Abstract
Respiratory distress syndrome (RDS), which is induced by insufficient production of surfactant, is the leading cause of mortality in preterm babies. Although several transcription factors are known to be involved in surfactant protein expression, the molecular mechanisms and signaling pathways upstream of these transcription factors have remained elusive. Here, using mammalian Hippo kinases (Mst1/2, mammalian sterile 20-like kinase 1/2) conditional knockout mice, we demonstrate that Mst1/2 kinases are critical for orchestration of transcription factors involved in surfactant protein homeostasis and prevention of RDS. Mice lacking Mst1/2 in the respiratory epithelium exhibited perinatal mortality with respiratory failure and their lungs contained fewer type I pneumocytes and more immature type II pneumocytes lacking microvilli, lamellar bodies, and surfactant protein expression, pointing to peripheral lung immaturity and RDS. In contrast to previous findings of YAP (Yes-associated protein)-mediated canonical Hippo signaling in the liver and intestine, loss of Mst1/2 kinases induced the defects in pneumocyte differentiation independently of YAP hyperactivity. We instead found that Mst1/2 kinases stabilized and phosphorylated the transcription factor Foxa2 (forkhead box A2), which regulates pneumocyte maturation and surfactant protein expression. Taken together, our results suggest that the mammalian Hippo kinases play crucial roles in surfactant homeostasis and coordination of peripheral lung differentiation through regulation of Foxa2 rather than of YAP.
Keywords: non-canonical Hippo pathway, lung development
Peripheral lung immaturity and deficiency of pulmonary surfactant cause many intractable pulmonary diseases. Deficit of surfactant because of preterm birth or genetic disorders of surfactant homeostasis induce respiratory distress syndrome (RDS) in the newborn period (1). Dysregulation of these genes also underlies the pathogenesis of many chronic lung diseases that have been considered idiopathic, such as interstitial lung disease, pulmonary alveolar proteinosis, and others (2). Elucidating the mechanism underlying regulation of surfactant would be very helpful for understanding the molecular basis of refractory lung diseases of both infants and adults.
Alveoli are composed of type I and type II pneumocytes. The type II pneumocyte is the progenitor cell of the type I pneumocyte and plays a central role in physiological pulmonary functions, especially surfactant production (3). Type I pneumocytes contact alveolar capillaries and participate in gas exchange. Lung morphogenesis in mice is a dynamic process that can be divided into embryonic [embryonic day (E) 9–11.5], pseudoglandular (E11.5–16.5), canalicular (E16.5–17.5), saccular [E17.5 to postnatal (PN) day 5], and alveolar (PN5–28) stages. During canalicular and saccular stages in late gestation, peripheral lungs containing type I and II pneumocytes fully differentiate in preparation for postnatal respiration (4).
Many transcription factors, including Foxa2 (forkhead box A2), TTF-1 (thyroid transcription factor-1)/Nkx2.1 (NK2 homeobox 1), and C/EBPα (CCAAT/enhancer binding protein-α), are known to be involved in surfactant production and homeostasis during late gestation (1, 4–7). Mice containing a mutation of TTF-1 or a deletion of Foxa2 or C/EBPα display immature type II pneumocytes and lack type I pneumocytes without an alteration in branching morphogenesis, resulting in RDS (8, 9). Foxa2, TTF-1, and C/EBPα reciprocally interact to regulate transcriptional targets of surfactant synthesis and peripheral lung maturation (5). Some upstream regulators, such as Nfatc3 (nuclear factor of activated T cells, cytoplasmic 3) and protein kinase A were identified (10, 11), but so far the mechanism regulating peripheral lung maturation and surfactant homeostasis are poorly understood and complex.
Mammalian sterile 20-like kinase 1 and 2 (Mst1/2) are upstream kinases involved in the Hippo pathway, the unique signaling pathway involved in organ development and cancer (12). Interestingly, Mst1/2- and 45kD WW domain protein (WW45)-knockout mice show a tendency to accumulate undifferentiated progenitor cells (13, 14). For example, liver-specific ablation of Mst1/2 causes an increase in the number of hepatocytes and facultative progenitor cells (oval cells) (15). Intestine-specific ablation of Mst1/2 also results in intestinal progenitor cell proliferation and subsequent colonic tumorigenesis (16, 17). The phenotypes of these organs are attributed to hyperactivity of Yes-associated protein (YAP), an oncogenic downstream effector molecule of the Hippo pathway. These studies suggest the interesting possibility that Yap-mediated Hippo signaling pathway, the so-called canonical Hippo pathway, works as critical inhibitor of epithelial progenitor cell proliferation in epithelial organs. Whether this paradigm is applicable to other epithelial organs is not yet clear. Recent studies have also demonstrated the role of Mst1/2 kinases in a YAP-independent mechanism, the so-called noncanonical Hippo pathway. For example, Mst1 suppresses lymphoma development by promoting faithful chromosome segregation, independently of YAP (18). Mst1/2 kinases also execute a variety of functions with multiple partners, such as Foxo1, Foxo3, Akt, and Aurora B, in different biological contexts (19–22).
We have now generated conditional double-knockout mice (dKO) in which Mst1 and Mst2 were deleted in the developing lung epithelium. Our results revealed that Mst1/2 acts through modulation of Foxa2 to play a critical role in the regulation of peripheral lung maturation and surfactant homeostasis with use of these mice.
Results
Lung-Specific Mst1/2-dKO Mice Succumb to RDS and Die in the Perinatal Period.
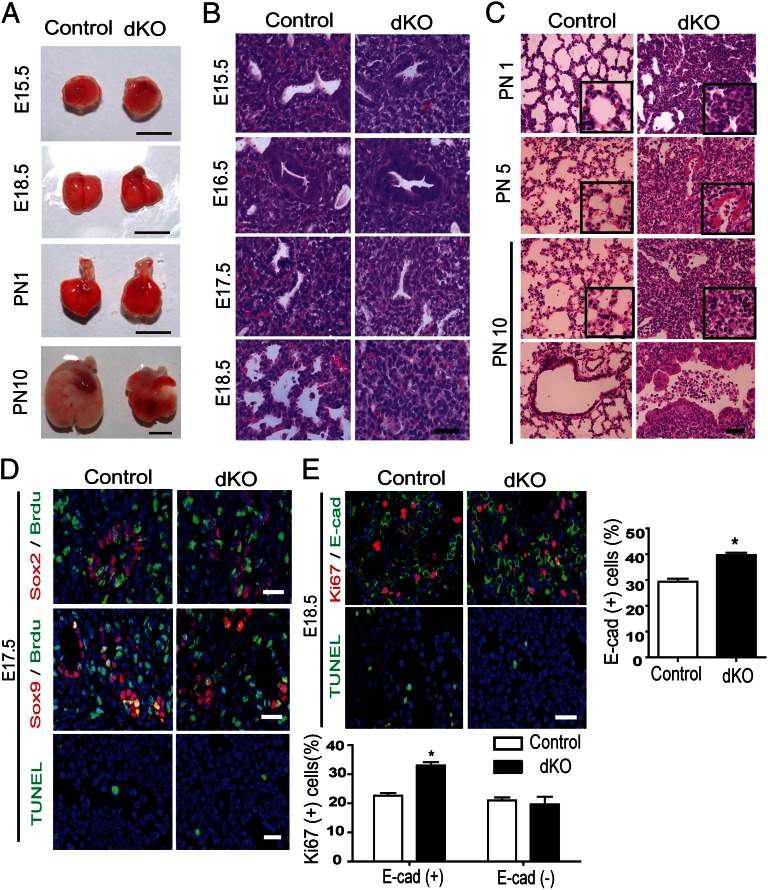
Mst1/2-null mice lacking both Mst1 and Mst2 genes begin dying in utero at embryonic day 8.5 because of defects in placental development and yolk sac/embryonic vascular patterning (21). To investigate the role of Mst1/2 in lung development, we crossed Mst1fl/fl;Mst2−/− mice with Nkx2.1-Cre mice to generate the lung-specific Mst1/2-knockout mouse, Mst1fl/fl;Mst2−/−;Nkx2.1-Cre, hereafter termed Mst1/2-dKO. Mst2−/− mice did not show any noticeable developmental or immunological defects (15). We confirmed the efficient deletion of Mst1 in the Mst2-null lung tissues (Fig. S1 A and B). LacZ staining showed that Nkx2.1 Cre was expressed in lung epithelial cells (Fig. S1C). Mst1/2-dKO mice were born at normal Mendelian ratio. Lung size and dry lung weight were comparable between Mst1/2 dKO mice and control mice at E15.5 and E18.5 (Fig. 1A and Fig. S2). However, Mst1/2-dKO mice exhibited a markedly sick appearance at birth and almost all mice died within the first 15 d after birth (Fig. S1D). At PN10, Mst1/2-dKO lung showed severe accumulation of inflammatory cells in the alveolar space and bronchiole with multiple atelectasis and emphysematous lesions (Fig. 1C and Fig. S3A), suggesting the Mst1/2-dKO mice died because of respiratory failure.
Fig. 1.
Mst1fl/fl;Mst2−/−;Nkx2.1-Cre mice exhibit perinatal mortality with RDS. (A) Representative lungs of E15.5, E18.5, PN1, and PN10 of control and Mst1/2-dKO mice. (Scale bar, 0.5 cm.) (B) Lung section from E15.5, E16.5, E17.5, and E18.5 of control and Mst1/2-dKO mice were stained with H&E, revealing that branching morphogenesis is normal in Mst1/2-dKO mice. (C) Lung sections from PN1, PN5, and PN10 mice were fixed and stained with H&E. (D) Evaluation of cell proliferation and apoptosis by immunodetection of Brdu and TUNEL assay, respectively, in sections of control and Mst1/2-dKO mice at E17.5. Distal progenitor marker (Sox9) and proximal progenitor marker (Sox2) were costained. (E) Ki67 and epithelial cell marker (E-cadherin) were costained to reveal the proliferation rates of epithelial cell and nonepithelial cell. TUNEL staining was performed to evaluate the apoptosis at E18.5. Mean ± SD was determined from three embryos per group. *P < 0.05. (Scale bars in B–E, 50 μm.)
We then determined the onset of aberrant phenotype from E15.5 to E18.5. Until E17.5, there were no significant differences between the lungs of Mst1/2-dKO mice and control mice (Fig. 1B). At that time, embryonic markers such as Sox9 (sex-determining region Y box 9, a marker for distal lung progenitor cells) and Sox2 (marker for proximal lung progenitor cells) were also normally expressed in Mst1/2 dKO lungs (23) (Fig.1D, and Fig. S4 A and B). However, alveoli of Mst1/2-dKO lungs at E18.5 and PN1 showed a remarkably reduced aerated space compacted with immature-looking cuboidal cells (Fig. 1 B and C). At around PN5, Mst1/2-dKO lungs displayed spontaneous infiltration of inflammatory cells and hyaline membrane formation, which are characteristics typical of RDS in preterm infants (1), at which point the destruction of lung became aggravated (Fig. 1C). No bacteria and fungi were detected in lung sections or in cytospins of bronchoalveolar lavage fluid, ruling out the possibility of infection (Fig. 1C and Fig. S3B).
We determined whether Mst1 and Mst2 affect cell proliferation and apoptosis by performing BrdU labeling and TUNEL assays, respectively, at E17.5. Proliferation and cell death in the Mst1/2-dKO mice lungs did not appeared significantly altered until E17.5 (Fig. 1D and Fig. S4C). The percentages of proliferating progenitor cells expressing Sox2 or Sox9 were similar between control and Mst1/2-dKO mice at E17.5 (Fig. 1D and Fig. S4D). However, E18.5 Mst1/2-dKO lungs showed a markedly increased number of proliferative cells and mildly diminished cell death (Fig. 1E and Fig. S4E). To confirm the increase of epithelial cell specific proliferation at E18.5, we costained the lung sections with Ki67 and E-cadherin (E-Cad). The percentage of Ki67+ cells among E-Cad+ epithelial cells was higher in Mst1/2 dKO versus control lungs at E18.5. In contrast, the percentage of Ki67+ cells among E-Cad− mesenchymal cells was similar in Mst1/2 dKO and control lungs (Fig. 1E). These observations suggest that the lung phenotype of Mst1/2 dKO mice is a result of the cell-autonomous effects of Mst1/2 deletion.
Deletion of Mst1/2 in Lung Epithelial Cells Causes Abnormal Lung Maturation: Impaired Surfactant Homeostasis and Delayed Differentiation of Type I and II Pneumocytes.
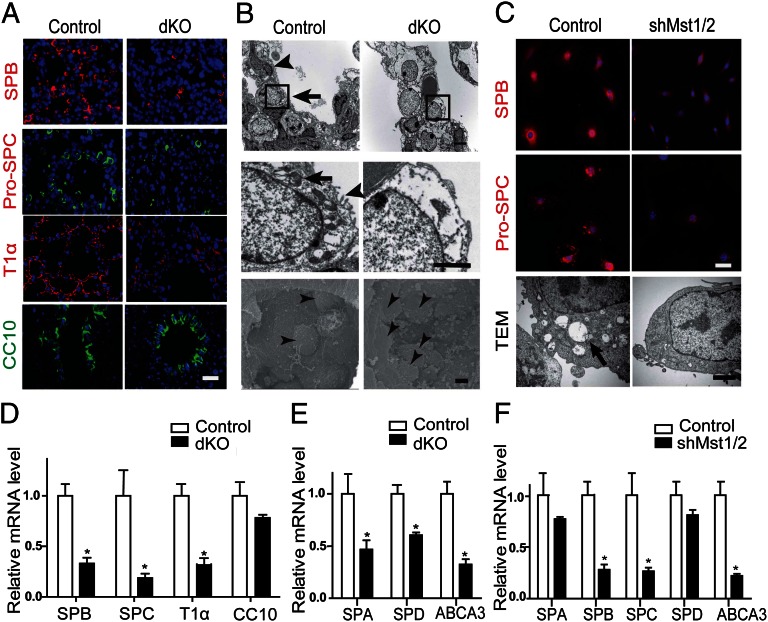
Given that deregulated maturation of the lung gives rise to perinatal lethality and RDS (1), which were observed in Mst1/2-dKO mice (Fig. 1 B and C), we examined differentiation markers to assess the degree of lung maturation. Normal maturation was observed in control lungs, which showed well-differentiated type II cells expressing the markers surfactant protein-B (SP-B) and prosurfactant protein-C (proSP-C), and type I cells, which were stained by T1α protein (Fig. 2A). In contrast, alveolar sacs in Mst1/2-dKO lungs at E18.5 were compacted with cuboidal cells lacking SP-B and proSP-C expression (Fig. 2A). These Mst1/2-dKO cuboidal cells seemed to be immature type II pneumocytes that did not fully mature to produce surfactant proteins normally. Staining for T1α demonstrated decreased numbers of squamous type I cells in Mst1/2-dKO lungs (Fig. 2A). However, the distribution and proliferation of CC10-expressing Clara cells and acetylated-tubulin expressing ciliated cells were similar between Mst1/2-dKO and control mice (Fig. 2A and Fig. S4F). These data suggested that proliferating E-Cad+ epithelial cells were mainly immature type II pneumocytes lacking SP-B and proSP-C expressions rather than airway epithelial cells. We did not see any abnormal proliferation of mesenchymal cells expressing smooth muscle actin (SMA) in Mst1/2-dKO lungs. Vascularization was normal in Mst1/2-dKO mice (Fig. S4G). We also examined the expression of mesenchyme-originated signaling factors, such as Fgf10 (fibroblast growth factor 10) and its targets, Spry2 (Sprouty homolog 2) and Bmp4 (Bone morphogenetic protein 4), and other cardinal factors of morphogenesis, including Wnt7b and Shh (Sonic Hedgehog) at E15.5 and E18.5 (23). The unaltered expressions of Fgf10, spry2, Bmp4, and Shh reflect that the effects of Mst1/2 deletion are cell autonomous, and not secondary result of a change in fundamental developmental pathway (Fig. S5).
Fig. 2.
Mst1/2-dKO mice exhibit defects in pulmonary surfactant protein expression and peripheral lung maturation. (A) SP-B, proSP-C, and T1α staining were decreased in lungs of E18.5 Mst1/2-dKO mice, whereas CC10 protein was unaltered. (Scale bar, 200 μm.) (B) Ultrastructure of lung epithelial cells in E18.5 control and Mst1/2-dKO lungs. TEM (Upper and Middle) showed squamous type I cells (arrowhead, Upper) and cuboidal type II cells (arrow, Upper) containing lamellar bodies (arrow, Middle) and microvilli (arrowhead, Middle) in the lungs of control mice. Type I cells were rarely observed in the lungs of Mst1/2-dKO mice. SEM (Lower) showed alveolar space. Arrowhead indicates type II cells. (Scale bars, 5 μm.) (C) MLE-12 mouse lung epithelial cells were infected with lentivirus encoding scrambled shRNA (control) or Mst1/Mst2 shRNA. Mst1/2-knockdown cells and control cells were immunostained for SP-B (red) and proSP-C (red) and counterstained with DAPI (blue, Upper and Middle) (Scale bar, 20 μm). (Lower) TEM of MLE-12 cells. (Scale bar, 5 μm.) (D and E) qRT-PCR of total RNA from E18.5 Mst1/2-dKO lungs. *P < 0.05. (F) Quantification of mRNA levels in control and Mst1/2-knockdown MLE-12 cells. *P < 0.05.
Transmission electronic microscopy (TEM) confirmed that Mst1/2-dKO mice have immature type II pneumocytes and few squamous type I pneumocytes. These immature type II pneumocytes lacked apical microvilli and lamellar bodies (Fig. 2B). Scanning electronic microscopy (SEM) also indicated much more filling of alveoli surfaces with cuboidal type II pneumocytes in Mst1/2-dKO mice compared with control mice (Fig. 2B). To further confirm these phenotypes of Mst1/2-dKO lungs, we measured the mRNA levels of several lung differentiation markers. A quantitative RT-PCR (qRT-PCR) analysis revealed a significant reduction in the levels of SP-A, SP-B, SP-C, SP-D, ABCA3 (lamellar body marker), and T1α mRNA in Mst1/2-dKO mice, but no change in the expression of CC10 (Fig. 2 D and E). Taken together, these findings demonstrate that the absence of Mst1 and Mst2 results in defective peripheral lung differentiation, characterized by impairments in alveolarization and delayed differentiation of type I and type II pneumocyte, finally causes RDS-like phenotype.
Mst1/2 Knockdown Decreases the Expression of SP-B and SP-C in MLE-12 Cells.
To confirm the hypothesis that Mst1 and Mst2 are indeed required for peripheral lung maturation and intrinsic surfactant protein production, we performed in vitro studies using MLE-12 (murine lung epithelial) cells expressing surfactant proteins (24). Consistently, depletion of Mst1/2 with small-interfering (hairpin) RNA (shRNA) suppressed the expression of SP-B and proSP-C (Fig. 2C). TEM revealed that the numbers of lamellar bodies, microvilli, and internal cellular organelles were markedly decreased in Mst1/2 knockdown MLE-12 (Fig. 2C), suggesting that these cells had become defective type II pneumocytes that cannot produce surfactant normally. Consistently, there was a significant reduction in the levels of SP-B, SP-C, and ABCA3 mRNA in Mst1/2 knockdown MLE-12 cells (Fig. 2F).
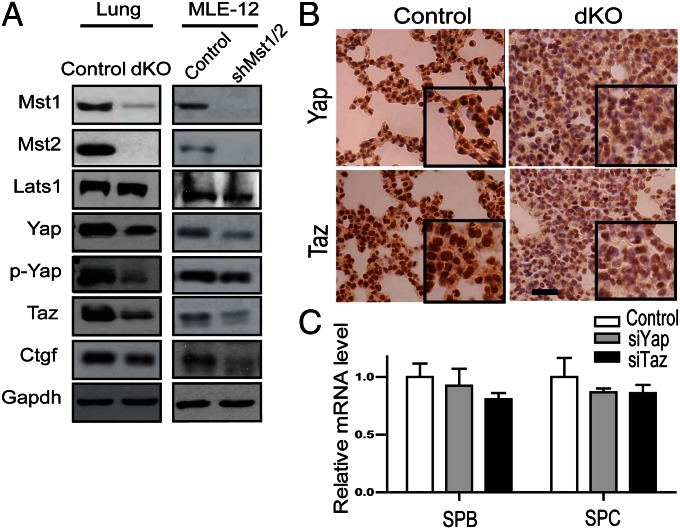
Mst1/2 Regulate Maturation of Type II Pneumocytes and Surfactant Production Independently of YAP/TAZ (transcriptional coactivator with PDZ-binding motif).
We next investigated the mechanism by which Mst1 and Mst2 regulate surfactant protein production and maturation of type II pneumocytes. We first tested the hyperactivity of YAP in lung tissues, because deletion of Mst1/2 in the liver and intestine induced hyperactivity of YAP with decrease in its phosphorylation (15, 16). Unexpectedly, the lung tissue of Mst1/2-dKO mice did not exhibit the hyperactivity of YAP or TAZ (transcriptional coactivator with PDZ-binding motif, a YAP paralog); instead, both phosphorylated and unphosphorylated forms of YAP were decreased (Fig. 3 A and B). The level of CTGF (connective tissue growth factor), which is a direct target of YAP/TAZ, was also decreased in Mst1/2-dKO lungs, indicating reduced YAP activity (Fig. 3A). In agreement with Mst1/2-dKO mice data, depletion of Mst1/2 in MLE-12 cells did not increase the level of YAP/TAZ or CTGF (Fig. 3A). In fact, siRNA-mediated knockdown of YAP and TAZ in MLE-12 cells did not affect the expression of SP-B or SP-C (Fig. 3C). Taken together, these findings indicate that Mst1 and Mst2 control the maturation of the type II pneumocytes and surfactant protein production by a cell-autonomous mechanism, independently of canonical Hippo signaling pathway through YAP/TAZ.
Fig. 3.
Mst1/2 regulate expression of surfactant proteins independently of the Hippo pathway. (A) Western blot of E18.5 lung lysates and MLE-12 cell lysates. Expression of Mst1, Mst2, Lats1, YAP, p-YAP (S127), TAZ, and CTGF were analyzed. MLE-12 mouse lung epithelial cells were infected with lentiviruses encoding scrambled (control) or Mst1/Mst2 shRNA. (B) Lung sections of control and Mst1/2-dKO mice at E18.5 were stained with antibodies to YAP and TAZ. (Scale bar, 200 μm.) Insets, 4× magnification. (C) MLE-12 cells were transfected with control, YAP, or TAZ siRNA. SP-B, and SP-C mRNA were measured by qRT-PCR. *P < 0.05.
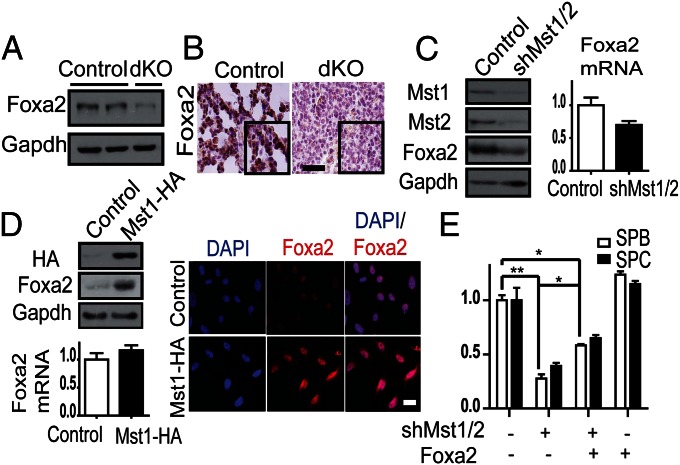
Mst1/2 Regulate Production of Surfactant Proteins Through Foxa2.
We next searched the potential partners to interact with Mst1/2 during peripheral lung maturation. As noted above, many mutant mice exhibit lung immaturity and RDS-like phenotype similar to that of Mst1/2-dKO mice. Among them, TTF-1, Foxa2, and C/EBPα are critical transcription factors for surfactant homeostasis. Because Mst1 phosphorylates Foxo1/3 in the forkhead domain (19, 25), which is highly conserved in Foxa2, it is possible that Mst1/2 may regulate Foxa2 during lung maturation. Thus, we checked the expression of Foxa2 in Mst1/2-dKO mice. Mst1/2-dKO lung tissue exhibited remarkably reduced expression of Foxa2 (Fig. 4 A and B), with no significant alteration of TTF-1 or C/EBPα (Fig. S6).
Fig. 4.
Deletion/knockdown of Mst1 and Mst2 decreases Foxa2 expression in mouse lungs and A549 cells. (A) Decreased Foxa2 expression in Mst1/2-dKO lungs at E18.5. (B) Lung sections from E18.5 control and Mst1/2-dKO mice were stained with an antibody against Foxa2. (Scale bar, 100 μm.) (C) A549 cells were infected with lentivirus encoding scrambled shRNA (control) or Mst1/Mst2 shRNA. Western blot revealed that Mst1/2 knockdown decreased endogenous Foxa2 expression. The level of mRNA of Foxa2 did not changed significantly (P = 0.7). (D) A549 cells were infected with empty (control) or Mst1-HA–expressing retrovirus. Western blotting for HA and Foxa2 showed that overexpression of Mst1 increased endogenous Foxa2 expression. The level of mRNA of Foxa2 was not changed significantly (P = 0.4). Mst1-overexpressing and control cells were immunostained for Foxa2 (red) and counterstained with DAPI (blue). (Scale bar, 20 μm.) (E) Mst1/2-knockdown MLE-12 cells were infected with empty (control) or Foxa2-expressing retrovirus. SP-B and SP-C mRNA levels were quantified by qPCR. Foxa2 expression partially rescued the deficiency of SP-B and SP-C in Mst1/2-knockdown MLE-12 cells. *P < 0.05, **P < 0.01.
Considering both the similarity in lung phenotypes between Foxa2-null mice and Mst1/2-dKO mice and the reduction of Foxa2 expression in Mst1/2-dKO lungs, it is possible that the perinatal phenotype of Mst1/2-dKO may be related to the decrease in Foxa2 expression. To confirm the functional relationship between Mst1/2 and Foxa2, we first checked for alterations of endogenous Foxa2 expression by Mst1/2 in A549 cells, which originate from type II pneumocytes. Consistent with in vivo data, Mst1/2 knockdown decreased the expression of endogenous Foxa2 and Mst1 overexpression increased that of Foxa2, but mRNA levels of Foxa2 did not change significantly (Fig. 4 C and D). To confirm the role of Foxa2 in Mst1/2-mediated surfactant production, we tested the effects of Foxa2 overexpression in Mst1/2-knockdown MLE-12 cells. Expectedly, overexpression of Foxa2 partially rescued the decrease in SP-B and SP-C in Mst1/2-knockdown MLE-12 cells (Fig. 4E), suggesting that Mst1/2 regulate surfactant protein production through Foxa2.
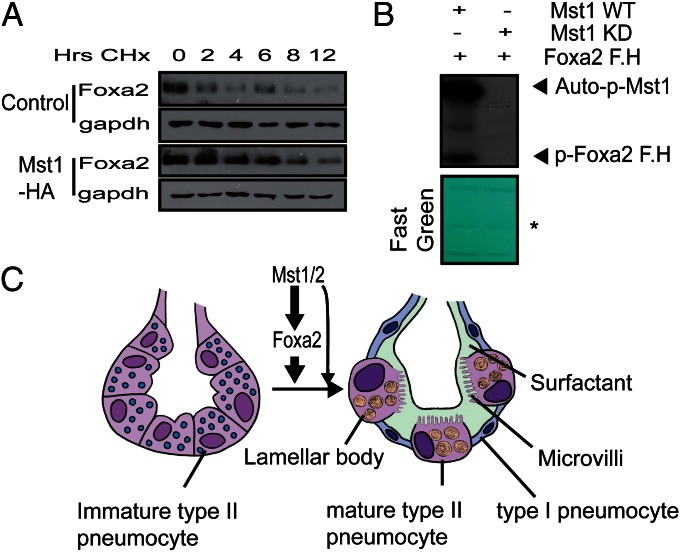
Next, we tested whether Mst1/2 affect the protein stability of Foxa2. With protein synthesis inhibited by cycloheximide, overexpression of Mst1 in A549 cells significantly increased the protein stability of Foxa2 (Fig. 5A). Next, we tested whether Mst1/2 phosphorylate Foxa2 because Mst1/2 are known to regulate Foxo1/3 activities through phosphorylation. In vitro kinase assays showed that the forkhead domain of Foxa2 was phosphorylated by WT, but not kinase-dead, Mst1 (Fig. 5B). However, unlike Mst1/2, large tumor suppressor 2 (Lats2) and their scaffold protein Mob1 did not phosphorylate the forkhead domain of Foxa2 (Fig. S7). These data support that Mst1/2 regulates Foxa2 independently of canonical Hippo pathway.
Fig. 5.
Mst1 increases the stability of Foxa2 protein and phosphorylates the forkhead domain of Foxa2. (A) A549 cells were infected with empty (control) or Mst1-HA–expressing retrovirus. The stability of endogenous Foxa2 protein with and without Mst1 overexpression was measured in cells in which new protein synthesis was inhibited with cycloheximide (30 μg/mL). Cells were harvested at the indicated times after addition of cycloheximide and analyzed for Foxa2 by Western blotting. Relative protein levels were compared with 0-h time points. (B) In vitro kinase assays. Anti-Flag immunoprecipitates prepared from 293T cell lysates expressing Flag-tagged WT or kinase-dead (KD) MST1 were assayed for kinase activity. GST fused with the forkhead domain of Foxa2 was used as a substrate. After autoradiography, the membrane was stained with Fast Green to reveal substrate input (Lower). *Ig heavy chain. (C) Schematic model of Mst1/2 and Foxa2 regulation of peripheral lung maturation. Mst1/2 and Foxa2 regulate type 2 pneumocytes to differentiate and produce lamellar bodies, surfactant, and microvilli. The differentiation of type II pneumocytes to type I pneumocytes requires Mst1/2 and Foxa2 signals.
Finally, to further characterize the contribution of Foxa2 in the Mst1/2-dKO mice phenotype, we analyzed the some representative Foxa2 target genes in Mst1/2-dKO mice. Published microarray data of Foxa2-null mice showed significant alteration of genes regulating antioxidant production (Sod3, Gstm5, and Mt1), lipid homeostasis (Abca3, Fabp5, Scd1, and Pon1), and innate host defense (Chi3l1, Hc, and Sftpa) (8). The qRT-PCR results showed that, like Foxa2-null mice, Mst1/2 dKO mice have decreased expression of genes related to innate host defense, such as Chi3l1 and Hc, and genes regulating lipid homeostasis, including Fabp5 and Abca3. These data suggested that the deregulation of Foxa2 contributes, at least in part, to the phenotype of Mst1/2 dKO mice. It was notable that some genes of antioxidant production including Sod3, Mt1, and Gstm5 were also decreased in contrast to the results of Foxa2 null mice (Fig. S8 and Table S1). We speculate that Mst1/2 can affect the differentiation of type II pneumocytes by itself or through other mediators, such as Foxo1/3, which regulate antioxidant production (21, 25).
Discussion
Deletion of Mst1/2 in the developing lung epithelium resulted in deregulated differentiation of type II pneumocytes and diminished production of surfactant proteins. These lung phenotypes are consistent with the RDS symptoms of human preterm babies (1). The immature type II pneumocytes were characterized by a lack of apical microvilli, lamellar bodies, and surfactant protein expression. Depletion of Mst1/2 in MLE-12 cells also caused a decrease in the expression of SP-B and SP-C.
Mst1/2 deletion in lung epithelial cells caused deregulated maturation of type II pneumocytes in the absence of YAP hyperactivity. These findings were surprising, given that Mst1/2 deletion has been found to increase the proliferation of intestinal and liver epithelial cells through YAP activation (15, 16, 26). In the liver and intestine, Mst1/2 functions mainly through YAP in the canonical Hippo pathway (12). However, in the context of the developing lung, Mst1/2 coordinates peripheral lung maturation, at least in part, through the transcription factor Foxa2 (Fig. 5C). We also evaluated the phenotype of WW45fl/fl;Nkx2.1-Cre mice. Whereas deletion of WW45 in the liver and intestine caused expansion of epithelial cells and eventually development of tumors, a phenotype similar to that of Mst1/2 mutant mice (13, 26, 27), lung-specific deletion of WW45 caused no abnormalities during development or in adults (Fig. S9). These data also support the suggestion that Mst1/2 regulates lung maturation through Foxa2 in the noncanonical Hippo pathway which is independent of YAP. TAZ-null mice show abnormal alveolarization during lung development, which causes airway enlargement, mimicking emphysema in adult mice (28). However, TAZ-null mice, unlike Mst1/2-dKO mice, express SP-C normally and lack an obvious RDS-like phenotype. Thus, the phenotype of Mst1/2-dKO mice is likely caused by a mechanism more complex than a simple decrease in TAZ. It is interesting to note that, unlike the liver and intestine, lung-specific deletion of Mst1/2 leads to reduction of YAP protein levels. It is possible that the lung context changed by Mst1/2 deletion (for example, altered mechanical strain or cell density) may affect the expression of YAP and TAZ. Future studies will be needed to investigate the exact mechanisms of YAP/TAZ regulation in developing lung.
Importantly, several studies have revealed the roles of the noncanonical Hippo pathway independent of YAP. In neural cells, Mst1 directly phosphorylates a conserved site in the forkhead domain of FoxO1/3 and thereby induces apoptosis (25). The Mst1-FoxO pathway is also important for protecting blood-naïve T cells from reactive oxygen species (19). Our study also confirmed that Mst1 phosphorylates the forkhead domain in Foxa2 (Fig. 5B) and increases Foxa2 protein stability. It is conceivable that the mammalian Hippo kinases Mst1/2 regulate other transcription factors with forkhead domain through phosphorylation as part of a noncanonical Hippo signaling pathway, but further mechanistic and functional studies will be needed to verify this in a specific cellular context. Foxa2 null mice and Mst1/2-dKO mice exhibit similar phenotype in the point of RDS. This similarity highlights the possibility that the decrease in Foxa2 expression may be responsible for the phenotypes in Mst1/2-dKO mice. More specifically, Mst1/2-dKO mice have many overlapping features, including immature type II pneumocytes, disorganized lamellar bodies, and a lack of type I pneumocytes. However, some features, such as goblet cell hyperplasia and decreased CC10 expression, are present only in Foxa2 mutant mice (6, 8). We think that these differences are caused by the fact that Mst1/2 dKO mice have decreased but retained Foxa2 expression. In addition, because the RDS phenotype of Mst1/2 dKO mice was fully penetrant, some postnatal phenotypes like goblet cell hyperplasia could not be efficiently observed. Postnatal deletion of Mst1/2 in lung may be required to accurately address these issues.
The transcription factor TTF-1 has been shown to be involved in lung development and is a target of rat Mst2 kinase (29). An in vitro study reported that Foxa2 regulates TTF-1 expression, but it was also reported that Foxa1−/−;Foxa2Δ/Δ mice exhibit no change in TTF-1 expression (7). Consistent with this latter study, we found that, although Foxa2 expression was markedly decreased in the lungs of Mst1/2-dKO mice, TTF-1 expression was not changed (Fig. S6).
Regulation of type II pneumocyte differentiation is important, not only in the developmental stage but also during tumorigenesis and lung regeneration after injury (3). However, lung developmental problems of mutant mice were ultimately fatal, preventing us from assessing effects of Mst1/2 deletion in adult animals, such as adult interstitial lung disease and tumorigenesis. A recent study revealed that K-Ras–induced distal lung adenocarcinomas originate from type II pneumocytes (30). It will thus be interesting to investigate the functions of Mst1 and Mst2 at the point of type II pneumocyte differentiation in K-Ras–induced lung tumorigenesis and lung regeneration after injury.
In conclusion, deletion of Mst1/2 from the respiratory epithelium caused deregulation of peripheral lung differentiation, prevented maturation of type II pneumocytes, reduced surfactant proteins, and decreased type I pneumocytes, causing RDS at the perinatal stage. The identification of Mst1/2 as unique regulators of peripheral lung differentiation may aid in the development of new strategies for early diagnosis and effective treatment of many peripheral lung diseases, such as RDS, interstitial lung disease, and lung cancer.
Materials and Methods
Generation Mst1fl/fl; Mst2−/−;Nkx2.1-Cre Mice.
The generation of Mst1fl/fl; Mst2−/−;Nkx2.1-Cre mice is described in SI Materials and Methods. Mouse care and experiments were performed in accordance with procedures approved by the Korea Advanced Institute of Science and Technology-Animal Care and Use Committee.
Histological and Electron Microscopy Analysis.
Lung tissue was fixed in 10% (vol/vol) formalin for paraffin sections and in 4% (wt/vol) paraformaldehyde for preparation of frozen sections. Sections (4-μm thickness) were stained with H&E or were subjected to immunohistochemical analysis using standard protocols. Detailed protocols and information on antibodies used for staining are presented in SI Materials and Methods. Electron microscopy analyses of lung and cell samples were performed according to standard protocols as previously described (14). Detailed procedures for electron microscopy analysis are described in SI Materials and Methods.
Cell Proliferation and Apoptosis.
Cell proliferation was analyzed by staining for Ki67 (Novocastra), and apoptosis was evaluated using an In Situ Cell Death Detection kit (Roche), as described previously (14).
Immunoblot Analysis.
Lung tissues were homogenized in Proprep lysis buffer (iNtRON Biotechnology) containing protease and phosphatase inhibitors. Lysates were centrifuged at 15,000 × g for 15 min at 4 °C, and the resulting supernatants were analyzed by immunoblotting. The antibodies used for detection are described in SI Materials and Methods.
qRT-PCR Analysis and in Vitro Kinase Assay.
Total RNA was isolated from lung tissues using Riboex (Geneall) according to the manufacturer’s protocol. Real-time reactions were performed as described previously (25). The relative concentration of mRNA was normalized to the concentration of GAPDH mRNA in each sample. GAPDH mRNA was quantified using the primers 5′-CTT CAC CAC CAT GGA GGA GGC-3′ and 5′-GGC ATG GAC TGT GGT CAT GAG-3′ primers. Other primer sequences and in vitro kinase assays are described in SI Materials and Methods and Tables S2–S4.
Foxa2 Protein Stability.
Cycloheximide was added to the cells at a final concentration of 30 μg/mL. The cells were harvested at the indicated times following cycloheximide treatment, and protein extracts were prepared. The protocols were performed as previously described (31), with some modifications.
Statistical Analysis.
Quantitative data are presented as means ± SDs unless indicated otherwise. Differences between means were evaluated using Student’s unpaired test. A P value < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Taechang Yang, Eun Soon Lee, and Seung Hye Yang for their technical assistance. This study was supported by National Creative Research Initiative Program Grant 20120001228.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1220603110/-/DCSupplemental.
References
- 1.Whitsett JA, Wert SE, Trapnell BC. Genetic disorders influencing lung formation and function at birth. Hum Mol Genet. 2004;13(Spec No 2):R207–R215. doi: 10.1093/hmg/ddh252. [DOI] [PubMed] [Google Scholar]
- 2.Whitsett JA, Wert SE, Weaver TE. Alveolar surfactant homeostasis and the pathogenesis of pulmonary disease. Annu Rev Med. 2010;61:105–119. doi: 10.1146/annurev.med.60.041807.123500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitsett JA, Weaver TE. Hydrophobic surfactant proteins in lung function and disease. N Engl J Med. 2002;347(26):2141–2148. doi: 10.1056/NEJMra022387. [DOI] [PubMed] [Google Scholar]
- 4.Maeda Y, Davé V, Whitsett JA. Transcriptional control of lung morphogenesis. Physiol Rev. 2007;87(1):219–244. doi: 10.1152/physrev.00028.2006. [DOI] [PubMed] [Google Scholar]
- 5.Martis PC, et al. C/EBPalpha is required for lung maturation at birth. Development. 2006;133(6):1155–1164. doi: 10.1242/dev.02273. [DOI] [PubMed] [Google Scholar]
- 6.Chen G, et al. Foxa2 programs Th2 cell-mediated innate immunity in the developing lung. J Immunol. 2010;184(11):6133–6141. doi: 10.4049/jimmunol.1000223. [DOI] [PubMed] [Google Scholar]
- 7.Wan H, et al. Compensatory roles of Foxa1 and Foxa2 during lung morphogenesis. J Biol Chem. 2005;280(14):13809–13816. doi: 10.1074/jbc.M414122200. [DOI] [PubMed] [Google Scholar]
- 8.Wan H, et al. Foxa2 is required for transition to air breathing at birth. Proc Natl Acad Sci USA. 2004;101(40):14449–14454. doi: 10.1073/pnas.0404424101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeFelice M, et al. TTF-1 phosphorylation is required for peripheral lung morphogenesis, perinatal survival, and tissue-specific gene expression. J Biol Chem. 2003;278(37):35574–35583. doi: 10.1074/jbc.M304885200. [DOI] [PubMed] [Google Scholar]
- 10.Davé V, et al. Calcineurin/Nfat signaling is required for perinatal lung maturation and function. J Clin Invest. 2006;116(10):2597–2609. doi: 10.1172/JCI27331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan C, Whitsett JA. Protein kinase A activation of the surfactant protein B gene is mediated by phosphorylation of thyroid transcription factor 1. J Biol Chem. 1997;272(28):17327–17332. doi: 10.1074/jbc.272.28.17327. [DOI] [PubMed] [Google Scholar]
- 12.Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19(4):491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu L, et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc Natl Acad Sci USA. 2010;107(4):1437–1442. doi: 10.1073/pnas.0911427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JH, et al. A crucial role of WW45 in developing epithelial tissues in the mouse. EMBO J. 2008;27(8):1231–1242. doi: 10.1038/emboj.2008.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou D, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16(5):425–438. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou D, et al. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proc Natl Acad Sci USA. 2011;108(49):E1312–E1320. doi: 10.1073/pnas.1110428108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren F, et al. Hippo signaling regulates Drosophila intestine stem cell proliferation through multiple pathways. Proc Natl Acad Sci USA. 2010;107(49):21064–21069. doi: 10.1073/pnas.1012759107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim TS, et al. Mammalian sterile 20-like kinase 1 suppresses lymphoma development by promoting faithful chromosome segregation. Cancer Res. 2012;72(20):5386–5395. doi: 10.1158/0008-5472.CAN-11-3956. [DOI] [PubMed] [Google Scholar]
- 19.Choi J, et al. Mst1-FoxO signaling protects naïve T lymphocytes from cellular oxidative stress in mice. PLoS ONE. 2009;4(11):e8011. doi: 10.1371/journal.pone.0008011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avruch J, et al. Protein kinases of the Hippo pathway: Regulation and substrates. Semin Cell Dev Biol. 2012;23(7):770–784. doi: 10.1016/j.semcdb.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oh S, et al. Crucial role for Mst1 and Mst2 kinases in early embryonic development of the mouse. Mol Cell Biol. 2009;29(23):6309–6320. doi: 10.1128/MCB.00551-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh HJ, et al. MST1 limits the kinase activity of aurora B to promote stable kinetochore-microtubule attachment. Curr Biol. 2010;20(5):416–422. doi: 10.1016/j.cub.2009.12.054. [DOI] [PubMed] [Google Scholar]
- 23.Janson R, Hogan BL. Epithelial progenitor cells in lung development, maintenance, repair, and disease. Annu Rev Cell Dev Biol. 2011;27:493–512. doi: 10.1146/annurev-cellbio-100109-104040. [DOI] [PubMed] [Google Scholar]
- 24.Sisson TH, et al. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am J Respir Crit Care Med. 2010;181(3):254–263. doi: 10.1164/rccm.200810-1615OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehtinen MK, et al. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell. 2006;125(5):987–1001. doi: 10.1016/j.cell.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 26.Lee KP, et al. The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc Natl Acad Sci USA. 2010;107(18):8248–8253. doi: 10.1073/pnas.0912203107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen L, Qin F, Deng X, Avruch J, Zhou D. Hippo pathway in intestinal homeostasis and tumorigenesis. Protein Cell. 2012;3(4):305–310. doi: 10.1007/s13238-012-2913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Makita R, et al. Multiple renal cysts, urinary concentration defects, and pulmonary emphysematous changes in mice lacking TAZ. Am J Physiol Renal Physiol. 2008;294(3):F542–F553. doi: 10.1152/ajprenal.00201.2007. [DOI] [PubMed] [Google Scholar]
- 29.Aurisicchio L, Di Lauro R, Zannini M. Identification of the thyroid transcription factor-1 as a target for rat MST2 kinase. J Biol Chem. 1998;273(3):1477–1482. doi: 10.1074/jbc.273.3.1477. [DOI] [PubMed] [Google Scholar]
- 30.Xu X, et al. Evidence for type II cells as cells of origin of K-Ras-induced distal lung adenocarcinoma. Proc Natl Acad Sci USA. 2012;109(13):4910–4915. doi: 10.1073/pnas.1112499109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rausa FM, 3rd, Hughes DE, Costa RH. Stability of the hepatocyte nuclear factor 6 transcription factor requires acetylation by the CREB-binding protein coactivator. J Biol Chem. 2004;279(41):43070–43076. doi: 10.1074/jbc.M407472200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.