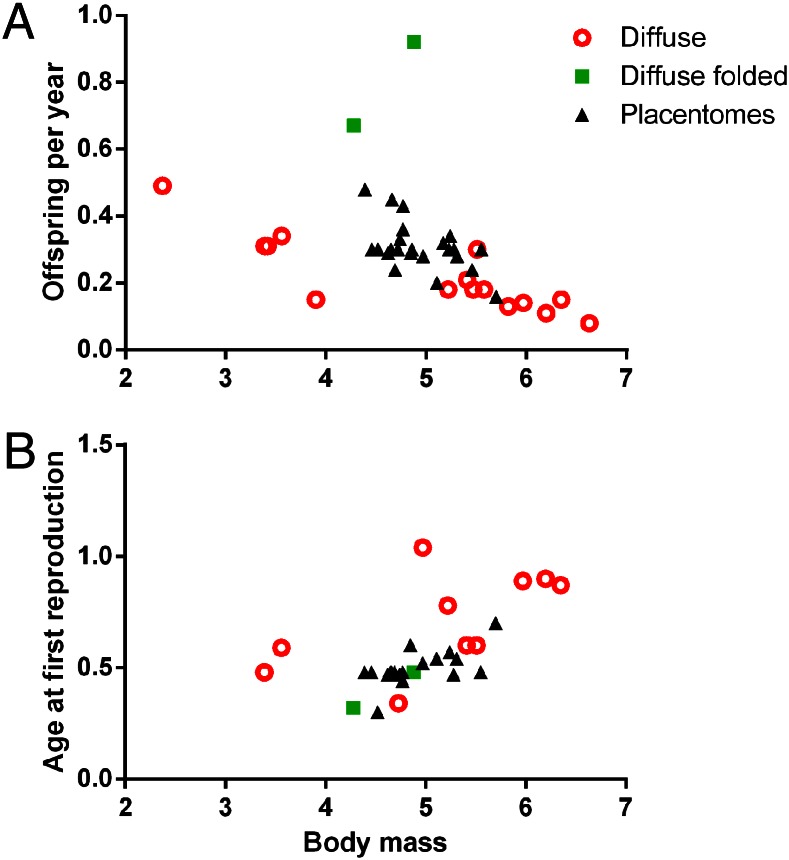
Fig. 4.
The relationships between annual fecundity (A), age at first reproduction (B), body mass and placenta surface area in species with an epitheliochorial placenta. Allowing for separate intercepts for species with each type of surface exchange area improves the fit of the data from a simple allometric model for each life history trait regressed against body mass (increase in fit for offspring per year: LRT2 = 16.90, P = 0.0002; age at first reproduction: LRT2 = 7.83, P = 0.020). Values for age at first reproduction and body mass were log-transformed, and offspring per year was transformed using a cubed root transformation.
Suidae are sometimes grouped into a completely separate “folded” category of interdigitation. Their placentas have retained a diffuse pattern, which does not appear to differ in relative villous surface area from other species with an epitheliochorial diffuse placenta when the combined weights of fetal and placental tissues are taken into account (19). However, an additional factor—macroscopic folding of the chorionic trophoblastic surface—governs the total surface area for exchange between suid mother and fetus (20). Transition to a folded placenta type is also associated with early age at first reproduction and greater numbers of offspring per year (Fig. 4).
Discussion
Our results reveal that changes in placental morphology over mammalian evolution are associated with variation in important life history traits. This finding is consistent with the prediction—derived from studies of the evolution of placenta-like structures in live-bearing fishes—that changes in placental morphology are driven by life history evolution (1, 10, 11, 17). Nonetheless, the possibility remains that changes in life history are enabled by the evolution of placental structures. The extent to which one transition drives the other remains an open question.
The life history traits that we have studied do not represent independent evolutionary dimensions, but rather a suite of coevolving traits, functionally associated via physiological and evolutionary trade-offs (21). Together, these traits contribute to the overall pace of life of an organism. Typically, compared with slow life histories, fast-paced life histories are characterized by shorter generation times, more offspring per female per year, younger first reproduction, shorter gestation periods, shorter maximum lifespans, younger onset of senescence, and more rapid senescence (22, 23). Faster life histories were associated with the ancestral labyrinthine interdigitation and the derived epitheliochorial form of invasiveness. Our results suggest that changes in placentation are associated with gross phylogenetic changes in the timing and degree of allocation to individual offspring and associated changes in senescence.
The association between changes in placental interdigitation and a slowdown in life history makes intuitive sense. A decrease in environmentally driven mortality might have favored a smaller placental surface area and a slowdown in life history, including longer gestation periods, decreased annual fecundity, and increased generation time. This reduced rate of offspring production in turn facilitates a longer lifespan and a slower rate of senescence for the mother (22). A transition to this maternal allocation pattern also will be favored by offspring, because the production of fewer offspring necessitates that each individual will receive more of the mother’s reproductive allocation.
The evolutionary association between life history and surface area for exchange is further supported by the secondary evolution of changes within the Cetartiodactyla. The placentomes in the Ruminantiamorpha and the folded placenta morphology of the Suinamorpha appear to be adaptations associated with a secondary acceleration of life history. Support from the Suinamorpha should be considered tentative, however, for two reasons. First, reliable life history data could be found for only two species (wild boar and collared peccary). Second, even though the evolution of folds is expected to be an adaptation to increase surface exhange area, reported comparisons with other species in terms of total surface area provided are limited (18) and do not include an accurate comparison of folding in the microscopic and macroscopic ranges.
After controlling for effects of interdigitation, we found no evidence that the transition from hemochorial to endotheliochorial placental invasiveness was associated with changes in life history. In both these placental types, the fetal trophoblast invades maternal tissues, but in endotheliochorial placentation the erosion of maternal tissues does not extend to the endothelial wall of the maternal blood vessels (3). Our results do, however, support a speeding up of life history with the transition from invasive placentas (hemochorial and endotheliochorial) to the noninvasive epitheliochorial placentas. In species with epitheliochorial placentation, there is no erosion of maternal tissues, and fetal tissues are apposed to the uterine epithelium (8). This leaves three layers of maternal tissue separating the fetus from the mother’s blood. Although placental invasiveness does not appear to consistently affect diffusion distance (8), the greater number of membranes in less-invasive placentas could potentially lower passive diffusion of some molecules or protect against fetal secretion of hormones that manipulate maternal physiology.
Our finding that species with epitheliochorial placentation have a faster pace of life contradicts the results of a previous study suggesting that hemochorial placentation is associated with the fastest pace of life (17). In that study, Lewitus and Soligo (17) used association analysis to determine the difference between the observed and the expected phylogenetic relationships for each placental trait and each life history character independently (17), thus examining the effects of invasiveness and interdigitation separately. Our analysis allows comparison of the effects of placental invasiveness and interdigitation within the same model, overcoming the tendency to confound the effects of labyrinthine interdigitation and hemochorial invasiveness in the ancestral character state.
Our results indicate that two different functional aspects of placental morphology are related to pace of life. Considering the effects of interdigitation and invasiveness combined, species can be ranked according to their overall placentation and its association with life history. At one end of the spectrum, species with the slowest pace of life have the most invasive placentas (hemochorial) and the smallest surface exchange area (villous); at the other end, those species with the least-invasive placentas (epitheliochorial) and potentially larger (placentomes, folds) surface areas have the fastest pace of life (Fig. S2). Variation in surface area within hemochorial and epitheliochorial placental types arises via different stuctures. Indeed, epitheliochorial invasiveness is evolutionarily correlated with villous interdigitation (7, 17), and species with this type of invasiveness never have labyrinthine interdigitation, perhaps because maternal tissue erosion is required to create the trophoblastic mesh network. Instead, other morplogical features have evolved, including placentomes, which increase the surface area. Endotheliochorial invasiveness is never associated with villous interdigitation and is evolutionarily correlated with labyrinthine interdigitation (22). There is wide variation in this structure (24), however, and this variation will likely cause some species to have smaller surface areas than others. When available, a comparison of the variation in surface exchange area within these species, and its relationship to life history, will offer another valuable test of our predictions.
Although our method controls for phylogenetic effects, owing to the small number of evolutionary transitions in placental morphology, a few species might exert strong leverage on our findings. Anthropoid primates are particularly notable for their slow pace of life and are one of only two phylogenetically independent groups of species with hemochorial villous placentation (the other being Xenarthra). In models of age at first reproduction, lifespan, rate of senescence, and generation time, in which effects of both invasiveness and interdigitation are found and compared in the same model, species with hemochorial villous placentas are compared with those with epitheliochorial villous placentas (which have evolved three times independently). In these models, after the removal of anthropoid primates, no significant effects of placentation are seen, as these species provide the major contrast (Table S1). Despite this, partitioned analyses in groups that do not contain arthropoid primates highlight the generality of our findings. Among species with epitheliochorial invasiveness, age at first reproduction, lifespan, and annual fecundity are all associated with secondary changes in placenta interdigitation. Likewise, when comparing species with relatively large surface areas for exchange (e.g., placentomes, labyrinthine interdigitation) but different types of invasiveness, species with epitheliochorial placentation are found to have shorter generation times, shorter lifespans, and earlier onset of senesence (Table S2), although this argument should be viewed with caution, given that the types of interdigitation are not strictly comparable.
Importantly, the effects of placentation on annual fecundity, duration of gestation, and onset of senescence remain statistically significant even when anthropoid primates are removed (Table S1). Here only one aspect of placentation explains variation in each life history trait, and removal of anthropoid primates still leaves four evolutionary transitions from labyrinthine to villous interdigitation, along with three transitions to epitheliochorial placentation from a more invasive placenta type. Species with villous interdigitation still have shorter gestations and produce fewer offspring than species with labyrinthine interdigitation, and those species with epitheliochoral invasiveness have an earlier onset of senescence compared with the other mammalian clades. Because epitheliochorial invasiveness is thought to be the derived state, we can expect that the consistently earlier onset of senescence seen in these species may be a by-product of evolution acting on life histories at earlier stages.
Conflict between mothers, fathers and offspring has been predicted to influence reproductive mode (12), particularly the evolution of placental morphology (5). The evidence that we present here, indicating that transitions in placental invasiveness and interdigitation are associated with life history evolution, does not refute a role for parent–offspring conflict. Indeed, we propose a counterintuitive role for conflict in driving the evolution of less-invasive epitheliochorial placentation from the highly invasive ancestral hemochorial invasiveness.
When environmentally driven mortality rises over evolutionary time, selection favors a faster pace of life, including higher lifetime fecundity for mothers. Such a transition requires the mother to allocate fewer resources to each individual offspring. However, alleles expressed by offspring (particularly paternal-derived alleles) should continue to demand greater investment from the mother, thereby increasing offspring quality at the expense of offspring quantity (25, 26). As a result, ceteris paribus, we would expect high environmentally driven mortality to elevate maternal–fetal conflict over provisioning, given that parent–offspring conflict is expected to be stronger in species with a high average number of offspring (25). The evolution of an epitheliochorial placenta, which separates the fetus from maternal blood, may result from more intense selection on mothers to limit offspring from taking more resources than they are optimally selected to provide. Mothers more liberated from fetal manipulation might then be able to spread their available resources for reproduction among more offspring.
The highly invasive hemochorial placenta is known to be a site of intense maternofetal conflict (5, 12, 27). Although such a placenta is expected to give the fetus an advantage in maternofetal conflict over resource provisioning (5), the extent of placental invasion in humans is known to be strongly influenced by the mother and by maternally inherited genes (28). We predict that among those taxa with villous interdigitation, hemochorial invasiveness might persist only in mammalian taxa with a relatively slow pace of life, because maternal and fetal interests do not diverge as dramatically in these species compared with faster-paced species. Thus, offspring may be allowed to retain an intimate association with the mother’s blood, because their evolutionary interests are quantitatively closer to those of the mother. This is not to downplay the importance of conflict in these species, but rather to predict that epitheliochorial placentation is a maternal adaptation to a more intense history of parent–offspring conflict. This prediction remains to be tested directly via within-species and experimental studies.
Materials and Methods
Our method is based on a previously published approach (7) that has been used to successfully determine whether placental diversity is related to gestation period and neonatal growth rate. To determine whether placentation is related to a species’ life history strategy, which corresponds to a covariation among a range of life history traits that describe the relative allocation to growth, reproduction, and mortality (21), we collected data from species for which complete demographic information was available. Although data for some life history traits had a larger sample size, with values extracted from published databases, the use of demographic data allowed us to estimate species-specific generation time, onset, and rate of senescence, which are key components in a detailed study of life history variation (14, 22). Complete lists of the species and data used are provided in Tables S3 and S4.
Maternal Body Mass and Life History Traits.
Maternal body mass (in grams), average litter size, number of litters per year, gestation length (length of an actively gestating fetus, not including any periods of delayed implantation, as observed in some carnivores and roe deer) were collected principally from two published datasets: the PANtheria database (29) and (30). Maximum species lifespan (in months) was collected principally from the AnAge database (31). The number of offspring per year, a measurement of annual fecundity, was calculated by multiplying average litter size by number of litters per year (32). For senescence measures, survival was modeled as a function of age using generalized additive models with the gam package in R version 2.51.2 (33). This method allowed us to estimate the age at which survival starts to decrease, that is, the age at onset of senescence (34). We then calculated the rate of actuarial senescence (referred to as rate of senescence) as the slope of the linear regression fitted from the age at onset of senescence to the maximum age for the species.
To estimate generation time, we built Leslie matrix models for each species with complete information on age-specific reproduction and survival. We used a prebreeding census matrix type in which the first row includes age-specific recruitment (i.e., the product of the probability of a female of age x to give birth, the number of females produced at birth by a female of age x, and the survival probability of females produced at birth by a female of age x to survive to 1 y of age) and the subdiagonal includes age-specific survival starting with the survival of females between 1 y and 2 y of age in the first column of the second row (see ref. 35 for further details). We then calculated the elasticity of each matrix element and we estimated generation time [mean weighted age of females that gave birth (36)] as the inverse of the sum of the elasticities of the recruitment parameters (i.e., the inverse of the sum of the elasticities of the matrix elements in the first row), as recommended by Brooks and Lebreton (37).
Placental Interdigitation and Invasiveness.
Placentas were defined on the basis of their full-term state, as described previously (3, 7, 16) and as outlined above. Owing to the presence of long-term error in datasets of other comparative studies of placental evolution, we included only species for which a primary reference could be found for the placenta of that species. In some instances, we cited a study that examined a different species of the same genus, and for eight species we used a reference that examined a species in the same family; however, restricting our analysis to species that themselves have been specifically studied does not influence our results (Table S5).
Statistical Analysis.
We used a comparative method that takes into account phylogenetic inertia to control for nonindependence between species owing to shared ancestry (38). We began by constructing a phylogeny of all species included in our database using a published phylogenetic supertree of mammals with topology and branch lengths (39). For all of our statistical analyses, we used a PGLS procedure, implemented in R (40), with a variance-covariance matrix extracted from the constructed phylogenetic tree using the R-package APE (41). This statistical method estimates an index of phylogenetic correlation, λ, that is introduced into the analysis to control for the phylogenetic dependence (15). Comparative analyses were conducted using R version 2.12.1 (R Development Core Team).
Given that most life history traits are strongly linked with body mass by allometric constraints (42), we used PGLS ANCOVAs, as outlined by Capellini et al. (7), to compare simple allometric models (i.e., with body mass as a covariate) of life history traits (43) with more complex models that included placentation variables. For invasiveness, we used the variable “hemochorial” as the reference variable, and for interdigitation, we used the variable “labyrinthine” (both coded 0 in our analyses). These nested models were compared using a likelihood ratio test (LRT), with likelihood ratio equal to −2 × log-likelihood [better-fitting model] – log-likelihood [worse-fitting model]. The statistical significance of this difference was determined using a χ2 distribution with degrees of freedom corresponding to the difference in the number of parameters between the two competing models.
We initially created models that allowed an interaction between female body mass and placentation, but these interaction terms were dropped because they did not improve the fit of the data. Similarly, we started with models that allowed three different types of placental invasiveness and three types of interdigitation. Because the intercepts of two different types of placental invasiveness and two types of interdigitation did not differ, we combined these to use a two-level variable for invasiveness (i.e., hemochorial and endotheliochorial combined as one variable, and epitheliochorial as another variable) and/or interdigitation.
Supplementary Material
Acknowledgments
We thank Bart Andriaenssens, Russell Bonduriansky, Elizabeth Cassidy, Camille Ferdenzi, Michael Kasumovic, and Terry Ord for comments on a draft manuscript. We also thank Christophe Bonenfant for his help with the artwork, Professor Rob Freckleton for the code for the phylogenetic analysis, and three anonymous referees for insightful comments on a previous draft. J.-F.L. is supported by a grant from the Fyssen Foundation, Paris. M.G. and R.C.B. are supported by the Australian Research Council.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.D.T. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1305018110/-/DCSupplemental.
References
- 1.Vogel P. The current molecular phylogeny of Eutherian mammals challenges previous interpretations of placental evolution. Placenta. 2005;26(8-9):591–596. doi: 10.1016/j.placenta.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Wildman DE. Review: Toward an integrated evolutionary understanding of the mammalian placenta. Placenta. 2011;32(Suppl 2):S142–S145. doi: 10.1016/j.placenta.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mossman HW. Vertebrate Fetal Membranes. New Brunswick, NJ: Rutgers Univ Press; 1987. [Google Scholar]
- 4.Hill JP. Croonian lecture: The developmental history of the primates. Philos Trans R Soc Lond B. 1932;221:45–46. [Google Scholar]
- 5.Haig D. Genetic conflicts in human pregnancy. Q Rev Biol. 1993;68(4):495–532. doi: 10.1086/418300. [DOI] [PubMed] [Google Scholar]
- 6.Wildman DE, et al. Evolution of the mammalian placenta revealed by phylogenetic analysis. Proc Natl Acad Sci USA. 2006;103(9):3203–3208. doi: 10.1073/pnas.0511344103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capellini I, Venditti C, Barton RA. Placentation and maternal investment in mammals. Am Nat. 2011;177(1):86–98. doi: 10.1086/657435. [DOI] [PubMed] [Google Scholar]
- 8.Benirschke K, Kaufman RJ. The Pathology of the Human Placenta. New York: Springer; 2000. [Google Scholar]
- 9.Trexler JC, DeAngelis DL. Resource allocation in offspring provisioning: An evaluation of the conditions favoring the evolution of matrotrophy. Am Nat. 2003;162(5):574–585. doi: 10.1086/378822. [DOI] [PubMed] [Google Scholar]
- 10.Pires MN, et al. Why do placentas evolve? An evaluation of the life-history facilitation hypothesis in the fish genus Poeciliopsis. Funct Ecol. 2011;25(4):757–768. [Google Scholar]
- 11.Pires MN, McBride KE, Reznick DN. Interpopulation variation in life-history traits of Poeciliopsis prolifica: Implications for the study of placental evolution. J Exp Zool A Ecol Genet Physiol. 2007;307(2):113–125. doi: 10.1002/jez.a.356. [DOI] [PubMed] [Google Scholar]
- 12.Crespi B, Semeniuk C. Parent-offspring conflict in the evolution of vertebrate reproductive mode. Am Nat. 2004;163(5):635–653. doi: 10.1086/382734. [DOI] [PubMed] [Google Scholar]
- 13.Elliot MG, Crespi BJ. Placental invasiveness and brain-body allometry in eutherian mammals. J Evol Biol. 2008;21(6):1763–1778. doi: 10.1111/j.1420-9101.2008.01590.x. [DOI] [PubMed] [Google Scholar]
- 14.Gaillard JM, et al. Generation time: A reliable metric to measure life-history variation among mammalian populations. Am Nat. 2005;166(1):119–123. doi: 10.1086/430330. [DOI] [PubMed] [Google Scholar]
- 15.Freckleton RP, Harvey PH, Pagel M. Phylogenetic analysis and comparative data: A test and review of evidence. Am Nat. 2002;160(6):712–726. doi: 10.1086/343873. [DOI] [PubMed] [Google Scholar]
- 16.Elliot MG, Crespi BJ. Phylogenetic evidence for early hemochorial placentation in eutheria. Placenta. 2009;30(11):949–967. doi: 10.1016/j.placenta.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Lewitus E, Soligo C. Life-history correlates of placental structure in eutherian evolution. Evol Biol. 2011;38(3):287–305. [Google Scholar]
- 18.Wooding FBP, Burton GJ. Comparative Placentation: Structures, Functions and Evolution. Berlin: Springer; 2008. [Google Scholar]
- 19.Baur R. Morphometry of the placental exchange area. Adv Anat Embryol Cell Biol. 1977;53(1):3–65. doi: 10.1007/978-3-642-66603-2. [DOI] [PubMed] [Google Scholar]
- 20.Hasselager E. Surface exchange area of the porcine placenta: Morphometry of anisotropic interdigitating microvilli. J Microsc. 1986;141(Pt 1):91–100. doi: 10.1111/j.1365-2818.1986.tb02703.x. [DOI] [PubMed] [Google Scholar]
- 21.Stearns SC. The Evolution of Life Histories. Oxford: Oxford Univ Press; 1992. [Google Scholar]
- 22.Jones OR, et al. Senescence rates are determined by ranking on the fast-slow life-history continuum. Ecol Lett. 2008;11(7):664–673. doi: 10.1111/j.1461-0248.2008.01187.x. [DOI] [PubMed] [Google Scholar]
- 23.Peron G, Gimenez O, Charmantier A, Gaillard J-M, Crochet P-A. Age at the onset of senescence in birds and mammals is predicted by early-life performance. Proc R Soc B Biol Sci. 2010;277:2849–2856. doi: 10.1098/rspb.2010.0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Enders AC, Carter AM. The evolving placenta: Convergent evolution of variations in the endotheliochorial relationship. Placenta. 2012;33(5):319–326. doi: 10.1016/j.placenta.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Bossan B, Hammerstein P, Koehncke A. We were all young once: An intragenomic perspective on parent–offspring conflict. Proc R Soc B Biol Sci. 2013;280:20122637. doi: 10.1098/rspb.2012.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Godfray HCJ, Parker GA. Clutch size, fecundity and parent offspring conflict. Philos Trans R Soc Lond B Biol Sci. 1991;332:67–79. [Google Scholar]
- 27.Papper Z, et al. Ancient origin of placental expression in the growth hormone genes of anthropoid primates. Proc Natl Acad Sci USA. 2009;106(40):17083–17088. doi: 10.1073/pnas.0908377106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frost JM, Moore GE. The importance of imprinting in the human placenta. PLoS Genet. 2010;6(7):e1001015. doi: 10.1371/journal.pgen.1001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones KE, et al. PanTHERIA: A species-level database of life history, ecology, and geography of extant and recently extinct mammals. Ecology. 2009;90(9):2648. [Google Scholar]
- 30.Ernest SKM. Life history characteristics of placental nonvolant mammals. Ecology. 2003;84(12):3401–3402. [Google Scholar]
- 31.de Magalhães JP, Costa J. A database of vertebrate longevity records and their relation to other life-history traits. J Evol Biol. 2009;22(8):1770–1774. doi: 10.1111/j.1420-9101.2009.01783.x. [DOI] [PubMed] [Google Scholar]
- 32.Allaine D, et al. The relationship between fecundity and adult body weight in homeotherms. Oecologia. 1987;73(3):478–480. doi: 10.1007/BF00385268. [DOI] [PubMed] [Google Scholar]
- 33.Hastie TJ. 2006. GAM: Generalized additive models (R package version 0.97). Available at http://www.r-project.org. Accessed January 5, 2011.
- 34.Hamilton WD. The moulding of senescence by natural selection. J Theor Biol. 1966;12(1):12–45. doi: 10.1016/0022-5193(66)90184-6. [DOI] [PubMed] [Google Scholar]
- 35.Caswell H. Prospective and retrospective perturbation analyses: Their roles in conservation biology. Ecology. 2000;81(3):619–627. [Google Scholar]
- 36.Leslie PH. Intrinsic rate of increase and overlap of successive generations in a population of guillemots (Uria aalge Pont.) J Anim Ecol. 1966;35(2):291–301. [Google Scholar]
- 37.Brooks EN, Lebreton JD. Optimizing removals to control a metapopulation: application to the yellow legged herring gull (Larus cachinnans) Ecol Modell. 2001;136(2-3):269–284. [Google Scholar]
- 38.Harvey PH, Pagel M. The Comparative Method in Evolutionary Biology. Oxford: Oxford Univ Press; 1991. [Google Scholar]
- 39.Bininda-Emonds ORP, et al. The delayed rise of present-day mammals. Nature. 2007;446:507–512. doi: 10.1038/nature05634. [DOI] [PubMed] [Google Scholar]
- 40.Gage MJG, Freckleton RP. Relative testis size and sperm morphometry across mammals: No evidence for an association between sperm competition and sperm length. Proc Biol Sci. 2003;270:625–632. doi: 10.1098/rspb.2002.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paradis E, Claude J, Strimmer K. APE: Analyses of Phylogenetics and Evolution in R language. Bioinformatics. 2004;20(2):289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
- 42.Peters RH. The ecological implications of size. Cambridge, UK: Cambridge Univ Press; 1983. [Google Scholar]
- 43.Thompson DA. In: On Growth and Form. Bonner J, editor. London: Cambridge Univ Press; 1961. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.