Abstract
Although primary cilia are well established as important sensory and signaling structures, their function in most tissues remains unknown. Obesity is a feature associated with some syndromes of cilia dysfunction, such as Bardet-Biedl syndrome (BBS) and Alström syndrome, as well as in several cilia mutant mouse models. Recent data indicate that obesity in BBS mutant mice is due to defects in leptin receptor trafficking and leptin resistance. Furthermore, induction of cilia loss in leptin-responsive proopiomelanocortin neurons results in obesity, implicating cilia on hypothalamic neurons in regulating feeding behavior. Here, we directly test the importance of the cilium as a mediator of the leptin response. In contrast to the current dogma, a longitudinal study of conditional Ift88 cilia mutant mice under different states of adiposity indicates that leptin resistance is present only when mutants are obese. Our studies show that caloric restriction leads to an altered anticipatory feeding behavior that temporarily abrogates the anorectic actions of leptin despite normalized circulating leptin levels. Interestingly, preobese Bbs4 mutant mice responded to the anorectic effects of leptin and did not display other phenotypes associated with defective leptin signaling. Furthermore, thermoregulation and activity measurements in cilia mutant mice are inconsistent with phenotypes previously observed in leptin deficient ob/ob mice. Collectively, these data indicate that cilia are not directly involved in leptin responses and that a defect in the leptin signaling axis is not the initiating event leading to hyperphagia and obesity associated with cilia dysfunction.
Obesity is a major health issue associated with complications that cause significant morbidity and mortality. Thus, identification of the protein hormone leptin in the spontaneous obese mouse mutant ob/ob was the source of much excitement (1), as leptin suppresses feeding activity and is secreted into serum in proportion to the amount of adipose tissue, the hormone’s primary source (2). However, the therapeutic potential of leptin was attenuated by a barrier to the action of leptin that must exist in an obese individual, as nearly all obese mice and human patients exhibit elevated levels of circulating leptin (2, 3). This barrier phenomenon is known as leptin resistance, the precise mechanisms of which are unknown. Furthermore, age impacts leptin sensitivity, as older rodents are less responsive to the anorectic effects of leptin than younger rodents (4–7). In the field of obesity research, one challenge becomes determining how individuals acquire leptin resistance and whether this is a primary cause of the obesity phenotype or simply a consequence.
Recent findings revealed that obesity is associated with mutations in proteins disrupting the function of primary cilia, which are small, immotile, microtubule-based appendages protruding from the surface of most mammalian cell types (8). Long thought to be vestigial, primary cilia are now known to serve as critical signaling hubs for diverse cellular pathways during development (9). The emergence of the primary cilium as a clinically important organelle was initiated by studies in model organisms such as Chlamydomonas reinhardtii and Caenorhabditis elegans. These studies led to the identification of proteins required for ciliogenesis through the bidirectional transport of cargo along the cilium in a process known as intraflagellar transport (IFT) (10). Because IFT-null mutations are embryonically lethal, research into the roles of cilia in adults was initially limited. However, the generation of conditional IFT alleles in mice and the realization that several human genetic syndromes, known as ciliopathies, result from cilia dysfunction has expanded our understanding of the organelle (11). Mutations affecting cilia function in humans lead to a spectrum of diseases. Ciliopathy clinical features range from prenatal lethality in Meckel-Grüber syndrome to obesity in Alström syndrome or Bardet-Biedl Syndrome (BBS).
BBS is a pleiotropic, genetically heterogeneous syndrome associated with retinopathy, cystic kidneys, cognitive deficits, polydactyly, anosmia, and obesity (8). Several of the implicated proteins form a complex called the BBSome that functions in cilia membrane trafficking (12–15). Numerous mouse models of BBS recapitulate many of the patients’ clinical features (16, 17). A major distinction between mouse BBS and IFT mutants is that BBS models possess primary cilia, whereas IFT mutations generally lead to cilia ablation. Furthermore, data from multiple model systems suggest that BBS-associated phenotypes result from defective cilia mediated signaling activity (12, 18, 19).
Recent data show that Bbs1, a BBSome component, binds the leptin receptor and may mediate leptin receptor trafficking (20). Furthermore, studies in Bbs2, Bbs4, and Bbs6 mutant mice reveal that they are hyperleptinemic, and importantly, fail to reduce food intake in response to leptin (21). Leptin resistance was observed in the mutant mice even after caloric restriction reduced fat mass and circulating leptin levels to those of control mice (20). Thus, defects in leptin signaling appear to contribute directly to BBS-associated obesity.
We previously showed that conditional disruption of Ift88 throughout an adult mouse using the ubiquitously expressed CAGG-CreER (actin promoter) transgene leads to hyperphagia and obesity (22). Intriguingly, the obesity phenotype can be recapitulated using proopiomelanocortin (POMC)-Cre to disrupt Ift88 in POMC neurons of the hypothalamus, a key satiety center that regulates leptin responses (22). Thus, our goal was to investigate whether cilia are directly involved in leptin signaling, whether cilia loss contributes to the development of leptin resistance, and whether the etiology of obesity in cilia mutant mice is similar to that observed in Bbs models where cilia are intact but dysfunctional.
Results
A longitudinal leptin sensitivity testing paradigm was designed to determine whether leptin resistance in cilia mutant mice is a direct result of cilia loss or a secondary consequence of obesity (Fig. 1A). To initiate cilia ablation in adult mice, a previously described conditional allele of Ift88 and ubiquitously expressed inducible CAGG-CreER line were used (22, 23). Cohorts were generated such that transgene CAGG-CreER–positive animals were always compared with control CAGG-CreER–negative littermates. Ift88 deletion was induced at 8 wk of age through tamoxifen injection (Fig. 1A). Genotyping and Western blotting confirmed CreER-mediated deletion of Ift88 within 10 d after tamoxifen administration (Fig. S1 A and C). Subsequent immunostaining for the neuronal cilia marker adenylate cyclase III (ACIII) (24) confirmed the absence of hypothalamic neuronal cilia throughout the study (Fig. S1B). These induced cilia mutant mice are hereafter referred to as Ift88Δ/Δ and control mice as Ift88flox/flox. Mice were challenged with i.p. injection of recombinant leptin, and subsequent feeding behavior was assessed using a Biodaq feeding monitoring system (25) to record food intake of individual mice in real time. Mice were injected with leptin at three time points at which they had different body and serum leptin compositions, hereafter referred to as time points I, II, and III (Fig. 1 B and C), corresponding to preobese, obese, and food-restricted nonobese states, respectively.
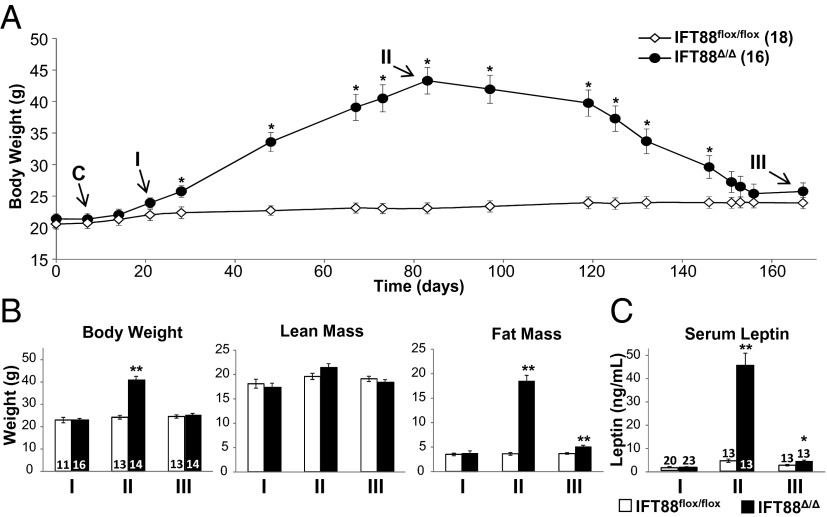
Fig. 1.
Paradigm of leptin sensitivity testing, body composition, and serum leptin in Ift88 conditional mutant mice. (A) Longitudinal paradigm showing body weights of Ift88Δ/Δ and Ift88flox/flox mice after Cre induction (C), lean time point I (I), obese time point II (II), and lean time point III (III). (B) Body weight, fat mass, and lean mass at each time point. (C) Serum leptin levels at each time point. All points and bars are means ± SEMs. *P < 0.05, **P < 0.01; Student t test. Numbers within parentheses or bars indicate number of animals.
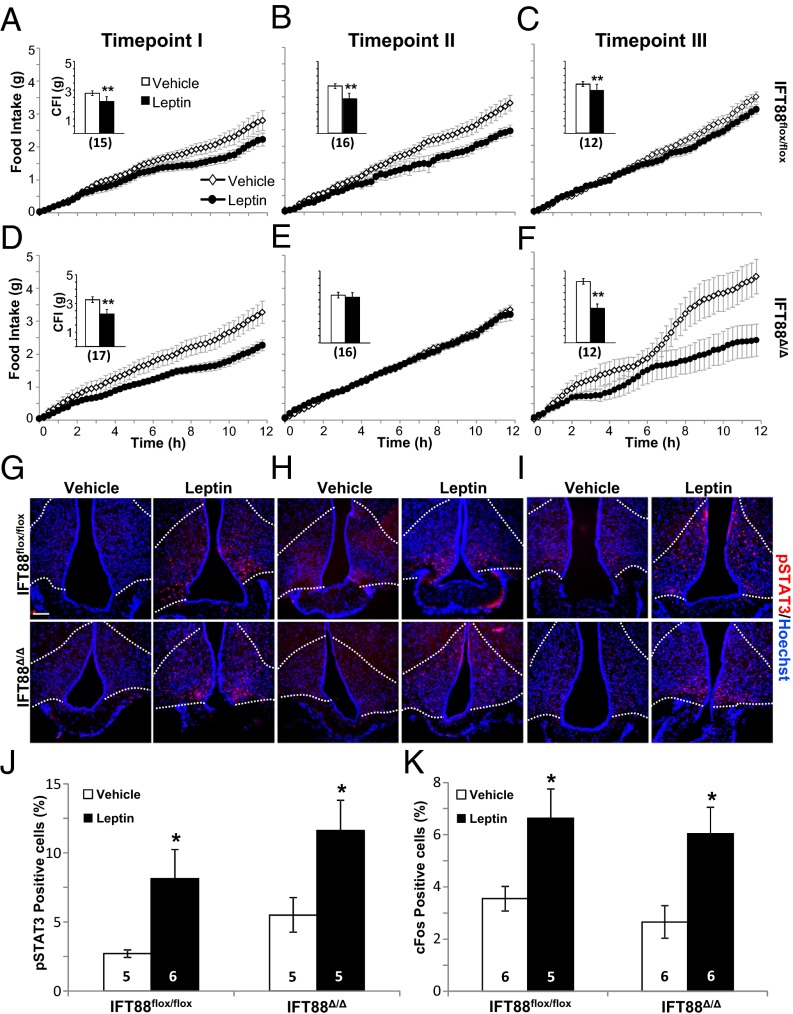
At time point I, Ift88Δ/Δ mice are hyperphagic but have not diverged significantly in body weight or fat mass from Ift88flox/flox controls. Serum leptin measurements demonstrated no significant difference between the two groups at this point (Ift88flox/flox, 1.07 ng/mL; Ift88Δ/Δ, 1.05 ng/mL; P = 0.94, Student t test; Fig. 1B). Unexpectedly, leptin reduced food intake for both Ift88Δ/Δ and Ift88flox/flox mice compared with vehicle injection (Fig. 2 A and D and Insets). This reduction would not be expected in Ift88Δ/Δ mice if leptin was acting through the primary cilium. Furthermore, the difference in food intake between Ift88Δ/Δ mice treated with leptin and control Ift88flox/flox mice treated with leptin was not significant (Ift88flox/flox, 2.22 g; Ift88Δ/Δ, 2.26 g; P = 0.93, Student t test; Fig. 2 A and D and Insets). Additionally, both Ift88flox/flox and Ift88Δ/Δ mice at time point I displayed increased phosphorylated Stat3 (pSTAT3) and cFos within the arcuate nucleus 90 min after injection, indicative of a response to leptin (Fig. 2 G, J, and K; Fig. S2A) (26). These results indicate that at time point I, after both the Ift88 protein and cilia have been lost, the response to leptin remains intact as measured by both behavioral activity and neuronal markers of activation in cilia mutant Ift88Δ/Δ mice.
Fig. 2.
Feeding behavior and arcuate nucleus pSTAT3 and cFos staining in Ift88 conditional mice after leptin injection. (A–F) Feeding data and cumulative food intake (CFI) after i.p. injection of leptin in Ift88flox/flox (A–C and Insets) and Ift88Δ/Δ (D–F and Insets) mice at time points I, II, and III. Insets show total CFI. (G–I) Representative images showing pSTAT3 staining in the arcuate nucleus after i.p. leptin or vehicle injection at time points I, II, and III. (Scale bar, 90 µm.) Dotted lines indicate approximate border of the arcuate nucleus. (J and K) Quantification of pSTAT3 and cFos staining in the arcuate nucleus after leptin or vehicle injection at time point I. *P < 0.05, **P < 0.01; Student t test. All points and bars in graphs represent means ± SEMs. Numbers within parentheses or bars indicate number of animals.
After 80 d of ad libitum feeding (time point II), Ift88Δ/Δ mutants are significantly heavier with greater fat mass and greater serum leptin levels (Fig. 1 A–C). Again, leptin was administered i.p., and as observed in many other models of increased adiposity and hyperleptinemia, the Ift88Δ/Δ mice did not diminish feeding in response to leptin, whereas control Ift88flox/flox responded with reduced food intake (Fig. 2 B and E and Insets). Both pSTAT3 and cFos labeling showed increased basal levels of staining in the arcuate nucleus in all groups, showing no significant effects of leptin (Fig. 2H; Fig. S2B). However, the ability to assess a response to leptin in rodents using markers such as pSTAT3 diminishes in aged animals (6, 7, 27). These results indicate that obese Ift88Δ/Δ mice have elevated leptin levels and display behavior consistent with hyperleptinemia-associated leptin resistance.
To test whether the increased fat mass and serum leptin observed at time point II drive the leptin resistant phenotype, mice were subjected to calorie restriction using a gradual stepwise process over a period of several weeks (Fig. 1 A and B). Calorie restriction restored adiposity and serum leptin levels to those observed in controls (hereafter referred to as time point III). The mice were then allowed ad libitum access to food for 10 d before testing the leptin response. Time point III leptin sensitivity was assessed before ad libitum–associated changes in body weight and serum leptin, a condition similar to time point I. Although the serum leptin levels and fat mass were significantly higher in Ift88Δ/Δ mice compared with Ift88flox/flox mice at time point III (Ift88flox/flox, 2.54 ng/mL and 3.69 g; Ift88Δ/Δ, 4.14 ng/mL and 5.05 g; P < 0.01, Student t test), they were not significantly different from the levels measured at time point I (Ift88Δ/Δ time point III, 4.14 ng/mL and 3.69 g; Ift88Δ/Δ time point I, 1.06 ng/mL and 3.91 g; one-way ANOVA followed by post hoc Tukey's HSD test; P = 0.72 and P = 0.57, respectively). There was also a greater than 10-fold decrease in serum leptin levels compared with time point II. Strikingly, Ift88Δ/Δ mice again demonstrated a strong anorectic behavior response to leptin administration (Fig. 2 C and F and Insets). Neither pSTAT3 nor cFos levels in the arcuate nucleus displayed differences in staining; however, as in time point II, this result is expected with older animals (Fig. 2I; Fig. S2C). Taken together, these results, along with those from time points I and II, show that the leptin resistance observed in a mouse model of cilia loss is a consequence of the obesity and hyperleptinemia and not a primary defect associated with cilia loss.
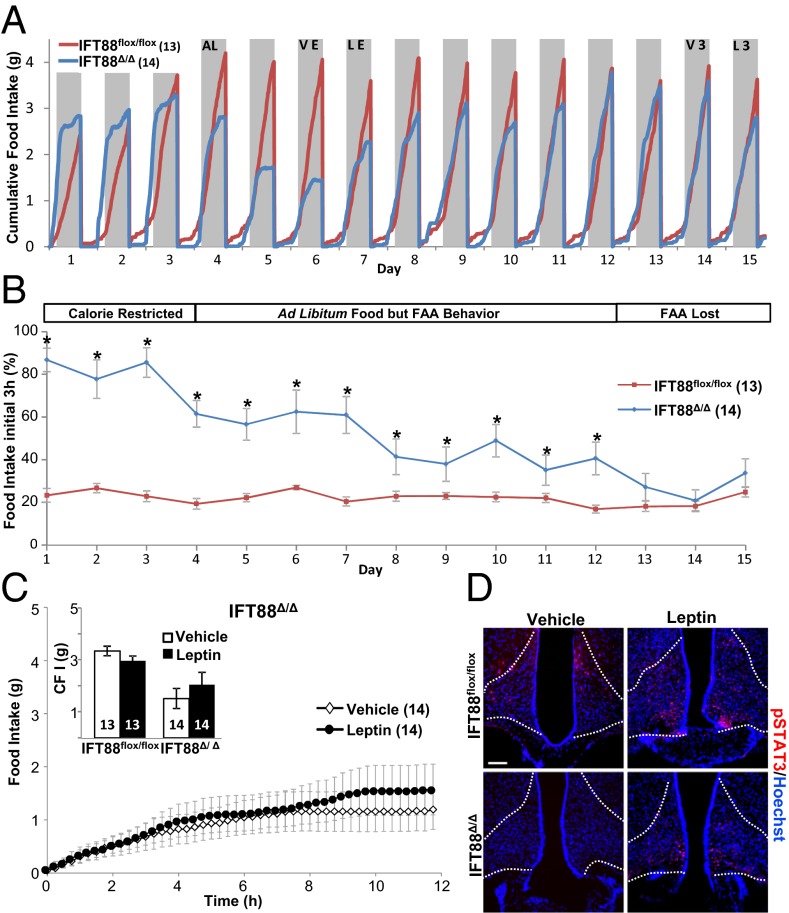
The finding that leptin administration causes a decrease in feeding behavior in adult cilia mutants (both before becoming obese and again after caloric restriction) was unexpected based on previous reports in the Bbs mutant mice (20, 21). To further explore potential causes of the discrepancy between Bbs and cilia null mice, we evaluated whether caloric restriction itself had effects on feeding behavior. This analysis revealed that calorie-restricted mice greatly alter the structure of their feeding pattern (Fig. 3A). These changes in feeding behavior are consistent with food anticipatory activity (FAA) observed previously in rodents (Fig. 3 A and B) (28–31). Ift88Δ/Δ mutant mice undergoing calorie restriction ate a majority of their daily food allowance within 3 h of access to food (Fig. 3B, days 1–3). This altered FAA meal structure persisted for 9 d after ad libitum food access was reinstated (Fig. 3 A and B, days 4–12). Another observation was that for 3 d after ad libitum food (Fig. 3A, days 5–7), the mutants exhibited a transient decrease in food consumption, possibly due to the absence of a cued response previously associated with food addition during calorie restriction. Surprisingly, this FAA behavior was stronger than the action of leptin (Fig. 3 A, day 7, and C and Inset). Although we observed positive pSTAT3 and cFos immunolabeling, similar to time points II and III, we could not determine whether this constituted a leptin response due to the high basal levels of immunostaining observed in aged animals (Fig. 3D; Fig. S2D) (6).
Fig. 3.
Food anticipatory activity (FAA) feeding behavior in ad libitum–fed Ift88 mutant mice that were calorie restricted. (A) Graphic output of real-time food intake. Days 1–3 show Ift88Δ/Δ FAA during calorie restriction, blue line (calorie restricted). Steep initial slope indicates that Ift88Δ/Δ consumed the majority of their food immediately. Ad libitum feeding began on day 4 (AL). FAA deteriorated after 9 d (ad libitum food but FAA behavior) and normal feeding resumed (FAA lost). Vehicle and leptin injection during FAA are indicated (VE and LE). Time point III vehicle and leptin injection nights are indicated (V3 and L3). Gray shading indicates dark cycle (night). (B) Percentage of food consumed between 5:00 and 8:00 PM (corresponding to the period just after food addition during the caloric restricted paradigm), indicating the persistence of FAA. Ift88Δ/Δ mice ate the majority of their ad libitum food during the first 3 h of night, compared with Ift88flox/flox mice that gradually ate approximately a quarter of their total intake during this same period. *P < 0.05, Student t test. (C and Inset) Feeding data and CFI after leptin injection in Ift88Δ/Δ during FAA. P = 0.07, P = 0.54; Student t test. All points and bars in graphs represent means ± SEMs. (D) pSTAT3 staining in the arcuate nucleus after IP leptin or vehicle injection during FAA. (Scale bar, 90 µm.) Dotted lines indicate approximate border of arcuate nucleus. Numbers within parentheses or bars indicate number of animals.
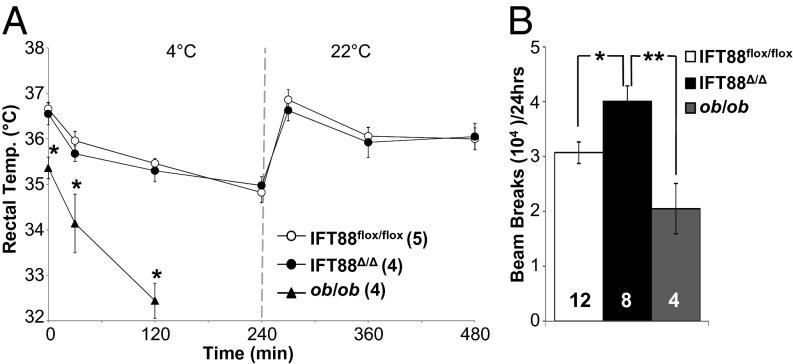
To further explore possible connections between leptin signaling defects in adult-induced cilia mutants, we performed both thermoregulation and activity experiments. Leptin mutant, ob/ob, mice display defects in thermogenesis, as evidenced by lower basal body temperature and the inability to maintain body temperature when challenged with a cold environment (32). To see whether conditional cilia mutant mice develop a cold intolerance phenotype associated with defects in leptin signaling, we cold-challenged Ift88Δ/Δ mutant mice. In contrast to the ob/ob mutant mice, Ift88Δ/Δ cilia mutant mice were able to regulate body temperature in response to cold challenge similar to Ift88flox/flox and C57BL/6 controls (Fig. 4A). Furthermore, activity analysis of Ift88Δ/Δ revealed a subtle hyperactivity before the onset of obesity and no changes in activity when obese (Fig. 4B). In contrast, ob/ob mice displayed diminished locomotor activity (Fig. 4B) (33, 34). Collectively these results indicate that cilia loss in Ift88Δ/Δ mutant mice does not result in phenotypes typically associated with leptin signaling deficits.
Fig. 4.
Thermoregulation and locomotor activity between Ift88 and ob/ob mice. (A) Body temperature of Ift88flox/flox, Ift88Δ/Δ, and ob/ob mice measured for baseline, after 30, 120, and 240 min of exposure to 4 °C, and at 270, 390, and 480 min during room temperature recovery. The ob/ob mice were pulled from the experiment due to an inability to thermoregulate. *P < 0.05, one-way ANOVA followed by post hoc Tukey's HSD test. (B) Locomotor activity at time point I comparing Ift88flox/flox, Ift88Δ/Δ, and ob/ob mice. *P < 0.05, **P < 0.01; one-way ANOVA followed by post hoc Tukey's HSD test. Numbers within parentheses or bars indicate number of animals.
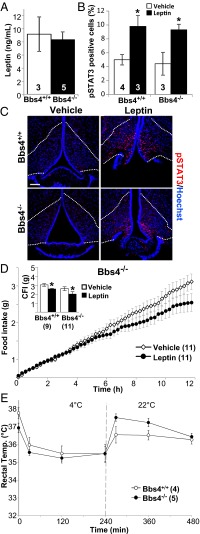
To determine whether the phenotypes observed in cilia loss models (Ift88Δ/Δ) differ from those observed in Bbs mouse models, leptin sensitivity and thermoregulation experiments were performed in Bbs4 mutants (congenital knockout of Bbs4, hereafter Bbs4−/−). To avoid confounding effects of calorie restriction and altered body composition, Bbs4−/− mutants were analyzed before the onset of obesity. Bbs4−/− mice genotyped null and showed loss of protein as previously described (Fig. S3 A and B) (16). Interestingly, much like Ift88Δ/Δ mice, preobese Bbs4−/− mice did not have significantly higher levels of serum leptin compared with controls (Bbs4+/+, 9.18 ng/mL; Bbs4−/−, 8.34 ng/mL; P = 0.75, Student t test; Fig. 5A), and responded to leptin injections compared with vehicle (Fig. 5D). Furthermore, increases in both pSTAT3 and cFos were detected within the arcuate nucleus of the young, preobese Bbs4−/− mutants, indicating a leptin response (Fig. 5 B and C; Fig. S2E). Also consistent with normal leptin signaling, nonobese Bbs4−/− mice did not display a defect in thermoregulation upon cold challenge (Fig. 5E). These results indicate that in both a model of cilia loss (Ift88Δ/Δ) and a model of defective cilia signaling (Bbs4−/−), leptin signaling is not directly affected. Importantly, these results indicate that a defect in a yet to be determined satiation pathway is dependent on the cilium.
Fig. 5.
Serum leptin, thermoregulation and leptin sensitivity analysis in Bbs4−/− mice. (A) Serum leptin levels of Bbs4+/+ and preobese Bbs4−/− mice. (B) Quantification of pSTAT3 staining in the arcuate nucleus of Bbs4+/+ and Bbs4−/− mice after IP leptin and vehicle. *P < 0.05, Student t test. (C) pSTAT3 staining in the arcuate nucleus after i.p. injection of leptin or vehicle in Bbs4+/+ and Bbs4−/− preobese mice. (Scale bar, 90 µm.) Dotted lines indicate approximate border of arcuate nucleus. (D) Feeding data after leptin and vehicle injection in Bbs4−/− mutant mice. (Inset) Cumulative food intake (CFI) for Bbs4+/+ and Bbs4−/− mice after leptin or vehicle i.p. injection. *P < 0.05, Student t test. (E) Body temperature of Bbs4+/+ and Bbs4−/− mice measured for baseline, and then at 30, 120, and 240 min of exposure to 4 °C, and at 270, 390, and 480 min during room temperature recovery. All points and bars represent means ± SEMs. Numbers within parentheses or bars indicate number of animals.
Discussion
We previously showed that loss of cilia on POMC cells in conditional mouse mutants resulted in hyperphagia and obesity (22). However, the molecular mechanisms behind this phenotype remain elusive. Recent studies in mouse models of BBS suggest that the complex of BBS proteins known as the BBSome is critical to leptin receptor trafficking and pathway activity (20). Interestingly, Bbs mutant mice retain their cilia, albeit with disrupted cilia receptor localization, whereas IFT mutations result in organelle loss (12, 22). Although both Bbs and IFT mutations lead to obesity, it is unclear whether similar mechanisms drive these phenotypes. Here we test whether the obesity phenotype observed in these mouse models is initiated by defects in leptin signaling.
To achieve this goal, the CAGG-CreER inducible transgene was used. Although this model does not lead to total loss of Ift88, it eliminates confounding effects of cilia loss during development, an advantage that the previously used POMC-Cre transgene does not possess (23, 35). It was previously shown that other obese leptin-resistant animals regain leptin sensitivity on regulation of body composition through controlled feeding (36, 37). Furthermore, the study presented here allowed for the repetitive assessment of leptin sensitivity in the same cohort of mice at different adiposity and leptin levels. Surprisingly, Ift88Δ/Δ mice are resistant to the actions of leptin when obese and have increased serum leptin but are sensitive to exogenous leptin both before weight gain and after weight loss.
Although the exact mechanism behind leptin action and resistance remains unclear, the downstream effects of leptin have been characterized. Leptin signaling leads to pSTAT3 and induction of Socs3, and subsequent neuronal activity induces nuclear cFos (38). In the young preobese states (time points I, Ift88Δ/Δ and Bbs4−/−), mice responded to leptin with an induction of both pSTAT3 and cFos in the arcuate nucleus, in contrast to what was shown for caloric-restricted lean Bbs mutants (20). In aged animals (time points II, III, and entrained), pSTAT3 and cFos staining were readily apparent in all groups, yet significant differences between vehicle and leptin treatment groups were not found. Similar results have been observed in several rodent models showing an age-dependent loss of leptin sensitivity (4, 39, 40). Furthermore, decreases or complete loss of leptin induced pSTAT3 response have been observed in aged rodents (6, 7, 27). This aspect of age-related emergence of leptin resistance is often overlooked in the literature. It is important to note that in age-associated diminished pSTAT3 induction, the anorectic behavioral response to leptin remains intact (6). It is interesting to note that in Bbs mutant mice, leptin-induced pSTAT3 is still observed, indicating that leptin signaling is not completely disrupted (20). Importantly, when Ift88Δ/Δ mice and Bbs4−/− mice are not hyperleptinemic, leptin injection elicits an anorectic effect on feeding. The Bbs4−/− mice were only analyzed before the onset of obesity in order to avoid confounding effects of altered body composition and behavioral changes that we observed as a result of caloric restriction. The cause of the altered behavior in the Bbs4 mutants is not known but may be associated with the neurodevelopmental and degenerative phenotypes that have been reported in these mice (41, 42). Collectively, the results obtained in the Bbs4 and Ift88 mutant mice indicate that the hyperphagia-associated obesity in models of cilia dysfunction is not initiated by defects in leptin signaling.
To further investigate whether other phenotypes associated with leptin signaling defects were observed in Ift88Δ/Δ mice, both thermoregulation and locomotor activity experiments were performed. Cold-challenged Ift88Δ/Δ mice demonstrated a normal thermoregulatory phenotype. This normal thermoregulation stands in stark contrast to leptin-deficient ob/ob mice, which are unable to thermoregulate (34). To further assess leptin signaling in cilia mutants, locomotor activity was evaluated. Ift88Δ/Δ mice were significantly more active in a 24-h period relative to ob/ob mice. These data indicate that conditional Ift88 cilia loss in adult mice does not lead to a primary defect in leptin signaling.
Previous reports demonstrated that restricted feeding in rodents can alter both behavior and physiology, independent of light-dark cycles (43). For example, in response to food restriction, mice are known to display FAA and alter their feeding behavior and meal structure (28). Thus, FAA can confound results in experiments if not taken into account. Our analysis of Ift88Δ/Δ mutant mice clearly revealed the FAA phenomenon. The mutants consume the majority of their calories within the first 3 h of the dark cycle during the paired feeding period (Fig. 3 A and B). In contrast, control mice on an ad libitum diet normally consume their calories gradually throughout the dark cycle. The FAA behavior in the mutants persisted for 9 d after ad libitum food access was initiated (Fig. 3B). In addition, after ad libitum access, the mutants experienced a short period of depressed feeding activity (days 5–7). This diminished feeding may result from the loss of cues established by daily food administration during caloric restriction. This cue would not occur during ad libitum access. If mutants are tested for leptin sensitivity during this FAA period, they appear leptin resistant with regard to their feeding. Thus, FAA is stronger than the appetite suppression effects of leptin. After mutants emerged from the FAA period and returned to a normal feeding pattern (time point III), they again exhibited an anorectic response to leptin. The persistence of the FAA represents an important and underappreciated aspect of feeding behavior analysis in the obesity field. Interestingly, FAA is dependent on the suprachiasmatic nuclei (SCN) and not the arcuate nucleus where ciliary function is needed for satiation responses (28). Future studies will address whether the FAA observed in Ift88 conditional mutants differs from that observed in WT mice.
In contrast to our findings, current dogma indicates that cilia are needed for normal leptin sensitivity based on previous studies in the Bbs ciliopathy mouse models. Data from Seo et al. used a caloric restriction paradigm up until leptin responsiveness was assessed to ensure that the Bbs mutant mice were kept lean (20). Although not directly addressed, this may have caused a FAA effect similar to what we observed in the Ift88Δ/Δ mice overriding the anorexigenic effects of leptin. To directly test whether Bbs mutant mice have a leptin signaling defect, we assessed both leptin sensitivity and thermoregulation in Bbs4−/− mice before the onset of obesity. Interestingly, Bbs4−/− mice responded to i.p. leptin injection and maintain body temperature when cold challenged. Collectively, these data suggest that the leptin signaling defect reported previously in the Bbs mice is only secondary to either weight gain or the FAA brought on by calorie restriction. These data ultimately leave the initiating molecular mechanism behind cilia dysfunction-associated obesity unknown.
Several potential molecular mechanisms for the obesity observed in ciliopathy mouse models exist. One must consider that the mechanism leading to obesity in Ift conditional models and Bbs models may be different. It is now well appreciated that the cilium functions as a key regulatory organelle for multiple pathways. Some of the potential pathways that could be involved in the obesity phenotype include altered G protein–coupled receptor (GPCR) signaling or abnormal regulation of mTor or hedgehog (Hh) signaling pathways. Importantly, the orexigenic GPCR melanin concentrating hormone receptor 1 (Mchr1) is present on neurons of the hypothalamus, but is mistargeted in the Bbs mutant mice (12). Thus, the possibility exists that altered Mchr1 signaling in the absence of cilia in the Ift88 mutants, or due to its exclusion from the cilia in the Bbs mutants, could result in hyperphagia induced obesity. Cilia loss alters mTOR activity, which has a well-documented role in energy homeostasis (44–46); however, altered mTor has not been evaluated in the Bbs mutant mice. Further, treatment of cilia mutant mice with rapamycin can partially correct some mutant phenotypes (47). Arguably, defects in the Hh pathway are currently the most directly associated with abnormal cilia function (48). Hh signaling is important for the development and patterning of numerous tissues, including the hypothalamus, and has critical roles during adult neurogenesis (49, 50). Thus, obesity in congenital Bbs mutants could arise through mispatterning of the hypothalamus. In fact, previous reports have indicated loss of POMC neurons in Bbs mutant mice (20). The loss of POMC neurons seems less likely in the adult inducible Ift88 mutant, as the hyperphagia phenotype is evident within 2 wk of inducing cilia loss. However, Hh pathway components are expressed in the hypothalamus of adult mice, and thus, it will be informative to evaluate whether feeding behavior and energy homeostasis can be altered by modulating Hh signaling activities specifically in the POMC neurons and whether this is influenced by the presence or absence of the cilium.
Methods
For methodological details, see SI Methods.
All mice were maintained in an Association for Assessment and Accreditation of Laboratory Animal Care-approved facility in accordance with Institutional Animal Care and Use Committee guidelines at University of Alabama at Birmingham. Ift88flox/flox; CAGG-CreER cohorts were generated, and tamoxifen induction of cilia loss was performed as previously described (22, 23). Bbs4 congenital mutant mice were obtained from Jackson Labs. Body composition was measured with an EchoMRI Quantitative Magnetic Resonance instrument as described previously (51). A BioDAQ episodic intake monitor (Research Diets) was used to measure food intake. Locomotor activity was measured using a tracker system (Phenotyper; Noldus).
Supplementary Material
Acknowledgments
We thank Dr. Mykytyn for the Bbs4 antibody, the University of Alabama at Birmingham small animal phenotyping core for body composition analyses (P30 DK056336 and P60 DK079626), and Mandy J. Croyle for technical assistance. This work was supported by the National Institutes of Health Grants RO1 DK075996 (to B.K.Y.), F32 DK088404 (to N.F.B.), T32 HL7578 (to E.B.M.), and T32 GM008111, (to R.C.P.). American Recovery and Reinvestment Act support (RO1DK075966-03S1) provided the Biodaq System.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. R.L.L. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1210192110/-/DCSupplemental.
References
- 1.Zhang Y, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 2.Considine RV, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334(5):292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 3.Maffei M, et al. Leptin levels in human and rodent: Measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1(11):1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 4.Jacobson L. Middle-aged C57BL/6 mice have impaired responses to leptin that are not improved by calorie restriction. Am J Physiol Endocrinol Metab. 2002;282(4):E786–E793. doi: 10.1152/ajpendo.00495.2001. [DOI] [PubMed] [Google Scholar]
- 5.Scarpace PJ, Matheny M, Moore RL, Tümer N. Impaired leptin responsiveness in aged rats. Diabetes. 2000;49(3):431–435. doi: 10.2337/diabetes.49.3.431. [DOI] [PubMed] [Google Scholar]
- 6.Morrison CD, et al. Increased hypothalamic protein tyrosine phosphatase 1B contributes to leptin resistance with age. Endocrinology. 2007;148(1):433–440. doi: 10.1210/en.2006-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scarpace PJ, Tümer N. Peripheral and hypothalamic leptin resistance with age-related obesity. Physiol Behav. 2001;74(4-5):721–727. doi: 10.1016/s0031-9384(01)00616-3. [DOI] [PubMed] [Google Scholar]
- 8.Zaghloul NA, Katsanis N. Mechanistic insights into Bardet-Biedl syndrome, a model ciliopathy. J Clin Invest. 2009;119(3):428–437. doi: 10.1172/JCI37041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berbari NF, O’Connor AK, Haycraft CJ, Yoder BK. The primary cilium as a complex signaling center. Curr Biol. 2009;19(13):R526–R535. doi: 10.1016/j.cub.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pedersen LB, Rosenbaum JL. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr Top Dev Biol. 2008;85:23–61. doi: 10.1016/S0070-2153(08)00802-8. [DOI] [PubMed] [Google Scholar]
- 11.Sharma N, Berbari NF, Yoder BK. Ciliary dysfunction in developmental abnormalities and diseases. Curr Top Dev Biol. 2008;85:371–427. doi: 10.1016/S0070-2153(08)00813-2. [DOI] [PubMed] [Google Scholar]
- 12.Berbari NF, Lewis JS, Bishop GA, Askwith CC, Mykytyn K. Bardet-Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc Natl Acad Sci USA. 2008;105(11):4242–4246. doi: 10.1073/pnas.0711027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nachury MV, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129(6):1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 14.Domire JS, et al. Dopamine receptor 1 localizes to neuronal cilia in a dynamic process that requires the Bardet-Biedl syndrome proteins. Cell Mol Life Sci. 2011;68(17):2951–2960. doi: 10.1007/s00018-010-0603-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin H, et al. The conserved Bardet-Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell. 2010;141(7):1208–1219. doi: 10.1016/j.cell.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mykytyn K, et al. Bardet-Biedl syndrome type 4 (BBS4)-null mice implicate Bbs4 in flagella formation but not global cilia assembly. Proc Natl Acad Sci USA. 2004;101(23):8664–8669. doi: 10.1073/pnas.0402354101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishimura DY, et al. Bbs2-null mice have neurosensory deficits, a defect in social dominance, and retinopathy associated with mislocalization of rhodopsin. Proc Natl Acad Sci USA. 2004;101(47):16588–16593. doi: 10.1073/pnas.0405496101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blacque OE, et al. Loss of C. elegans BBS-7 and BBS-8 protein function results in cilia defects and compromised intraflagellar transport. Genes Dev. 2004;18(13):1630–1642. doi: 10.1101/gad.1194004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lechtreck KF, et al. The Chlamydomonas reinhardtii BBSome is an IFT cargo required for export of specific signaling proteins from flagella. J Cell Biol. 2009;187(7):1117–1132. doi: 10.1083/jcb.200909183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seo S, et al. Requirement of Bardet-Biedl syndrome proteins for leptin receptor signaling. Hum Mol Genet. 2009;18(7):1323–1331. doi: 10.1093/hmg/ddp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rahmouni K, et al. Leptin resistance contributes to obesity and hypertension in mouse models of Bardet-Biedl syndrome. J Clin Invest. 2008;118(4):1458–1467. doi: 10.1172/JCI32357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davenport JR, et al. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr Biol. 2007;17(18):1586–1594. doi: 10.1016/j.cub.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: A tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244(2):305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- 24.Bishop GA, Berbari NF, Lewis J, Mykytyn K. Type III adenylyl cyclase localizes to primary cilia throughout the adult mouse brain. J Comp Neurol. 2007;505(5):562–571. doi: 10.1002/cne.21510. [DOI] [PubMed] [Google Scholar]
- 25.Farley C, Cook JA, Spar BD, Austin TM, Kowalski TJ. Meal pattern analysis of diet-induced obesity in susceptible and resistant rats. Obes Res. 2003;11(7):845–851. doi: 10.1038/oby.2003.116. [DOI] [PubMed] [Google Scholar]
- 26. Cui H, Cai F, Belsham DD (2006) Leptin signaling in neurotensin neurons involves STAT, MAP kinases ERK1/2, and p38 through c-Fos and ATF1. FASEB J 20(14):2654–2656. [DOI] [PubMed]
- 27.Scarpace PJ, Matheny M, Shek EW. Impaired leptin signal transduction with age-related obesity. Neuropharmacology. 2000;39(10):1872–1879. doi: 10.1016/s0028-3908(00)00014-9. [DOI] [PubMed] [Google Scholar]
- 28.Pitts S, Perone E, Silver R. Food-entrained circadian rhythms are sustained in arrhythmic Clk/Clk mutant mice. Am J Physiol Regul Integr Comp Physiol. 2003;285(1):R57–R67. doi: 10.1152/ajpregu.00023.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martínez-Merlos MT, et al. Dissociation between adipose tissue signals, behavior and the food-entrained oscillator. J Endocrinol. 2004;181(1):53–63. doi: 10.1677/joe.0.1810053. [DOI] [PubMed] [Google Scholar]
- 30.Escobar C, Cailotto C, Angeles-Castellanos M, Delgado RS, Buijs RM. Peripheral oscillators: The driving force for food-anticipatory activity. Eur J Neurosci. 2009;30(9):1665–1675. doi: 10.1111/j.1460-9568.2009.06972.x. [DOI] [PubMed] [Google Scholar]
- 31.Li X, Cope MB, Johnson MS, Smith DL, Jr, Nagy TR. Mild calorie restriction induces fat accumulation in female C57BL/6J mice. Obesity (Silver Spring) 2010;18(3):456–462. doi: 10.1038/oby.2009.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mayer J, Barrnett RJ. Sensitivity to cold in the hereditary obese-hyperglycemic syndrome of mice. Yale J Biol Med. 1953;26(1):38–45. [PMC free article] [PubMed] [Google Scholar]
- 33.Medina-Gomez G, et al. PPAR gamma 2 prevents lipotoxicity by controlling adipose tissue expandability and peripheral lipid metabolism. PLoS Genet. 2007;3(4):e64. doi: 10.1371/journal.pgen.0030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trayhurn P, Thurlby PL, James WP. Thermogenic defect in pre-obese ob/ob mice. Nature. 1977;266(5597):60–62. doi: 10.1038/266060a0. [DOI] [PubMed] [Google Scholar]
- 35.Xu AW, Ste-Marie L, Kaelin CB, Barsh GS. Inactivation of signal transducer and activator of transcription 3 in proopiomelanocortin (Pomc) neurons causes decreased pomc expression, mild obesity, and defects in compensatory refeeding. Endocrinology. 2007;148(1):72–80. doi: 10.1210/en.2006-1119. [DOI] [PubMed] [Google Scholar]
- 36.Enriori PJ, et al. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab. 2007;5(3):181–194. doi: 10.1016/j.cmet.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Shi H, et al. Diet-induced obese mice are leptin insufficient after weight reduction. Obesity (Silver Spring) 2009;17(9):1702–1709. doi: 10.1038/oby.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woods AJ, Stock MJ. Leptin activation in hypothalamus. Nature. 1996;381(6585):745. doi: 10.1038/381745a0. [DOI] [PubMed] [Google Scholar]
- 39.Gabriely I, Ma XH, Yang XM, Rossetti L, Barzilai N. Leptin resistance during aging is independent of fat mass. Diabetes. 2002;51(4):1016–1021. doi: 10.2337/diabetes.51.4.1016. [DOI] [PubMed] [Google Scholar]
- 40.Ma XH, et al. Aging is associated with resistance to effects of leptin on fat distribution and insulin action. J Gerontol A Biol Sci Med Sci. 2002;57(6):B225–B231. doi: 10.1093/gerona/57.6.b225. [DOI] [PubMed] [Google Scholar]
- 41.Davis RE, et al. A knockin mouse model of the Bardet-Biedl syndrome 1 M390R mutation has cilia defects, ventriculomegaly, retinopathy, and obesity. Proc Natl Acad Sci USA. 2007;104(49):19422–19427. doi: 10.1073/pnas.0708571104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eichers ER, et al. Phenotypic characterization of Bbs4 null mice reveals age-dependent penetrance and variable expressivity. Hum Genet. 2006;120(2):211–226. doi: 10.1007/s00439-006-0197-y. [DOI] [PubMed] [Google Scholar]
- 43.Mistlberger RE. Food-anticipatory circadian rhythms: Concepts and methods. Eur J Neurosci. 2009;30(9):1718–1729. doi: 10.1111/j.1460-9568.2009.06965.x. [DOI] [PubMed] [Google Scholar]
- 44.Berbari NF, et al. Mutations in Traf3ip1 reveal defects in ciliogenesis, embryonic development, and altered cell size regulation. Dev Biol. 2011;360(1):66–76. doi: 10.1016/j.ydbio.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boehlke C, et al. Primary cilia regulate mTORC1 activity and cell size through Lkb1. Nat Cell Biol. 2010;12(11):1115–1122. doi: 10.1038/ncb2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cota D, et al. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312(5775):927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 47.Shillingford JM, et al. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci USA. 2006;103(14):5466–5471. doi: 10.1073/pnas.0509694103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goetz SC, Anderson KV. The primary cilium: A signalling centre during vertebrate development. Nat Rev Genet. 2010;11(5):331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Szabo NE, et al. (2009) Role of neuroepithelial Sonic hedgehog in hypothalamic patterning. J Neurosci 29(21):6989–7002. [DOI] [PMC free article] [PubMed]
- 50.Breunig JJ, et al. Primary cilia regulate hippocampal neurogenesis by mediating sonic hedgehog signaling. Proc Natl Acad Sci USA. 2008;105(35):13127–13132. doi: 10.1073/pnas.0804558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jones AS, Johnson MS, Nagy TR. Validation of quantitative magnetic resonance for the determination of body composition of mice. Int J Body Compos Res. 2009;7(2):67–72. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.