Abstract
Membrane transporters rely on highly coordinated structural transitions between major conformational states for their function, to prevent simultaneous access of the substrate binding site to both sides of the membrane—a mode of operation known as the alternating access model. Although this mechanism successfully accounts for the efficient exchange of the primary substrate across the membrane, accruing evidence on significant water transport and even uncoupled ion transport mediated by transporters has challenged the concept of perfect mechanical coupling and coordination of the gating mechanism in transporters, which might be expected from the alternating access model. Here, we present a large set of extended equilibrium molecular dynamics simulations performed on several classes of membrane transporters in different conformational states, to test the presence of the phenomenon in diverse transporter classes and to investigate the underlying molecular mechanism of water transport through membrane transporters. The simulations reveal spontaneous formation of transient water-conducting (channel-like) states allowing passive water diffusion through the lumen of the transporters. These channel-like states are permeable to water but occluded to substrate, thereby not hindering the uphill transport of the primary substrate, i.e., the alternating access model remains applicable to the substrate. The rise of such water-conducting states during the large-scale structural transitions of the transporter protein is indicative of imperfections in the coordinated closing and opening motions of the cytoplasmic and extracellular gates. We propose that the observed water-conducting states likely represent a universal phenomenon in membrane transporters, which is consistent with their reliance on large-scale motion for function.
Keywords: major facilitator superfamily, LeuT-fold transporters, ABC transporters, neurotransmitter transporters
Membrane transporters provide efficient mechanisms for exchange of diverse molecular species across the cellular membrane generally in an active manner. By coupling various forms of chemical energy in the cell to a set of highly coordinated conformational transitions, membrane transporters fuel vectorial translocation of their respective substrates across the membrane. From a structural perspective, the overall process of transport is furnished through transitions between two major conformational states—namely, the outward-facing (OF) and inward-facing (IF) states (1)—during which substrate accessibility is shifted from one side of the membrane to the other. Central to efficient transport and to preventing dissipation of the substrate down the electrochemical gradient is to ensure that the substrate binding site will not be accessible simultaneously to both sides of the membrane, a mechanism best known as the alternating access model (2). This model, which has received substantial support from biochemical, kinetic, and structural studies (1, 3, 4), relies on a high level of coordination between two gating mechanisms, a cytoplasmic and an extracellular one, exposing the substrate binding site to only one side of the membrane at a time. This key mechanistic aspect defines the main distinction of transporters from membrane channels, in which opening of a single gate usually creates the transmembrane substrate permeation pathway to connect both sides of the membrane simultaneously (5).
Interestingly, various experimental studies have reported water transport and uncoupled ion transport for several membrane transporters (6–8), a phenomenon that might appear to contradict the traditional understanding of the alternating access model. Water exchange mediated by membrane transporters has been attributed to two fundamentally different mechanisms: passive, osmosis-driven water exchange (9) and active, stoichiometrically related cotransport of water (6). Though the involvement of osmosis-driven mechanism has been well established (9), the role of active water cotransport is still a subject of debate, and various models have been proposed to account for it (6). These models include the carrier-mediated model, which relies on the uptake of a large number of water molecules along with the substrate into the binding region/lumen of the transporter and their simultaneous pumping to the other side of the membrane (10–12), and cotransport of water as a result of osmotic imbalance introduced by the local accumulation of the nonaqueous substrate (13–15).
A detailed investigation of the mechanism(s) for transporter-mediated water exchange across the membrane requires not only high-resolution structures of membrane transporters, but also a dynamical description of the protein and transported species. Recent years have witnessed a sharp increase in the number of crystal structures of membrane transporters in different conformational states, thereby providing valuable insight into their mechanisms (3, 4). Though several of these structures are reported at resolutions high enough to gain information on the position of a few water molecules, none seems to offer a convincing mechanism for water transport. Thus, it appears necessary to examine the dynamics of the protein as well as water to gain insight into the mechanistic details of the process. In this context, molecular dynamics (MD) offers a powerful tool to capture conformational fluctuations of the transporter, which are closely coupled to formation of water permeation pathways, and to describe the dynamics of smaller species, such as water, substrate, and ions during the structural breathing of the protein (16).
In this study, we used MD simulations to study a diverse set of membrane transporters (Table 1) with the primary goal of characterizing the mechanism of water transport. Extended equilibrium simulations of membrane-embedded structures are performed for transporters from the solute sodium symporter (SSS), solute carrier 1 (SLC1), major facilitator superfamily (MFS), nucleobase-cation-symport-1 (NCS1), and ATP binding cassette (ABC) families/superfamilies—namely, Na+-coupled glucose transporter (vSGLT), glutamate transporter (GlT), glycerol-3-phosphate transporter (GlpT), benzyl hydantoin transporter (Mhp1), and maltose transporter, respectively. We note that for some of the transporter families studied here, the phenomenon of water transport has not yet been reported experimentally. We observe the formation of water-conducting states in all cases, describe their nature and mechanism of formation, and quantify the rate of water transport through these states. To our knowledge, this report provides previously undescribed formation of water-conducting states in multiple transporter families, suggesting that the phenomenon might represent a universal aspect of membrane transporter function.
Table 1.
Simulated systems
| Transporter | Substrate/ion | Family or superfamily | State | Simulation time |
| vSGLT | Na+/glucose | SSS | IF substrate-bound | 1.15 μs |
| Gltph | Na+/glutamate | SLC1 | intermediate apo | 160 ns |
| GlpT | Glycerol-3-phosphate/Pi | MFS | IF substrate-bound | 200 ns |
| Mhp1 | Na+/benzyl-hydantoin | NCS1 | OF substrate-free | 1.2 μs |
| Maltose transporter | Maltose | ABC | OF substrate-bound | 100 ns |
Results and Discussion
Water-Conducting States of SGLT.
The best-documented case of a water-conducting transporter is SGLT, with several studies reporting its characteristic leakiness to small species, such as water, and even ions (9, 11, 13, 17–20). Water transport has also been reported for Na+-I− symporter (NIS) (12) and lactate transporter (MCT1) (21), both belonging to the same family (SSS) of transporters as SGLT. More importantly, other leucine transporter (LeuT)-fold transporters have also been reported to exhibit water transport (22, 23). The availability of a crystal structure for the substrate-bound IF state of a bacterial SGLT (vSGLT) (24) provides the opportunity to study the molecular mechanism of water transport in a transporter family and structural topology that are well established experimentally to exhibit the phenomenon.
During a 1.15-μs MD simulation of vSGLT, a large number of water permeation events were detected. On examining the origin and structural basis for water transport, we detected frequent formation of water-conducting states during the simulation (Fig. 1). A clear relationship exists between the degree of the opening of the water-conducting state at its narrowest point (the “constriction point”), and the number of permeating water molecules (Fig. 1C).
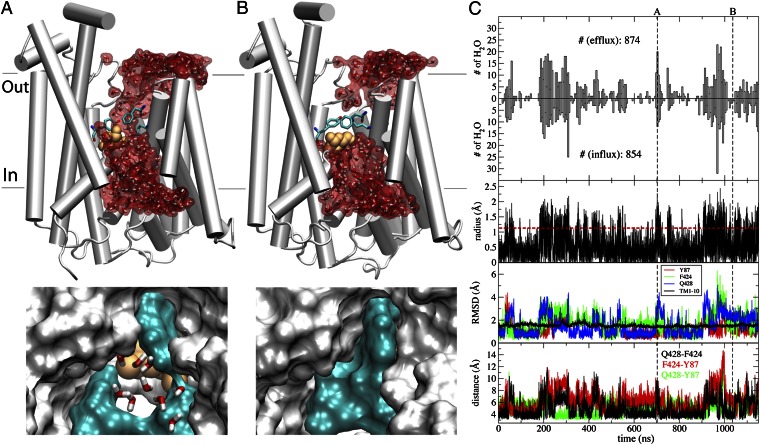
Fig. 1.
Water-conducting states and water transport in vSGLT. (A) Water-permeable state of vSGLT. (Upper) Overall view of the water-conducting state and the continuous water channel formed within the transporter lumen. The protein is drawn in white cartoon, with some of the helices removed for clarity. Water molecules are drawn in both sticks and surface (red) representations, and the substrate in orange van der Waals. The three gating residues controlling water flow— namely, Y87, F424, and Q428—are shown in sticks and colored by atom type. (Lower) Close-up view of the same conformation, highlighting the narrowest part of the water permeation pathway. Protein is shown in white surface, except for the three gating residues, which are in cyan surface. (B) Water-impermeable conformation of vSGLT. (Upper and Lower) Same as in A, but for a water-impermeable state formed due to side-chain motions of the gating residues (cyan surface) closing the narrowest point along the water permeation pathway. (C) Protein dynamics and water transport. The number of observed water permeation events in either direction (influx or efflux) is plotted against the simulation time in the top two panels. The third panel depicts the time series of the minimum radius of the aqueous pathway. The fourth panel shows the rmsd of gating residues and of the core transmembrane domain (10 TMs). The bottom panel shows the time evolution of the distances between the gating residues, i.e., Q428:Nε2–F424:Cε2, F424:Cε2–Y87:Cε1, and Q428:Nε2–Y87:Cε1. The vertical black dashed lines in C mark the snapshots shown in A and B, respectively, and the horizontal red dashed line is used to indicate the minimum radius needed to accommodate a water molecule.
The water-conducting states are transient in nature, forming and disappearing frequently, thereby resulting in the fluctuation of the number of permeating water molecules (Fig. 1). The aqueous pathway in these states coincides with the putative substrate translocation pathway. This observation is in agreement with experimentally measured inhibitory action of phlorizin, a competitive inhibitor of substrate transport, on water flux, suggesting that these may share the same pathway (9). Remarkably, the radius of the constriction point of the water pore formed within the lumen never reaches above 2.5 Å, which is larger than the radius of water (∼1.15 Å) but smaller than that of the primary substrate (∼4.0 Å), thus preventing it from leaking back down its chemical gradient.
During the simulation, the protein maintains its IF state as in the crystal structure; this is indicated by the low structural deviation (rmsd < 2 Å) during the simulation (Fig. 1C) for the 10 transmembrane (TM) helices. Meanwhile, structural deviation of side chains lining the constriction point, namely Y87, F424, and Q428, and the resulting change in their distances, show a high correlation with the water transport (Fig. 1C). Y87 and F424 have been proposed to form the extracellular gate (24), and Q428 has been implicated in direct substrate binding (24). Therefore, in addition to their role in controlling substrate access, these three residues appear to also act as the “gate” for water transport in the IF state of SGLT. The formation of the water-conducting states, in SGLT, therefore, is mainly a result of local conformational changes of a few side chains of these gating residues.
The osmotic permeability (Pf) of vSGLT estimated from these simulations is 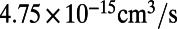 , which is an order of magnitude higher than the experimentally measured value of
, which is an order of magnitude higher than the experimentally measured value of 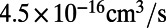 for rSGLT (25). It may be noted here that the Pf values obtained from these simulations are only based on a partial view of the complete transport cycle, i.e., only the IF substrate-bound state, and therefore do not reproduce the average permeability of the complete cycle, reflected in the experimental Pf value. It is expected that increased sampling of more states would bring experiment and simulation results closer to each other. The larger gap between the Pf values for vSGLT calculated from a shorter simulation (200 ns) (19) and the experimental value is in line with this notion.
for rSGLT (25). It may be noted here that the Pf values obtained from these simulations are only based on a partial view of the complete transport cycle, i.e., only the IF substrate-bound state, and therefore do not reproduce the average permeability of the complete cycle, reflected in the experimental Pf value. It is expected that increased sampling of more states would bring experiment and simulation results closer to each other. The larger gap between the Pf values for vSGLT calculated from a shorter simulation (200 ns) (19) and the experimental value is in line with this notion.
Water-Conducting States in Other Transporter Families.
Examining the dynamics of water in other transporter systems, we were surprised to find that the formation of water-conducting states is not unique to vSGLT. In fact, we observed water transport in four other transporter systems, namely, Gltph, GlpT, Mhp1, and maltose transporter (Figs. 2 and 3). Unlike vSGLT, water-conducting states in these transporters appear to be associated with the transition of the transporters from one major functional state to another, due either to the intermediate nature of the initial crystal structure or to large structural changes induced during the simulations by mechanistically relevant molecular events as described below (and detailed in SI Text).
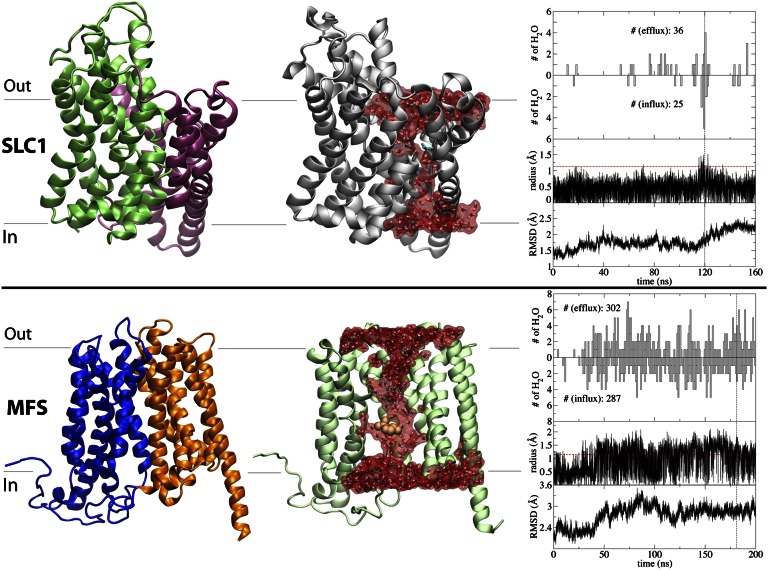
Fig. 2.
Water-conducting states for Gltph (EAAT) of the SLC1 family (Upper) and GlpT from the MFS superfamily (Lower). (Left) Topology of the protein using the crystal structure, highlighting in different colors functionally relevant domains and repeats. Though Gltph is simulated as a trimer, here only the monomer in the intermediate state is depicted, with the transport and trimerization domains colored in green and purple, respectively. (Center) Representative water-conducting frame from the simulations. (Right) Time series for the number of water permeation events along the efflux and influx directions, radius of the narrowest part along of the aqueous lumen, and Cα rmsd of the TM region. The vertical black dashed lines represent the snapshots shown in the molecular images, and the horizontal red dashed lines show the minimal radius to accommodate a water molecule.
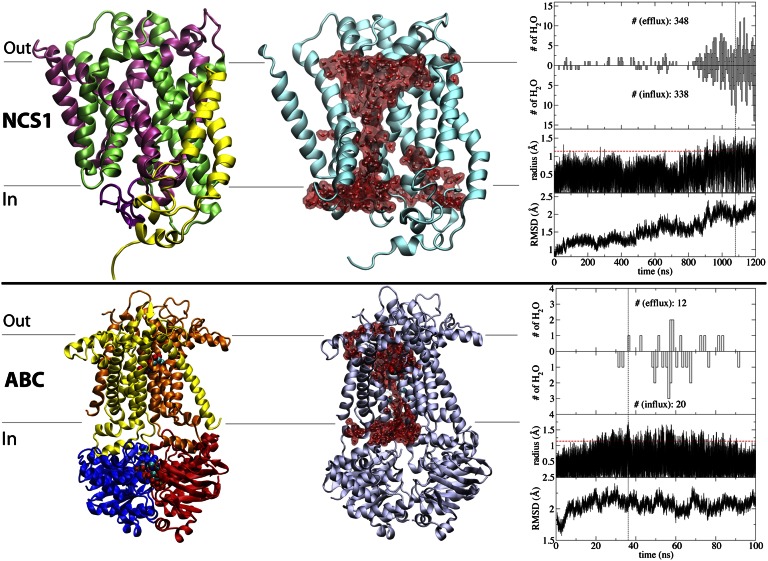
Fig. 3.
Water-conducting states for Mhp1 of the NCS1 family (Upper) and maltose transporter of the ABC superfamily (Lower). The panels are arranged in the same manner as in Fig. 2. (Left) crystal structure. (Center) Representative water-conducting state. (Right) Time series for key events. For clarity here, only the TM domains and NBDs are depicted in maltose transporter.
GlTs [also termed excitatory amino acid transporters (EAATs)] belong to the SLC1 family of neurotransmitter transporters, which are responsible for the clearance of neurotransmitters from the synapse after their release from the presynaptic neurons. Several states of a bacterial GlT homolog (Gltph) have been structurally resolved, i.e., the OF (26), IF (27), and intermediate (28) states. GlT is an experimentally well-documented case of a transporter mediating water exchange across the membrane during its function, and both osmosis-driven and cotransport mechanisms have been proposed for it (29–31).
Using the trimeric structure of Gltph (28) with one monomer in the intermediate state (and the other two monomers in the IF state), we have performed a 160-ns MD simulation of the apo (substrate/ion-free) state. These simulations have been performed under the exact same conditions used previously in our laboratory to simulate the OF and IF states of Gltph (32, 33). During the simulation, numerous water permeation events occur along a continuous aqueous pathway formed at the interface between the transport domain [specifically, helical hairpin 1 (HP1)] and the trimerization domain, but only in the monomer, which is in the intermediate state (Fig. 2). The characterized pathway and mechanism are consistent with a proposed mechanism for water transport in a human GlT (31) and the crystal structure of Gltph in the intermediate state (28), suggesting that water transport through GlT is mediated via a putative Cl− leak pathway of the transporter. The radius of the constriction point in the intermediate Gltph (∼1.5 Å) remains always smaller than that of the substrate (Fig. 2). Although the intermediate nature of this monomer well accounts for the most part of the water permeation pathway, further conformational fluctuation of HP1 seems to be necessary for the formation of the full water channel (see SI Text for details). No water exchange was observed in the other two monomers, or in our previous simulations performed on trimeric states of either OF or IF states in numerous different bound states. Together, these results strongly associate the observation of the water-conducting state with the intermediate character of Gltph.
The third studied membrane transporter is GlpT, one of the few structurally known members of the MFS superfamily, the largest superfamily of secondary active transporters (34). Although no study implicating GlpT in water transport currently exists, other MFS transporters, most prominently glucose transporters GLUT1 and GLUT2, have been reported to exhibit water transport (35, 36).
During a 200-ns simulation of substrate-bound, IF-state GlpT (37, 38), we observed copious water transport events (Fig. 2). Similar to vSGLT, water permeation in GlpT occurs via an aqueous pathway running through the substrate binding site, and the radius of the constriction point remains smaller than that of the main substrate (Fig. 2). However, the scale of conformational changes preceding the formation of the water-conducting state differs from vSGLT. It is important to note that water permeation in GlpT was only observed following two functionally relevant events—namely, substrate binding, which happened spontaneously in equilibrium simulations (37, 38), and a subsequent proton transfer reaction, which was modeled by the change of the protonation state of a conserved histidine in the binding pocket. These molecular events are deemed to be necessary for triggering/facilitating large-scale structural transitions of GlpT from the crystallographically captured state to other major states, the early stages of which seem to have been captured in our simulation (see SI Text for more detail). These structural changes include the rearrangements of the two TM domains with respect to each other, resulting in partial opening and closure in the extracellular and cytoplasmic halves, respectively, forming an intermediate state between the OF and IF states, exhibiting water transport. The results offer a putative mechanism of water transport for other MFS members as well, in particular for GLUT1 and GLUT2, where water transport has been demonstrated experimentally (35, 36).
The next transporter studied here, Mhp1, is a Na+-hydantoin transporter in the NCS1 family, which mediates the uptake of nucleobases and related metabolites (39). Structurally (40, 41), Mhp1 belongs to the LeuT-fold family of transporters. However, though other LeuT-fold transporters, e.g., SGLT1 and LeuT, have been experimentally characterized as water-transporting proteins (17, 22), until now, no water transport has been reported for the NCS1 family.
Transient water-conducting states were observed during a 1.2-μs simulation of the OF state of Mhp1 in its substrate-free form (Fig. 3). As in vSGLT and GlpT, the aqueous pathway overlaps with the putative substrate pathway, whereas the radius of the constriction point (∼1.5 Å) is consistently smaller than the substrate (∼4.0 Å; Fig. 3), thereby preventing free diffusion of the substrate. Mhp1 is the another transporter discussed here for which the formation of water-conducting states is associated with large-scale structural changes of the protein, in contrast to more localized fluctuations, which seem to be sufficient for the formation of water pores in vSGLT. A significant rearrangement of the cytoplasmic half of the protein appears to accompany the formation of the observed water-conducting state in Mhp1 (Fig. 3). This change is triggered by the spontaneous unbinding of the Na+ ion from its binding site during the equilibrium simulation (see SI Text for details). The presence of the Na+ ion has been demonstrated experimentally to directly affect the stability of the OF state in LeuT-fold transporters (42, 43).
The last transporter examined for water transport is the Escherichia coli maltose transporter (44). Distinct from the secondary transporters described above, maltose transporter is a primary active transporter from the ABC transporter superfamily, with two TM domains forming the substrate translocation pathway (Fig. 3), whose conformational states are determined by ATP binding and hydrolysis within two cytoplasmic nucleotide-binding domains (NBDs; Fig. 3).
Starting from the crystal structure of maltose transporter in the OF state (45), a 100-ns simulation was performed after removing ATP from the two NBDs, as an efficient method to trigger the structural transition toward the IF state (46, 47). The removal of ATP leads to a rapid separation of the NBDs, which in turn translates into the opening of the cytoplasmic ends of the closely coupled TM domains (47). Because the periplasmic end of the transporter is already open in the crystal structure (OF state), the above-described molecular events result in the formation of a water-conducting state through which water permeation events were observed (t > 30 ns; Fig. 3). We note that in control simulations performed on the ATP-bound state, no large conformational changes or water permeation events were observed (see SI Text for details).
The maximal radius recorded at the constriction point of the aqueous pathway during the simulations is ∼1.8 Å, which is sufficiently large to allow water exchange intermittently but not the translocation of the much larger transported substrate (maltose or maltodextrin). As was the case for most of the transporters studied here, the aqueous pathway in maltose transporter runs through the substrate-binding site (Fig. 3). Herein, we report previously undescribed water transport for a primary active transporter.
Mechanistic Features of Water Transport in Membrane Transporters.
The results presented in this study illustrate the presence of a common molecular mechanism of water transport in diverse transporters. Earlier experimental studies on members of SSS, the neurotransmitter sodium symporter (NSS), SLC1, MFS, and other families have detected conduction for water and/or ions (6–9, 22, 30, 35, 36). Together with the results of the present study demonstrating the phenomenon in diverse transporter families, the accumulated evidence on leakiness of membrane transporters suggest that the coordination between opening and closing motions of the two gates in these molecular machines might not be as perfect as generally perceived. Water transport is mediated by well-characterized water-conducting states (pores) that may arise at different steps of the transport cycle, most likely during the transition between major functional states. In all studied cases except one (GlT), the aqueous pathway forms along the putative substrate pathway. The observed formation of a water pore in a different region in Gltph (between the transport and trimerization domains) is an example highlighting the possibility that such permeation pathways can arise at any mechanically active interface due to various structural defects induced by large-scale transitions of membrane transporters.
In the absence of well-resolved structural and mechanistic descriptions for water and ion leak, the phenomenon might appear initially detrimental to the transporter function, because such leaky states could potentially dissipate substrate gradient across the membrane. Our results, however, clearly demonstrate how such water-conducting states can form frequently in response to different structural fluctuations without interfering with the tight control of the alternating access for the substrate. In all cases, the observed water-conducting states remain impermeable to the main substrate, thereby conforming with the required alternating access model, a principle that only needs to be obeyed by the substrate. Though structural fluctuations of the protein, whether localized gating-like motions or more global transitions, mediate the formation of the water-conducting states, the constriction point within the transporter lumen is found to always remain sufficiently small to prevent the substrate from leaking.
The results presented here provide a structural basis only for a passive (osmosis-driven) mode of water transport, which is also the only mode of water transport consistent with the structures available for membrane transporters. These structures, particularly those captured in an occluded state, e.g., LeuT (48), vSGLT (24), Gltph (28), and Na+/betaine symporter BetP (49), indicate that the occluded cavity is too small to accommodate more than a few water molecules to be transported along with the substrate in a carrier-mediated fashion. This low capacity differs by an order of magnitude from the number of water molecules transported per turnover in most transporters (6). Nevertheless, because neither our simulations nor the available crystal structures can claim to have fully examined a transport cycle in its entirety, the involvement of an active (carrier-mediated) mode of transport cannot be discarded based on the currently available data. We note that the water-conducting states captured in the simulations can readily account for a high ratio of water/substrate transport. The amount of water transported through these leaky states is simply a function of their lifetime and average water permeability.
Finally, we note that our simulations do not have any indication of a biased water flux across the membrane in either direction. The total amount of water efflux and influx are always almost the same as in all of the studied transporters (Figs. 1–3). This observation, which is expected given the absence of an osmotic gradient or hydrostatic pressure difference across the simulated membrane, also supports a completely passive mode of water transport in membrane transporters.
Universality of Water-Conducting States in Transporters.
One of the unique aspects of the present study is the demonstration of water-conducting states in diverse families and distinct functional states of membrane transporters: vSGLT and Mhp1 represent, respectively, a substrate-bound IF state and an OF state of LeuT-fold transporters; Gltph and GlpT represent, respectively, an intermediate state of an SLC1 and an IF state of an MFS transporter; and maltose transporter is an OF state of ABC transporters. The simulations describe the phenomenon not only for several experimentally demonstrated cases (6–9, 22, 30, 35, 36) but also for a number of additional transporters.
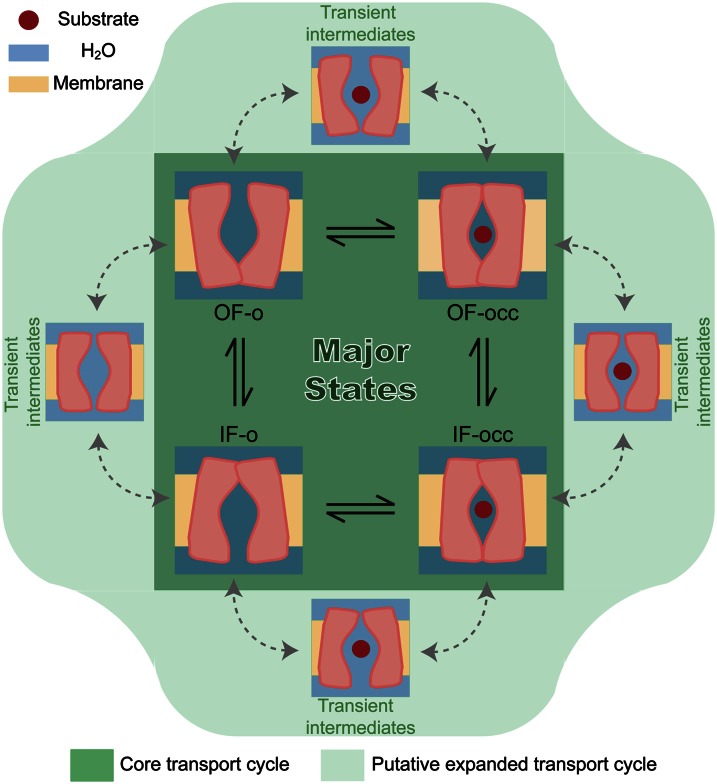
Although the formation of water-conducting states in vSGLT is mediated by mostly localized structural fluctuations, for the remainder of the studied transporters, the phenomenon appears to be correlated with more global structural changes, which are necessary for cycling through major functional states. This observation is in line with the notion that water-conducting states may generally arise as short-lived intermediates every time the protein undergoes a transition from one major state to another (Fig. 4).
Fig. 4.
Formation of water-conducting states during the transport cycle. The core transport cycle of a membrane transporter (dark green region) relies on interconversion of the protein structure between major known functional states—namely, outward-facing open (OF-o), outward-facing occluded (OF-occ), inward-facing open (IF-o), and inward-facing occluded (IF-occ) states, which have been observed in various crystal structures (24, 34, 40, 41, 45). An expanded view of the transport cycle (light green area) would involve a number of additional intermediates that arise during the transition between the major states, which due to their transient nature have not yet been structurally characterized by experiments, but can account for the uncoupled water and ion transport during the transport cycle.
Membrane transporters face an extremely challenging task to function effectively: a transporter needs to achieve highly coordinated gating motions at the cytoplasmic and extracellular gates, which are often spatially far from each other. Only a perfect coordination of the two gates can ensure the formation of a truly occluded state that would prevent even the leak of small species such as water. Given the soft mechanical properties of transporter proteins, it comes as no surprise to observe harmless imperfections in the overall gating motions, which manifest themselves in the formation of water-conducting states. It would, of course, be a concern if these channels were large enough to leak the substrate, and/or long-lived to allow very large amounts of smaller species to permeate across the membrane. Neither of these aspects appears to be the observed in our results, because the leaky states are only large enough for small species such as water. Furthermore, it appears that these states only transiently rise during the transport cycle, an attribute that might make them difficult to capture experimentally. Viewing the formation of these leaky states as a consequence of deviation of a transporter protein from a perfect, machine-like behavior, it is not surprising that such mechanical defects might not necessarily arise during each and every large-scale transition of every transporter. In fact, in several other transporter systems studied in our laboratory by comparably long simulations, we did not observe the formation of such water-conducting states. Furthermore, several experimental studies have also illustrated the occurrence of water and ion leak only for specific states of the studied transporters (6, 8, 20, 50). Depending on the protein architecture, degree of conformational changes involved in the cycle, and even the nature of the substrate, some transporters and some transitions might be more prone to the formation of water-conducting states.
Materials and Methods
A summary of the studied transporters is provided in Table 1. All of the transporter proteins were simulated in the explicit presence of membrane, water, and ions. Simulations were performed using NAMD 2.6 (51) or Desmond on Anton (52), using the CHARMM force field (53) for proteins, lipids, ions, and substrates, and TIP3P model for explicit water (54). After the initial minimization and equilibration, the systems were subjected to production simulations in the constant pressure and constant temperature (NPT) ensemble (Table 1; see SI Materials and Methods for more detail).
Supplementary Material
Acknowledgments
Simulations in this study have been performed using National Science Foundation’s Extreme Science and Engineering Discovery Environment (XSEDE) Resources Grant MCA06N060 and Anton at Pittsburg Supercomputing Center Grant MCB100017P. Support was also provided by National Institutes of Health Grants R01-GM086749, U54-GM087519, and P41-GM104601.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1218986110/-/DCSupplemental.
References
- 1.DeFelice LJ. Transporter structure and mechanism. Trends Neurosci. 2004;27(6):352–359. doi: 10.1016/j.tins.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Jardetzky O. Simple allosteric model for membrane pumps. Nature. 1966;211(5052):969–970. doi: 10.1038/211969a0. [DOI] [PubMed] [Google Scholar]
- 3.Forrest LR, Krämer R, Ziegler C. The structural basis of secondary active transport mechanisms. Biochim Biophys Acta. 2011;1807(2):167–188. doi: 10.1016/j.bbabio.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 4.Oldham ML, Davidson AL, Chen J. Structural insights into ABC transporter mechanism. Curr Opin Struct Biol. 2008;18(6):726–733. doi: 10.1016/j.sbi.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gadsby DC. Ion channels versus ion pumps: The principal difference, in principle. Nat Rev Mol Cell Biol. 2009;10(5):344–352. doi: 10.1038/nrm2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeuthen T. Water-transporting proteins. J Membr Biol. 2010;234(2):57–73. doi: 10.1007/s00232-009-9216-y. [DOI] [PubMed] [Google Scholar]
- 7.DeFelice LJ, Goswami T. Transporters as channels. Annu Rev Physiol. 2007;69:87–112. doi: 10.1146/annurev.physiol.69.031905.164816. [DOI] [PubMed] [Google Scholar]
- 8.Andrini O, Ghezzi C, Murer H, Forster IC. The leak mode of type II Na(+)-P(i) cotransporters. Channels (Austin) 2008;2(5):346–357. doi: 10.4161/chan.2.5.6900. [DOI] [PubMed] [Google Scholar]
- 9.Loo DD, et al. Passive water and ion transport by cotransporters. J Physiol. 1999;518(Pt 1):195–202. doi: 10.1111/j.1469-7793.1999.0195r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeuthen T, Stein WD. Cotransport of salt and water in membrane proteins: Membrane proteins as osmotic engines. J Membr Biol. 1994;137(3):179–195. doi: 10.1007/BF00232587. [DOI] [PubMed] [Google Scholar]
- 11.Meinild A, Klaerke DA, Loo DD, Wright EM, Zeuthen T. The human Na+-glucose cotransporter is a molecular water pump. J Physiol. 1998;508(Pt 1):15–21. doi: 10.1111/j.1469-7793.1998.015br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeuthen T, Belhage B, Zeuthen E. Water transport by Na+-coupled cotransporters of glucose (SGLT1) and of iodide (NIS). The dependence of substrate size studied at high resolution. J Physiol. 2006;570(Pt 3):485–499. doi: 10.1113/jphysiol.2005.100933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duquette PP, Bissonnette P, Lapointe JY. Local osmotic gradients drive the water flux associated with Na(+)/glucose cotransport. Proc Natl Acad Sci USA. 2001;98(7):3796–3801. doi: 10.1073/pnas.071245198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gagnon MP, Bissonnette P, Deslandes LM, Wallendorff B, Lapointe JY. Glucose accumulation can account for the initial water flux triggered by Na+/glucose cotransport. Biophys J. 2004;86(1 Pt 1):125–133. doi: 10.1016/S0006-3495(04)74090-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naftalin RJ. Osmotic water transport with glucose in GLUT2 and SGLT. Biophys J. 2008;94(10):3912–3923. doi: 10.1529/biophysj.107.122531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khalili-Araghi F, et al. Molecular dynamics simulations of membrane channels and transporters. Curr Opin Struct Biol. 2009;19(2):128–137. doi: 10.1016/j.sbi.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loo DD, Zeuthen T, Chandy G, Wright EM. Cotransport of water by the Na+/glucose cotransporter. Proc Natl Acad Sci USA. 1996;93(23):13367–13370. doi: 10.1073/pnas.93.23.13367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeuthen T, Zeuthen E, Klaerke DA. Mobility of ions, sugar, and water in the cytoplasm of Xenopus oocytes expressing Na(+)-coupled sugar transporters (SGLT1) J Physiol. 2002;542(Pt 1):71–87. doi: 10.1113/jphysiol.2001.014530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choe S, Rosenberg JM, Abramson J, Wright EM, Grabe M. Water permeation through the sodium-dependent galactose cotransporter vSGLT. Biophys J. 2010;99(7):L56–L58. doi: 10.1016/j.bpj.2010.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sasseville LJ, Cuervo JE, Lapointe JY, Noskov SY. The structural pathway for water permeation through sodium-glucose cotransporters. Biophys J. 2011;101(8):1887–1895. doi: 10.1016/j.bpj.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamann S, Kiilgaard JF, la Cour M, Prause JU, Zeuthen T. Cotransport of H+, lactate, and H2O in porcine retinal pigment epithelial cells. Exp Eye Res. 2003;76(4):493–504. doi: 10.1016/s0014-4835(02)00329-9. [DOI] [PubMed] [Google Scholar]
- 22.Santacroce M, Castagna M, Sacchi VF. Passive water permeability of some wild type and mutagenized amino acid cotransporters of the SLC6/NSS family expressed in Xenopus laevis oocytes. Comp Biochem Physiol A Mol Integr Physiol. 2010;156(4):509–517. doi: 10.1016/j.cbpa.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 23.MacAulay N, Zeuthen T, Gether U. Conformational basis for the Li(+)-induced leak current in the rat gamma-aminobutyric acid (GABA) transporter-1. J Physiol. 2002;544(Pt 2):447–458. doi: 10.1113/jphysiol.2002.022897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faham S, et al. The crystal structure of a sodium galactose transporter reveals mechanistic insights into Na+/sugar symport. Science. 2008;321(5890):810–814. doi: 10.1126/science.1160406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zampighi GA, et al. A method for determining the unitary functional capacity of cloned channels and transporters expressed in Xenopus laevis oocytes. J Membr Biol. 1995;148(1):65–78. doi: 10.1007/BF00234157. [DOI] [PubMed] [Google Scholar]
- 26.Boudker O, Ryan RM, Yernool D, Shimamoto K, Gouaux E. Coupling substrate and ion binding to extracellular gate of a sodium-dependent aspartate transporter. Nature. 2007;445(7126):387–393. doi: 10.1038/nature05455. [DOI] [PubMed] [Google Scholar]
- 27.Reyes N, Ginter C, Boudker O. Transport mechanism of a bacterial homologue of glutamate transporters. Nature. 2009;462(7275):880–885. doi: 10.1038/nature08616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verdon G, Boudker O. Crystal structure of an asymmetric trimer of a bacterial glutamate transporter homolog. Nat Struct Mol Biol. 2012;19(3):355–357. doi: 10.1038/nsmb.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacAulay N, Gether U, Klaerke DA, Zeuthen T. Water transport by the human Na+-coupled glutamate cotransporter expressed in Xenopus oocytes. J Physiol. 2001;530(Pt 3):367–378. doi: 10.1111/j.1469-7793.2001.0367k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacAulay N, Gether U, Klaeke DA, Zeuthen T. Passive water and urea permeability of a human Na(+)-glutamate cotransporter expressed in Xenopus oocytes. J Physiol. 2002;542(Pt 3):817–828. doi: 10.1113/jphysiol.2002.020586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vandenberg RJ, Handford CA, Campbell EM, Ryan RM, Yool AJ. Water and urea permeation pathways of the human excitatory amino acid transporter EAAT1. Biochem J. 2011;439(2):333–340. doi: 10.1042/BJ20110905. [DOI] [PubMed] [Google Scholar]
- 32.Huang Z, Tajkhorshid E. Dynamics of the extracellular gate and ion-substrate coupling in the glutamate transporter. Biophys J. 2008;95(5):2292–2300. doi: 10.1529/biophysj.108.133421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang Z, Tajkhorshid E. Identification of the third Na+ site and the sequence of extracellular binding events in the glutamate transporter. Biophys J. 2010;99(5):1416–1425. doi: 10.1016/j.bpj.2010.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang Y, Lemieux MJ, Song J, Auer M, Wang DN. Structure and mechanism of the glycerol-3-phosphate transporter from Escherichia coli. Science. 2003;301(5633):616–620. doi: 10.1126/science.1087619. [DOI] [PubMed] [Google Scholar]
- 35.Fischbarg J, et al. Glucose transporters serve as water channels. Proc Natl Acad Sci USA. 1990;87(8):3244–3247. doi: 10.1073/pnas.87.8.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeuthen T, Zeuthen E, Macaulay N. Water transport by GLUT2 expressed in Xenopus laevis oocytes. J Physiol. 2007;579(Pt 2):345–361. doi: 10.1113/jphysiol.2006.123380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Enkavi G, Tajkhorshid E. Simulation of spontaneous substrate binding revealing the binding pathway and mechanism and initial conformational response of GlpT. Biochemistry. 2010;49(6):1105–1114. doi: 10.1021/bi901412a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Law CJ, Enkavi G, Wang DN, Tajkhorshid E. Structural basis of substrate selectivity in the glycerol-3-phosphate: Phosphate antiporter GlpT. Biophys J. 2009;97(5):1346–1353. doi: 10.1016/j.bpj.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pantazopoulou A, Diallinas G. Fungal nucleobase transporters. FEMS Microbiol Rev. 2007;31(6):657–675. doi: 10.1111/j.1574-6976.2007.00083.x. [DOI] [PubMed] [Google Scholar]
- 40.Weyand S, et al. Structure and molecular mechanism of a nucleobase-cation-symport-1 family transporter. Science. 2008;322(5902):709–713. doi: 10.1126/science.1164440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shimamura T, et al. Molecular basis of alternating access membrane transport by the sodium-hydantoin transporter Mhp1. Science. 2010;328(5977):470–473. doi: 10.1126/science.1186303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao Y, et al. Substrate-modulated gating dynamics in a Na+-coupled neurotransmitter transporter homologue. Nature. 2011;474(7349):109–113. doi: 10.1038/nature09971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Claxton DP, et al. Ion/substrate-dependent conformational dynamics of a bacterial homolog of neurotransmitter:sodium symporters. Nat Struct Mol Biol. 2010;17(7):822–829. doi: 10.1038/nsmb.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bordignon E, Grote M, Schneider E. The maltose ATP-binding cassette transporter in the 21st century—towards a structural dynamic perspective on its mode of action. Mol Microbiol. 2010;77(6):1354–1366. doi: 10.1111/j.1365-2958.2010.07319.x. [DOI] [PubMed] [Google Scholar]
- 45.Oldham ML, Khare D, Quiocho FA, Davidson AL, Chen J. Crystal structure of a catalytic intermediate of the maltose transporter. Nature. 2007;450(7169):515–521. doi: 10.1038/nature06264. [DOI] [PubMed] [Google Scholar]
- 46.Newstead S, et al. Insights into how nucleotide-binding domains power ABC transport. Structure. 2009;17(9):1213–1222. doi: 10.1016/j.str.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wen PC, Tajkhorshid E. Conformational coupling of the nucleotide-binding and the transmembrane domains in ABC transporters. Biophys J. 2011;101(3):680–690. doi: 10.1016/j.bpj.2011.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh SK, Yamashita A, Gouaux E. Antidepressant binding site in a bacterial homologue of neurotransmitter transporters. Nature. 2007;448(7156):952–956. doi: 10.1038/nature06038. [DOI] [PubMed] [Google Scholar]
- 49.Perez C, Koshy C, Yildiz O, Ziegler C. Alternating-access mechanism in conformationally asymmetric trimers of the betaine transporter BetP. Nature. 2012;490(7418):126–130. doi: 10.1038/nature11403. [DOI] [PubMed] [Google Scholar]
- 50.Schicker K, et al. Unifying concept of serotonin transporter-associated currents. J Biol Chem. 2012;287(1):438–445. doi: 10.1074/jbc.M111.304261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Phillips JC, et al. Scalable molecular dynamics with NAMD. J Comput Chem. 2005;26(16):1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shaw DE, et al. 2009. Millisecond-scale molecular dynamics simulations on Anton. SC ’09: Proceedings of the Conference on High Performance Computing Networking, Storage and Analysis (Assoc for Computing Machinery, Portland, OR), pp 39:1–39:11.
- 53.MacKerell AD, Jr, et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J Phys Chem B. 1998;102:3586–3616. doi: 10.1021/jp973084f. [DOI] [PubMed] [Google Scholar]
- 54.Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML. Comparison of simple potential functions for simulating liquid water. J Chem Phys. 1983;79:926–935. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.