Abstract
Host innate immune responses to DNA viruses involve members of the nucleotide-binding domain, leucine-rich repeat and pyrin domain containing protein (NLRP) family, which form “inflammasomes” that activate caspase-1, resulting in proteolytic activation of cytokines interleukin (IL)-1β and IL-18. We hypothesized that DNA viruses would target inflammasomes to overcome host defense. A Vaccinia virus (VACV) B-cell CLL/lymphoma 2 (Bcl-2) homolog, F1L, was demonstrated to bind and inhibit the NLR family member NLRP1 in vitro. Moreover, infection of macrophages in culture with virus lacking F1L (ΔF1L) caused increased caspase-1 activation and IL-1β secretion compared with wild-type virus. Virulence of ΔF1L virus was attenuated in vivo, causing altered febrile responses, increased proteolytic processing of caspase-1, and more rapid inflammation in lungs of infected mice without affecting cell death or virus replication. Furthermore, we found that a hexapeptide from F1L is necessary and sufficient for inhibiting the NLRP1 inflammasome in vitro, thus identifying a peptidyl motif required for binding and inhibiting NLRP1. The functional importance of this NLRP1-binding motif was further confirmed by studies of recombinant ΔF1L viruses reconstituted either with the wild-type F1L or a F1L mutant that fails to bind NLRP1. Cellular infection with wild-type F1L reconstituted virus-suppressed IL-1β production, whereas mutant F1L did not. In contrast, both wild-type and mutant versions of F1L equally suppressed apoptosis. In vivo, the NLR nonbinding F1L mutant virus exhibited an attenuated phenotype similar to ΔF1L virus, thus confirming the importance of F1L interactions with NLRP1 for viral pathogenicity in mice. Altogether, these findings reveal a unique viral mechanism for evading host innate immune responses.
Keywords: virus infection, virology, innate immunity, poxviruses
Nucleotide-binding domain and leucine-rich repeat containing receptors (NLRs) constitute a large family of intracellular innate immunity proteins involved in host defense (1). Upon activation, NLRs form large protein complexes called “inflammasomes” that bind and activate caspase-1 family proteases, resulting in proteolytic activation of proinflammatory cytokines (which include pro–IL-1β and pro–IL-18) and sometimes causing a caspase-1–dependent form of cell death known as pyroptosis (2, 3). Members of the NLR family possess a conserved architecture that includes leucine-rich repeats (LRRs) that are thought to act as receptors for various pathogen-derived molecules. Certain NLRs, and several other types of innate immunity proteins, are involved in detecting foreign double-stranded DNA (dsDNA) in cells, a function linked to host defense against DNA viruses (4–9).
Poxviruses constitute a viridae known for their large, complex DNA genomes. Vaccinia virus (VACV) is a prototypical member of the poxvirus family, which replicates in the cytoplasm of host cells and encodes numerous proteins that manipulate the host response to infection (10). VACV encodes several viral homologs of the cellular antiapoptotic protein B-cell CLL/lymphoma 2 (Bcl-2), including A46R, A52R, B14R, C1L, C6L, C16/B22R, F1L, K7R, N1L, and N2L (11, 12). Despite low sequence similarity to cellular Bcl-2 family members, several of these viral homologs were discovered to adopt an α-helical fold similar to Bcl-2 (13-17). However, not all of the VACV-encoded Bcl-2–like proteins possess antiapoptotic activity (18), implying that they have other functions. Indeed, the viral Bcl-2–like proteins A52, B14, N1, and K7 were found to inhibit NF-κB pathways at various checkpoints (11).
Recently, cellular Bcl-2 family proteins have been demonstrated to interact with NLRs to impact innate immunity responses (19, 20). We therefore hypothesized that viral Bcl-2 homologs encoded by VACV might similarly inhibit inflammasome signaling during infection. Our data provide evidence that the viral Bcl-2 homolog F1L (but not N1L) is a suppressor of NLR family proteins involved in IL-1β activation. Furthermore, by testing genetically engineered VACV in a mouse model, our findings also demonstrate a role for NLR suppression in the pathogenic mechanism by which F1L contributes to virulence in vivo.
Results
VACV Bcl-2 Homolog, F1L, Binds NLRP1.
Of the viral Bcl-2 homologs encoded by VACV, only F1L and N1L have been reported to display antiapoptotic activity and to bind proapoptotic members of the cellular Bcl-2 family such as Bcl-2–associated X protein (Bax) (14, 18, 21). We explored the ability of F1L to bind various NLR family members, making comparisons with apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), absent in melanoma 2 (AIM2), and other proteins involved in innate immune defense against viruses. In cell transfection experiments, F1L coimmunoprecipitated with NLRP1, a NLR member inhibited by Bcl-2 and Bcl-XL (20), but not with NLR family members NLRP3 and nucleotide-binding oligomerization domain-containing protein 2 (NOD2) (NLRC2) and not with AIM2 or ASC (Fig. 1A and Fig. S1A). Colocalization of F1L with NLRP1 was also demonstrated by immunofluorescence confocal microscopy (Fig. S1C). In contrast to F1L, the viral N1L protein did not associate with NLRP1 as determined by coimmunoprecipitation (co-IP) assay and by immunofluorescence confocal microscopy (Fig. 1A and Fig. S1C).
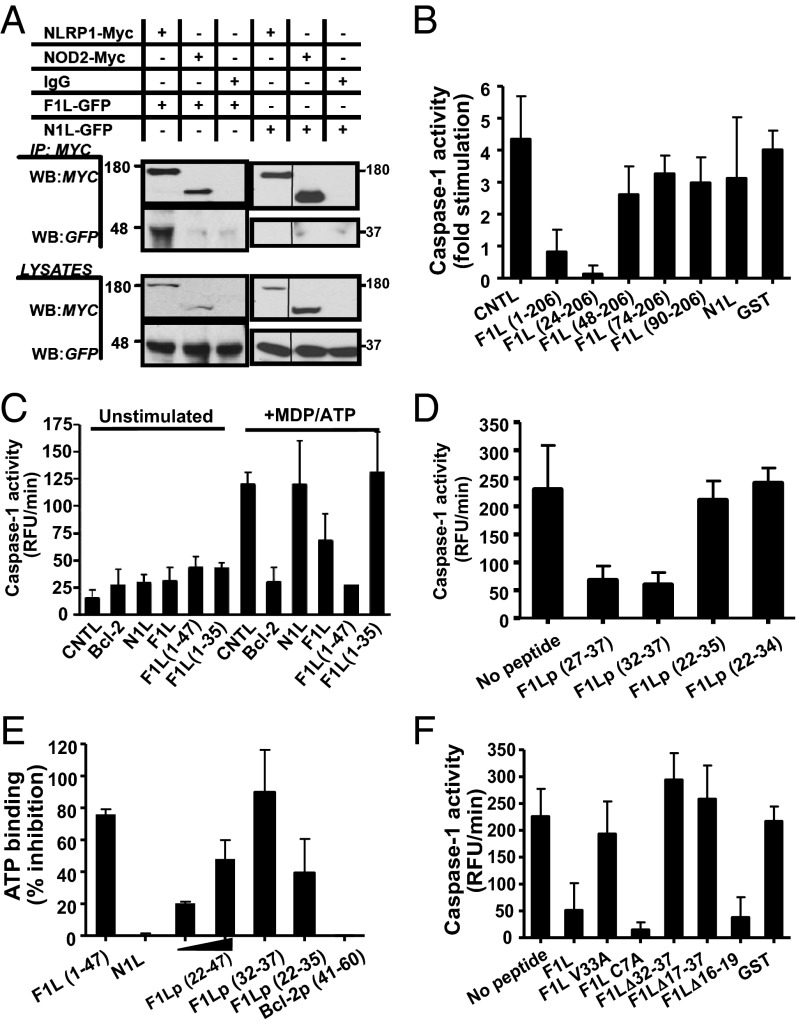
Fig. 1.
F1L binds NLRP1. (A) HEK293T cells were transiently cotransfected with plasmids encoding Myc-tagged NLRP1 or NOD2 and either GFP-tagged N1L or GFP-tagged F1L. Approximately 106 cells were lysed and subjected to IP by using protein-G anti-GFP. Immune-complexes (Upper) and cell lysates (Lower) were separated by SDS/PAGE and analyzed by immunoblotting using anti-Myc and anti-GFP antibodies. (B–D and F) Reactions contained His6-NLRP1, procaspase-1, ATP, mM Mg2+, MDP and GST-Bcl-2, N1L, or various GST-F1L constructs. Caspase-1 activity was measured by hydrolysis of Ac-WEHD-AMC substrate. (B) The version of F1L protein used here (residues 1–206) lacks the C-terminal transmembrane domain. Results are reported as fold stimulation induced by MDP/ATP (mean ± SD; n = 3). (C) F1L residues 1–47 are sufficient to inhibit NLRP1. Caspase-1 activity is reported as measuring relative fluorescence unit (RFU) generated per minute (mean ± SD, n = 3). (D) Caspase-1 activity is reported as measuring relative fluorescence unit (RFU) generated per minute (mean ± SD, n = 3). (E) F1L inhibits ATP binding to NLRP1. His6-NLRP1 was incubated for 5 min on ice in the presence of different F1L peptides. The mixture was then incubated for an additional 5 min with 1 μM MDP, FL-conjugated ATP analog, and Mg2+ (23). ATP binding was analyzed by fluorescence polarization (milliPolars; mP), and the percentage of inhibition was determined vs. NLRP1 incubated only with MDP (mean ± SD; n = 3). (F) Various recombinant GST-F1L mutants were produced and tested in the in vitro reconstituted NLRP1 inflammasome for inhibitory activity as above (mean ± SD, n = 3).
The interaction of F1L with NLRP1 was further confirmed by in vitro protein binding assays, using GST-tagged recombinant proteins. Recombinant F1L bound to full-length NLRP1 but not a deletion mutant lacking the LRRs (Fig. S1B), binding characteristics similar to Bcl-2 and Bcl-XL (20, 22). Comparisons of essentially full-length F1L (residues 1–206, in which the C-terminal transmembrane domain was removed for solubility purposes) with various truncation mutants (lacking progressively longer segments from the N-terminal end of F1L) showed that deletion of the first 47 amino acids from F1L abolished binding to NLRP1 in vitro (Fig. S2A).
F1L Inhibits NLRP1 in Vitro.
We explored whether F1L can inhibit NLRP1 activity by using an in vitro reconstituted system. We reconstituted the NLRP1 inflammasome in vitro by using purified recombinant proteins, showing that the combination of NLRP1 ligand muramyl dipeptide (MDP) and ATP induces NLRP1 oligomerization and caspase-1 activation in a Bcl-2/Bcl-XL-suppressible manner (22, 23). Adding recombinant F1L to these in vitro reactions suppressed NLRP1-driven caspase-1 activity, whereas N1L and GST control proteins did not (Fig. 1B). Comparison of various fragments of F1L (truncated from the N terminus) suggested that residues between aa 24 and 48 of F1L are required for NLRP1 suppression (Fig. 1B), which correlated with binding to NLRP1 (Fig. S2A). Additional experiments using a GST-F1L (1–47) fusion protein showed that the first 47 amino acids of F1L are sufficient for suppressing NLRP1 activity in vitro (Fig. 1C).
Next, we chemically synthesized peptides (Table S1) corresponding to residues 1–47 of F1L and various shorter segments, examining their effects on the reconstituted NLRP1 inflammasome in vitro. The addition of the F1L peptide 1–47 and shorter peptides (residues 22–47 and 27–37) inhibited NLRP1-mediated caspase-1 activation to baseline levels (Fig. 1D and Fig. S2B). However, these F1L peptides were neither active against the gain-of-function mutant NLRPΔLRR (which does not bind F1L protein) nor against purified active recombinant caspase-1, showing NLRP1 dependence (Fig. S2 C and D). Furthermore, the F1L peptide (22–47) binds to NLRP1, as determined by fluorescence polarization assay using a fluorochrome-conjugated peptide (Fig. S2E). In addition to using a fluorigenic peptide substrate (Ac-WEHD-AMC) to monitor caspase-1 activity, we also assessed NLRP1-induced proteolytic processing of caspase-1 by immunoblotting, showing that both synthetic F1L peptide (22–47) and recombinant GST-F1L (1–47) protein inhibited proteolytic processing of caspase-1 driven by NLRP1, but not by mutant constitutively active NLRP1ΔLRR (Fig. S2F).
By testing still shorter peptides, F1L residues 32–37 were ultimately determined to be sufficient to inhibit NLRP1 in vitro (Fig. 1D). This hexapeptide potently suppressed NLRP1 activity in vitro, with IC50 value of 18.8 ± 6.7 nM, similar to reported results for the Bcl-2 and Bcl-XL proteins (20) (Fig. S2G). In addition to inhibiting caspase-1 activation mediated by NLRP1, active F1L peptides also suppressed MDP-induced binding of ATP to NLRP1 (Fig. 1E), but N1L did not. Structure-activity analysis of the F1L 32–37 peptide conducted by using a series of alanine substitutions showed that alanine substitutions of residues 32, 33, 35, and 37 abolished NLRP1 inhibitory activity, whereas residues 34 and 36 tolerated these substitutions (Fig. S2H). These findings were confirmed in the context of the F1L protein by mutagenesis experiments (Fig. 1F and Fig. S2I). Thus, the region 32–37 of F1L is essential for binding to and inhibition of NLRP1 in vitro.
F1L Inhibits Virus- and NLR-Mediated IL-1β Production in Cells.
Several approaches were taken to assess the cellular activity of F1L as a potential inhibitor of NLRP1. First, cell transfection experiments were performed in which inflammasome components (NLRP1, ASC, procaspase-1, and mouse pro–IL-1β) were expressed from plasmids in HEK293T cells, then mouse interleukin-1β (mIL-1β) secretion was measured after NLRP1 stimulation with MDP. The full-length F1L protein inhibited MDP-induced mIL-1β production in these assays, as described for Bcl-2 and Bcl-XL (20). Comparison of various mutants of F1L demonstrated a correlation between NLRP1 binding and suppression of MDP-induced mIL-1β. For example, F1LΔ57–78, a mutant that fails to bind Bcl-2 family proteins but that retains NLRP1 binding activity, successfully suppressed mIL-1β production, whereas non–NLRP1-binding mutant F1LΔ1–44 did not (Fig. 2 A and B).
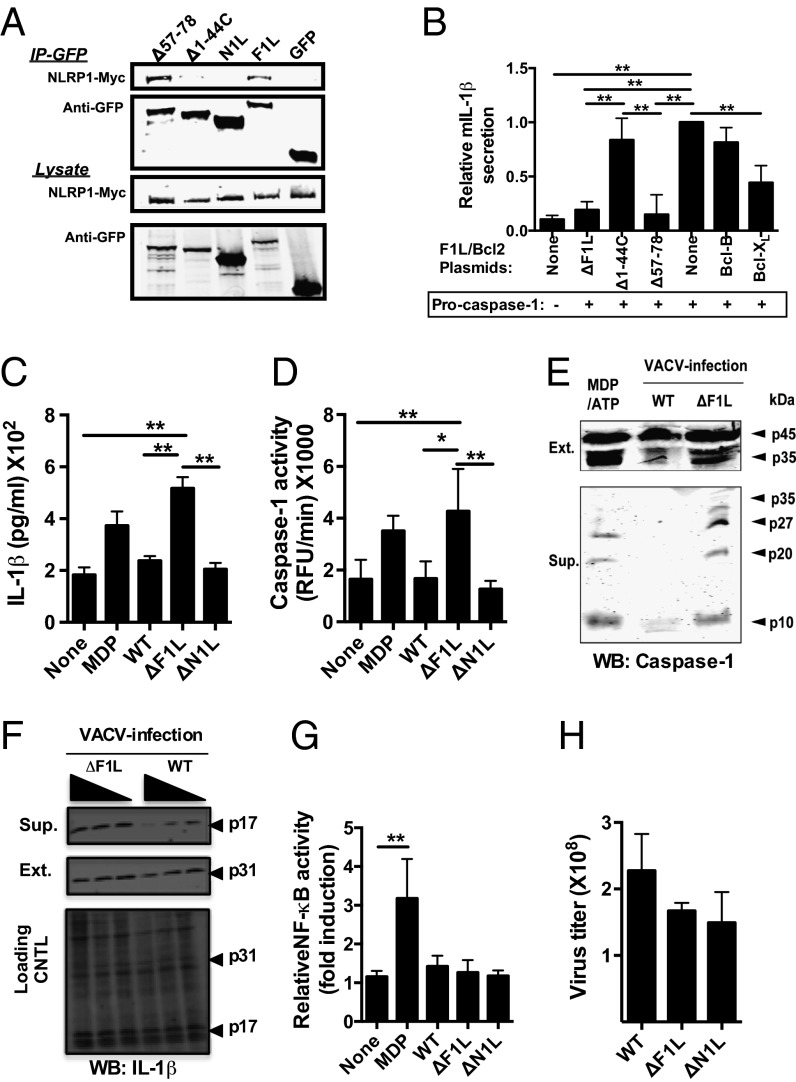
Fig. 2.
Increased caspase-1 cleavage and IL-1β processing in macrophage cultures infected with F1L-deficient virus. (A) HEK293T cells were transiently cotransfected with plasmids encoding Myc-tagged NLRP1 and various GFP-tagged F1L constructs. Cell lysates were normalized for protein content, and co-IPs were performed as above. (B) F1L inhibits NLRP1 activity in cells. HEK293T cells were cotransfected in 12-well plates with plasmids encoding 400 ng of mouse proIL-1β, 25 ng of pro–Caspase-1, 20 ng of ASC, 200 ng of NLRP1, and 600 ng of various GFP-tagged F1L constructs, maintaining a total DNA amount of 1 µg by addition of control (empty) plasmid. At 18 h after transfection, cells were stimulated with MDP (5 μg/mL) for 8 h. Cell culture supernatants were analyzed by ELISA for secreted mIL-1β. As controls, we used pcDNA3-Myc (None), Bcl-B-Myc (negative), and Bcl-XL-Myc (positive). NLRP1 induced mIL-1β secretion was determined by subtracting spontaneous secretion (unstimulated cells) from MDP-stimulated selection. Data were normalized relative to pcDNA3 control-transfected cells (=1.0). All results are mean ± SD; n =3. Statistical significance was determined by unpaired t tests. (C and D) 12-D-tetradecanoylphorbol-13-acetate (TPA)-differentiated THP-1-Blue cells (106) were infected (MOI = 1) with VACV-WR (WT), ΔF1L, or ΔN1L viruses. Supernatants were collected 18 h later for analysis. (C) IL-1β secretion was measured by using ELISA. (D) Caspase-1 activity in culture supernatants was measured by hydrolysis of Ac-WEHD-Rhodamine substrate. As positive control for C and D, macrophages were stimulated for 18 h with MDP (5 μg/mL). All results are mean ± SD; n = 3. (E) TPA-differentiated THP-1 cells were infected as above. Cleaved fragments of caspase-1 were detected in either cell extracts (Upper) or culture supernatants (Lower) by immunoblotting using a mixture of anti-p10 and p-20 antibodies. As a positive control for caspase-1 cleavage, macrophages were primed 12 h with 50 ng/mL LPS, incubated for 4 h with 2 μg/mL MDP, then stimulated with 2.5 mM ATP for 20 min. (F) Pro–IL-1β (p31) and mature IL-1β (p17) cytokine were detected as for caspase-1. (G) NF-κB activity from C was measured by alkaline phosphatase reporter gene assays. (H) Viral titers were measured in THP-1 cells from C.
Second, we compared effects of wild-type (WT) VACV [Western Reserve (WR) strain], F1L-deficient (ΔF1L), and N1L-deficient (ΔN1L) viruses (18, 21) on caspase-1 and IL-1β by using a cell culture infection model. Infection of human acute monocytic leukemia cell line (THP-1) macrophages with ΔF1L virus resulted in greater caspase-1 activity and IL-1β secretion compared with WT and ΔN1L virus (Fig. 2 C and D). Experiments using liopopdysaccharide (LPS) priming of THP-1 cells (to induce more robust pro–IL-1β production) resulted in similar conclusions, as did experiments using primary cultured human peripheral blood mononuclear cells (PBMCs) primed with LPS (Fig. S3 A and C). We also assessed proteolytic processing of caspase-1 in ΔF1L virus-infected cells, making comparison with infection with WT virus. Cells infected with ΔF1L contained markedly higher levels of processed caspase-1, both when measured in the intracellular compartment (where levels of the protein (p)35 processed subunit of procaspase-1 were clearly higher in ΔF1L virus compared with WT virus-infected cells) and the secreted compartment (where levels of both p20 and p10 processed forms of mature caspase-1 were clearly higher in ΔF1L virus compared with WT virus-infected cells) (Fig. 2E). The inhibition of IL-1β processing from its proform (P31) to its mature form (P17) by WT virus was dose dependent (multiplicity of infection; MOI), contrasting with ΔF1L virus infection (Fig. 2F).
Importantly, neither NF-κB activity nor production of the NF-κB–inducible cytokine TNFα was different among these viruses (Fig. 2G and Fig. S3 B and D), showing the specificity of these results. Furthermore, the efficiency of THP-1 cell infection was not significantly different for WT, ΔF1L, or ΔN1L viruses, as monitored by either plaque assay or by expression of viral antigens (Fig. 2H and Fig. S3E), thus excluding a trivial explanation for the differences in caspase-1 and IL-1β observed for ΔF1L virus. We conclude therefore that, in the absence of F1L, VACV-infected cells generate higher levels of caspase-1 activity, produce more processed caspase-1, and secrete more IL-1β.
Dissociation of Antiapoptotic Activity of F1L from Its Ability to Suppress IL-1β Production.
To determine the relationship of F1L’s antiapoptotic activity to its ability to suppress IL-1β production, we performed virus reconstitution experiments wherein F1L was restored in ΔF1L mutant virus by a DNA recombination “knock in” strategy. Furthermore, to elucidate the role of NLRP1 binding by F1L, we knocked in either wild-type F1L or a mutant (N32A, H35A) that completely lacks NLRP1 binding activity—both with Flag epitope tags (Fig. S4 A–C). Using these reconstituted viruses, we then compared their ability to suppress cell death by measuring apoptotic markers in cells infected with WT and various mutant and reconstituted viruses (21). Whereas ΔF1L virus failed to suppress staurosporine (STS)-mediated induction of apoptosis [as measured by cleavage of caspase-3 and proteolytic processing of the caspase substrate poly-ADP ribosyl polymerase (PARP)], the WT virus and Flag-F1L and Flag-F1L(N32A,H35A) viruses suppressed proteolytic events associated with apoptosis (Fig. 3A). Moreover, analysis of cell viability in the infected cultures showed that F1L(N32A,H35A) reconstituted virus was equally effective as wild-type F1L virus at preserving host cell survival (Fig. 3B).
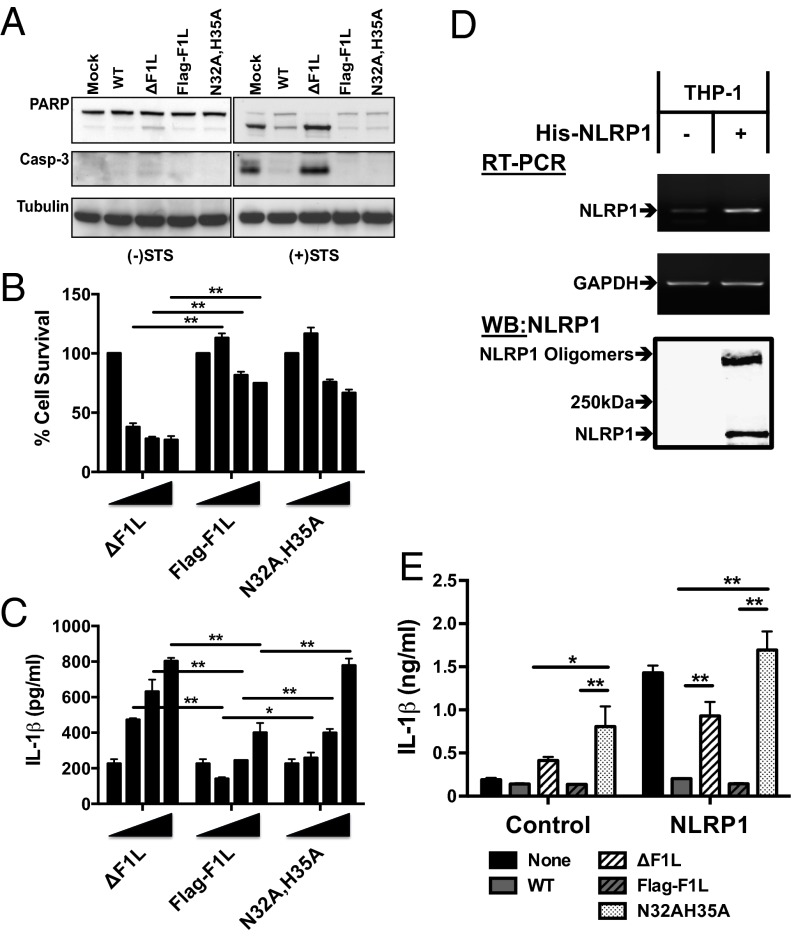
Fig. 3.
VACV expressing a NLR-nonbinding mutant induces increased IL-1β secretion in cell cultures. (A) HeLa cells were infected (MOI = 1) for 6 h with the wild-type VACV strain Western Reserve (WR) or various VACV F1L mutants, then cultured for 2 h without (Left) or with (Right) STS. PARP and Caspase-3 cleavage were assessed in cell lysates by immunoblotting of lysates normalized for total protein content. (B and C) TPA-differentiated THP-1-Blue cells (106) were infected (MOI = 0.5–5) with VACV ΔF1L, Flag-F1L, or Flag-F1L(N32A,H35A) viruses. Supernatants were collected at 18 h for analysis. (B) Cell viability was estimated by using a fluoriogenic assay for ATP. (C) IL-1β secretion was measured by using ELISA (mean ± SD; n ≥ 3). (D) THP-1-Blue cells stably overexpressing His6-tagged NLRP1 were analyzed for NLRP1 mRNA levels by using RT-PCR (Top and Middle) and protein expression by immunoblotting (Bottom). For immunoblot analysis, His6-NLRP1 was recovered from cell lysates by using Ni-NTA agarose beads, and blots were probed with rabbit anti-NLRP1 antibody. (E) NLRP1 overexpressing THP-1-Blue cells were infected at MOI = 2 and analyzed 18 h later for IL-1β secretion by ELISA (mean ± SD; n ≥ 3). Statistical significance at the level of P < 0.05 (*) and P < 0.01 (**) is indicated.
VACV Expressing non–NLRP1-Binding Mutant F1L Shows Defective Suppression of IL-1β Production.
Having observed that the F1L(N32A,H35A) reconstituted virus was equally effective as WT F1L-reconstituted virus at preserving host cell survival, we then compared effects of these viruses on IL-1β production. When THP-1 macrophages were infected with ΔF1L, Flag-F1L, or Flag-F1L(N32A, H35A) viruses, ∼twofold higher levels of IL-1β secretion were observed in ΔF1L virus-infected and Flag-F1L(N32A, H35A) virus-infected cultures compared with Flag-F1L (Fig. 3C). In contrast, levels of NF-κB activity were not different among these various virus-infected cells (Fig. S4D). Importantly, levels of Flag-F1L and Flag-F1L(N32A, H35A) protein production were comparable in infected cells (Fig. S4B). Comparisons of the various VACV mutants using a plaque assay revealed no significant differences in viral infectivity (Fig. S4C). We therefore conclude that reconstitution of ΔF1L virus with a non–NLRP1-binding mutant restores antiapoptotic activity but fails to restore competency to suppress IL-1β production induced during viral infection of cultured cells.
Additionally, we generated THP-1 macrophages stably overexpressing NLRP1 as a model for comparing effects of WT and ΔF1L virus (Fig. 3D). These His6-NLRP1–overexpressing cells spontaneously produce IL-1β without differences in TNFα secretion (Fig. 3E and Fig. S4 E–G), consistent with constitutively active NLRP1 protein. NLRP1 in these cells was already at least partially active under routine cultures conditions, as suggested by molecular analysis of His6-NLRP1 recovered from these cells, which showed a population of SDS-resistant oligomers (Fig. 3D). Infection of NLRP1-overexpressing THP-1 macrophages with WT virus or WT Flag-F1L reconstituted virus potently suppressed IL-1β production, whereas ΔF1L virus and Flag-F1L(N32A,H35A) reconstituted viruses failed to suppress IL-1β secretion by these NLRP1 overexpressing cells (Fig. 3E). In contrast to IL-1β, levels of TNFα production did not correlate with F1L status (Fig. S4E). Taken together, these experiments show that the F1L(N32A,H35A) mutant retains its antiapoptotic activity despite defective IL-1β suppression, thus dissociating F1L’s antiapoptotic activity from its ability to interfere with caspase-1 activation and IL-1β production.
VACV Expressing NLRP1-Binding Defective F1L Exhibits Reduced Virulence in Mice.
To explore the role of F1L in virus virulence in vivo, we used a mouse model of VACV infection (24), after confirming by co-IP assay that F1L can interact with NLRP1b, the murine counterpart of human NLRP1 (Fig. 4A). Intranasal (i.n.) administration of virus results in acute, productive infection of the lung. Deletion of the F1L gene (ΔF1L) from VACV resulted in a dose-dependent attenuated phenotype, characterized by dramatically improved survival rates of infected mice and less severe body weight reductions, without significantly impacting in vivo viral replication (Fig. 4 B–D).
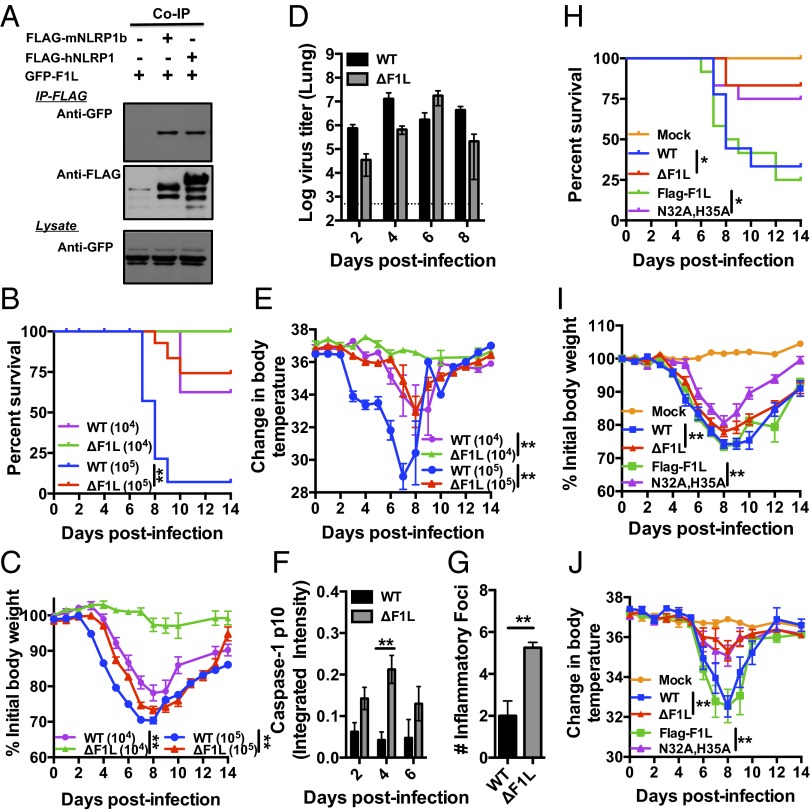
Fig. 4.
VACV expressing a NLR-nonbinding mutant displays reduced virulence in mice. (A) HEK293T cells were transiently cotransfected with plasmids encoding GFP-F1L together with FLAG-tagged human NLRP1 (hNLRP1), mouse Nlrp1b (mNLRP1b), or empty Myc plasmid, which served as negative controls. Coimmunoprecipitations was preformed as in Fig. 1A. (B–G) Mice were infected i.n. with 104 (n = 6–8 mice per group) or 105 (n = 30 mice per group) pfu of wild-type VACV (WT) or F1L deleted virus (ΔF1L), measuring survival (B) (where mice that lost ≥ 30% of initial body were killed), body weight (mean ± SEM) (C), and body temperature (rectal) (mean ± SEM) (E). (D and F) At various days after infection, four mice from each group (105 pfu) were killed; BAL fluids and lungs were collected. (D) Viral titers were measured in the homogenized lung. (F) Concentrated BAL fluids (10×) were analyzed by SDS/PAGE immunoblotting for caspase-1 cleavage by using anti-p10 antibody. Results were obtained by using a fluorescence imaging system using near infrared dye-conjugated secondary antibodies, quantifying results by integrated fluorescence intensity measurements of bands (mean ± SEM; n = 4). (G) Lung tissue from killed mice (105 pfu) was analyzed by hematoxylin/eosin staining, quantifying number of inflammatory foci per unit area (mean ± SEM; n = 4). P values for all graphs were determined by pairwise comparisons using t tests. (H–J) Mice were infected intranasally with 5 × 104 of various VACV (WT and mutants) or PBS (Mock), then monitored for survival (H), body weight (I), and body temperature (rectal) (J), (mean ± SEM; n = 9–12 mice per group). Statistical analysis was performed by using log-rank test for survival, two-way ANOVA for body weight, and Bonferroni's tests for temperature, *P < 0.05, **P < 0.01.
Others have reported that VACV infection results in reduced body temperature due to the viral gene B15R, which encodes the VACV IL-1β receptor —a neutralizer of IL-1β, endogenous pyrogen (25). Interestingly, we observed reduced body temperatures in virus-infected mice, as expected, but ΔF1L-infected mice showed only a slight reduction in body temperature (Fig. 4E). This observation thus implies that mice infected with ΔF1L virus may produce more IL-1β, in excess of the neutralizing viral proteins. We therefore examined the status of caspase-1 processing in bronchial alveolar lavage (BAL) fluids recovered from the infected animals. The processed p10 subunit of caspase-1 was detected at higher levels in BAL fluids from ΔF1L virus-infected mice compared with WT virus-infected mice at early stages of infection (Fig. 4F and Fig. S5A).
Because IL-1β is a proinflammatory cytokine, we examined the inflammatory response to viral infection, analyzing lung histology to determine the number of inflammatory foci at various times postinfection. Interestingly, an increase in inflammatory foci was present earlier in ΔF1L-infected mice. At day 4 postinfection, when little inflammatory infiltrate was found in mice infected with WT virus, mice infected with mutant virus already had impressive leukocyte infiltrate in lung. In contrast, mice infected with WT virus did not develop such lung leukocytosis until 6–8 d after infection (Fig. 4G and Fig. S5C). Thus, an earlier host inflammatory response to VACV is observed for F1L deficient virus.
Although VACV-infected cells are more sensitive to apoptosis when F1L is deleted from the viral genome (21, 26), we did not detect an increase in % apoptotic cells in the lungs of ΔF1L virus-infected mice compared with animals infected with WT virus (Fig. S5B). In this regard, redundancy may be provided by other viral apoptosis suppressors (13, 27, 28).
Having observed diminished virulence of the ΔF1L virus, we next compared the in vivo virulence of recombinant viruses reconstituted with wild-type Flag-F1L versus the NLRP1 nonbinding Flag-F1L(N32A,H35A) mutant. Mice infected with ΔF1L or Flag-F1L(N32A, H35A) viruses displayed attenuated phenotypes, marked by dramatically improved survival of mice and less severe body temperature reductions (Fig. 4 H–J). By comparison, the reconstituted Flag-F1L virus showed virulence properties comparable to WT virus, resulting in rapid killing of mice and profound alteration in body temperature (Fig. 4 H–J). Altogether, these studies of the Flag-F1L(N32A, H35A) virus (encoding a F1L mutant that is unable to bind NLRP1 but that maintains antiapoptotic activity) demonstrates the importance of NLRs as a target of F1L for successful viral pathogenesis in vivo.
Discussion
In this report, we demonstrate that F1L contributes to the virulence of VACV in vivo (using a mouse model and ΔF1L virus), and we dissected by mutagenesis a region on the F1L protein (residues 32–37) that is functionally required for suppression of caspase-1 activation and IL-1β production in virus-infected cells in culture and in mice in vivo. This functionally important region defines a binding site for NLPR1, based on in vitro protein interaction experiments and on experiments assessing suppression of NLRP1-driven caspase-1 activation by using an in vitro reconstituted system by recombinant F1L proteins and synthetic peptides. Moreover, our data demonstrate that F1L possesses at least two separable functions—suppression of apoptosis (which is presumably advantageous for maintaining host cell survival for viral replication or latency) and inhibition of NLR family proteins for reducing host inflammatory responses. Importantly, while we focused on NLRP1 as a prototypical target among NLRs, it is possible that F1L inhibits additional members of this large protein family (22 in humans and 33 in mice; ref. 29).
VACV strains all encode several Bcl-2 homologs, among which only F1L appears to have incontrovertible antiapoptotic activity (18). For those viral Bcl-2 homologs lacking antiapoptotic activity, it is conceivable that they instead operate as NLR antagonists. Unlike F1L, we found that the N1L protein (another viral homolog of Bcl-2) neither bound NLRP1 nor suppressed IL-1β production. Thus, structural differences between F1L and N1L presumably account for their differential functions.
By using an in vitro reconstituted NLRP1 inflammasome, we mapped the minimal region of F1L that is necessary and sufficient for inhibiting NLRP1-mediated caspase-1 activation to a hexapeptide motif. The ability of a small peptide ligand to inhibit NLRP1 is analogous to Bcl-2, for which a decapeptide sequence was identified that suppresses this NLR family member (22). Interestingly, however, the Bcl-2 and F1L peptides bear no similarity to each other in sequence, implying that they either inhibit NLRP1 through different mechanisms or that divergent sequences are capable of engaging a regulatory site on NLRP1. Nevertheless, the mechanism by which F1L and Bcl-2 peptides inhibit NLRP1 appears to be similar, in that both prevent MDP-induced ATP binding. In this regard, we previously described a two-step mechanism for NLRP1 activation, in which MDP binding to NLRP1 induces a conformational change, rending NLRP1 competent to bind ATP, which then stimulates NLRP1 oligomerization (23). Upon oligomerization of NLRP1, caspase-1 monomers associate with NLRP1, resulting in protease activation, presumably via an induced dimerization mechanism (30). Thus, we hypothesize that F1L and Bcl-2 peptides either interfere with MDP binding or prevent the conformational change induced by MDP that confers competence to bind ATP. The site on NLRP1 where F1L and Bcl-2 peptides bind is unclear, but association of F1L (and Bcl-2 and Bcl-XL) with NLRP1 requires the LRRs, which maintain many NLRs in an inactive conformation until bound by an activating ligand such as MDP. NLRP1ΔLRR neither binds nor is inhibited by F1L (or by Bcl-2 or Bcl-XL; ref. 20). Further elaboration of the structural basis for modulation NLRs by peptidyl ligands derived from viral and cellular Bcl-2 family proteins may reveal future strategies for antiinflammatory drug discovery, based on mimicking these ligands with synthetic chemical inhibitors.
Caspase-1 plays several important roles in innate immune responses to viruses. Similar to NLRs, the AIM2 protein forms an inflammasome with the adaptor protein ASC to activate caspase-1 (5–7, 31). AIM2 directly binds to dsDNA (including VACV DNA) to induce IL-1β secretion and pyroptosis (a caspase-1–dependent form of cell death). We determined that F1L does not bind AIM2 and showed that WT virus as well as ΔF1L reduces IL-1β production induced by other inflammasome stimuli, such as poly(dA:dT), monosodium urate (MSU), and LPS (Fig. S4H). Thus, AIM2 is likely to be only one of the host proteins involved in cellular responses to DNA viruses and F1L only one of the viral factors that suppress the innate immune machinery of infected host cells. Of note, it was shown that AIM2 is not expressed in THP-1 cells unless pretreated with IFN-γ (7), which we did not apply in our experiments. In addition to AIM2, a role for NLRP3 has been reported in host responses to VACV in some cellular contexts (32). Thus, different inflammasome-initiating proteins may play greater or lesser roles in host response to this family of DNA viruses, depending on cell lineage and other variables. One limitation of our study is that we were unable to directly demonstrate that VACV infection causes NLRP1 activation (even when using ΔF1L virus), largely due to the inadequacy of antibodies available for detecting endogenous NLRP1 protein (which also stymied our attempts to directly demonstrate interaction of endogenous F1L with endogenous NLRP1 in the context of virus-infected cells). Attempts to express epitope (or His6) tagged NLRP1 in cells resulted in constitutively active NLRP1, thus impairing our ability to determine whether viral infection stimulates NLRP1. An important goal for future research is to identify the virus-derived molecules (besides DNA) that are responsible for NLR family protein activation.
F1L represents a viral NLR-suppressing factor that demonstrates an in vivo role in viral virulence. Recently, a NLR-binding protein [open reading frame 63 (Orf63)] was identified in Kaposi Sarcoma Virus [human herpesvirus 8 (HHV8)], but studies of this NLR inhibitor were limited to cell culture experiments (33) and, thus, their in vivo relevance is unclear. The measles virus V protein was similarly reported to bind and inhibit NLRP3 in cultured cells (34). The genomes of VACV and many other poxviruses encode several inhibitors of the caspase-1/IL-1β axis, including (i) cytokine response modifier A (CrmA) (SP1-2;B13R), which directly inhibits caspase-1; (ii) viral soluble receptor for IL-1β (vIL-1βR; B15R), which is a IL-1R decoy that neutralizes IL-1; and (iii) viral pyrin domain only protein M13L (vPOP) that competes with ASC (10, 25, 27, 35–38). Interestingly, genomic comparisons reveal that the gene encoding F1L is not found in Poxvirus genera that have the ASC antagonist M13L and vice versa, suggesting that either F1L or M13L may be sufficient to disarm inflammasomes. With the addition of F1L, poxviruses would appear to be capable of inhibiting at five levels (NF-κB; NLRs; ASC; caspase-1; IL-1β) to suppress production of or neutralize this important cytokine. Although the relative importance of each of these points of regulation may vary depending on the host-pathogen context and timing after infection, the observation that cells infected with F1L-deficient or F1L mutant (N32A, H35A) viruses secrete more IL-1β argues that F1L is an important defense that these viruses have evolved to interfere with host innate immune responses.
Materials and Methods
Additional details are provided as SI Materials and Methods.
VACV Production.
A VACV WR strain lacking only the F1L coding sequence (ΔF1L) has been described (13). The revertant F1L viruses [Flag-F1L and Flag-F1L(N32A, H35A)] were generated by introducing wild-type or mutant F1L genes in frame with a pE/L-Cherry selection cassette. The ΔN1L virus was generated by replacing nucleotides 1–275 of the N1L Orf with a pE/L-gpt-Cherry selection cassette. The fidelity of the resulting recombinant viruses was verified by DNA sequencing. Expression of the Flag-F1L proteins and loss of expression of N1L was documented by immunoblot analysis of infected cells. The VACV strains were grown in HeLa cells and titered on VeroE6 cells. For measuring virus titers in various types of infected cells, host cells were suspended in a minimal volume of PBS then disrupted by three freeze-thaw cycles. Samples were then centrifuged at 800 × g for 10 min to produce supernatants that were titered on VeroE6 cells.
VACV-Titer Assay.
Virus titers were determined as described (39).
VACV Challenge.
BALB/c mice were anesthetized by inhalation of isoflurane and inoculated by the i.n. route with VACV-WR, as well as a mutant of WR in which only the F1L gene was deleted, VACV-ΔF1L (104 or 105 pfu per mice). Body weight and rectal temperature of mice were measured daily before and after infection. At various times after infection, four mice from each group were killed and lungs and BAL fluids were collected. Mice were euthanized when they lost 30% of their initial body weight or were severely hypothermic (<32 °C) on two consecutive days.
Supplementary Material
Acknowledgments
We thank M. Hanaii for manuscript preparation. This work was supported by National Institutes of Health Grants AI-091967, AI-056324, and AI-078048 (to J.C.R.); AI-067341 (to M.C.); and AI-077079 (to S.S-A.); and by the Crohn's and Colitis Foundation of America (M.G. and B.F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1215995110/-/DCSupplemental.
References
- 1.Martinon F, Mayor A, Tschopp J. The inflammasomes: Guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 2.Franchi L, Eigenbrod T, Muñoz-Planillo R, Nuñez G. The inflammasome: A caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10(3):241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: Host cell death and inflammation. Nat Rev Microbiol. 2009;7(2):99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schroder K, Muruve DA, Tschopp J. Innate immunity: Cytoplasmic DNA sensing by the AIM2 inflammasome. Curr Biol. 2009;19(6):R262–R265. doi: 10.1016/j.cub.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Roberts TL, et al. 2009. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science 323(5917):1057–1060.
- 6.Hornung V, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458(7237):514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458(7237):509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muruve DA, et al. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452(7183):103–107. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- 9.Donehower LA, et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356(6366):215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 10.Haga IR, Bowie AG. Evasion of innate immunity by vaccinia virus. Parasitology. 2005;130(Suppl):S11–S25. doi: 10.1017/S0031182005008127. [DOI] [PubMed] [Google Scholar]
- 11.Bahar MW, et al. How vaccinia virus has evolved to subvert the host immune response. J Struct Biol. 2011;175(2):127–134. doi: 10.1016/j.jsb.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.González JM, Esteban M. A poxvirus Bcl-2-like gene family involved in regulation of host immune response: Sequence similarity and evolutionary history. Virol J. 2010;7(1):59. doi: 10.1186/1743-422X-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aoyagi M, et al. Vaccinia virus N1L protein resembles a B cell lymphoma-2 (Bcl-2) family protein. Protein Sci. 2007;16(1):118–124. doi: 10.1110/ps.062454707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooray S, et al. Functional and structural studies of the vaccinia virus virulence factor N1 reveal a Bcl-2-like anti-apoptotic protein. J Gen Virol. 2007;88(Pt 6):1656–1666. doi: 10.1099/vir.0.82772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kvansakul M, et al. 2008. Vaccinia virus anti-apoptotic F1L is a novel Bcl-2-like domain-swapped dimer that binds a highly selective subset of BH3-containing death ligands. Cell Death Differ 15(10):1564–1571.
- 16.Graham SC, et al. Vaccinia virus proteins A52 and B14 Share a Bcl-2-like fold but have evolved to inhibit NF-kappaB rather than apoptosis. PLoS Pathog. 2008;4(8):e1000128. doi: 10.1371/journal.ppat.1000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalverda AP, et al. Poxvirus K7 protein adopts a Bcl-2 fold: Biochemical mapping of its interactions with human DEAD box RNA helicase DDX3. J Mol Biol. 2009;385(3):843–853. doi: 10.1016/j.jmb.2008.09.048. [DOI] [PubMed] [Google Scholar]
- 18.Postigo A, Way M. The vaccinia virus-encoded Bcl-2 homologues do not act as direct Bax inhibitors. J Virol. 2012;86(1):203–213. doi: 10.1128/JVI.05817-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeretssian G, et al. Non-apoptotic role of BID in inflammation and innate immunity. Nature. 2011;474(7349):96–99. doi: 10.1038/nature09982. [DOI] [PubMed] [Google Scholar]
- 20.Bruey JM, et al. Bcl-2 and Bcl-XL regulate proinflammatory caspase-1 activation by interaction with NALP1. Cell. 2007;129(1):45–56. doi: 10.1016/j.cell.2007.01.045. [DOI] [PubMed] [Google Scholar]
- 21.Postigo A, Cross JR, Downward J, Way M. Interaction of F1L with the BH3 domain of Bak is responsible for inhibiting vaccinia-induced apoptosis. Cell Death Differ. 2006;13(10):1651–1662. doi: 10.1038/sj.cdd.4401853. [DOI] [PubMed] [Google Scholar]
- 22.Faustin B, et al. Mechanism of Bcl-2 and Bcl-X(L) inhibition of NLRP1 inflammasome: Loop domain-dependent suppression of ATP binding and oligomerization. Proc Natl Acad Sci USA. 2009;106(10):3935–3940. doi: 10.1073/pnas.0809414106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faustin B, et al. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol Cell. 2007;25(5):713–724. doi: 10.1016/j.molcel.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 24.Reading PC, Smith GL. A kinetic analysis of immune mediators in the lungs of mice infected with vaccinia virus and comparison with intradermal infection. J Gen Virol. 2003;84(Pt 8):1973–1983. doi: 10.1099/vir.0.19285-0. [DOI] [PubMed] [Google Scholar]
- 25.Alcamí A, Smith GL. A mechanism for the inhibition of fever by a virus. Proc Natl Acad Sci USA. 1996;93(20):11029–11034. doi: 10.1073/pnas.93.20.11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gill C, Dowling C, O’Neill AJ, Watson RW. Effects of cIAP-1, cIAP-2 and XIAP triple knockdown on prostate cancer cell susceptibility to apoptosis, cell survival and proliferation. Mol Cancer. 2009;8:39. doi: 10.1186/1476-4598-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartlett N, Symons JA, Tscharke DC, Smith GL. The vaccinia virus N1L protein is an intracellular homodimer that promotes virulence. J Gen Virol. 2002;83(Pt 8):1965–1976. doi: 10.1099/0022-1317-83-8-1965. [DOI] [PubMed] [Google Scholar]
- 28.Postigo A, Martin MC, Dodding MP, Way M. Vaccinia-induced epidermal growth factor receptor-MEK signalling and the anti-apoptotic protein F1L synergize to suppress cell death during infection. Cell Microbiol. 2009;11(8):1208–1218. doi: 10.1111/j.1462-5822.2009.01327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reed JC, et al. RIKEN GER Group GSL Members Comparative analysis of apoptosis and inflammation genes of mice and humans. Genome Res. 2003;13(6B):1376–1388. doi: 10.1101/gr.1053803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salvesen GS, Riedl SJ. Caspase mechanisms. Adv Exp Med Biol. 2008;615:13–23. doi: 10.1007/978-1-4020-6554-5_2. [DOI] [PubMed] [Google Scholar]
- 31.Bürckstümmer T, et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10(3):266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 32.Qi J, et al. The ubiquitin ligase Siah2 regulates tumorigenesis and metastasis by HIF-dependent and -independent pathways. Proc Natl Acad Sci USA. 2008;105(43):16713–16718. doi: 10.1073/pnas.0804063105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gregory SM, et al. Discovery of a viral NLR homolog that inhibits the inflammasome. Science. 2011;331(6015):330–334. doi: 10.1126/science.1199478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Komune N, Ichinohe T, Ito M, Yanagi Y. Measles virus V protein inhibits NLRP3 inflammasome-mediated interleukin-1β secretion. J Virol. 2011;85(24):13019–13026. doi: 10.1128/JVI.05942-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bowie A, et al. A46R and A52R from vaccinia virus are antagonists of host IL-1 and toll-like receptor signaling. Proc Natl Acad Sci USA. 2000;97(18):10162–10167. doi: 10.1073/pnas.160027697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kettle S, et al. Vaccinia virus serpin B13R (SPI-2) inhibits interleukin-1beta-converting enzyme and protects virus-infected cells from TNF- and Fas-mediated apoptosis, but does not prevent IL-1beta-induced fever. J Gen Virol. 1997;78(Pt 3):677–685. doi: 10.1099/0022-1317-78-3-677. [DOI] [PubMed] [Google Scholar]
- 37.Symons JA, et al. The vaccinia virus C12L protein inhibits mouse IL-18 and promotes virus virulence in the murine intranasal model. J Gen Virol. 2002;83(Pt 11):2833–2844. doi: 10.1099/0022-1317-83-11-2833. [DOI] [PubMed] [Google Scholar]
- 38.Johnston JB, et al. A poxvirus-encoded pyrin domain protein interacts with ASC-1 to inhibit host inflammatory and apoptotic responses to infection. Immunity. 2005;23(6):587–598. doi: 10.1016/j.immuni.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Zhai D, et al. High-throughput fluorescence polarization assay for chemical library screening against anti-apoptotic Bcl-2 family member Bfl-1. J Biomol Screen. 2012;17(3):350–360. doi: 10.1177/1087057111429372. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.