Significance
Understanding the molecules that govern production of specific cell types from adult tissue stem cells is a major challenge, and Drosophila follicle stem cells (FSCs) are an outstanding model. We report identification of a gene, castor, which is required for FSC maintenance. Castor also functions in a genetic circuit with two other genes, hedgehog and eyes absent, to determine specific progeny cell fates. Our studies provide a marker for the earliest cell-fate decisions in this model and insight into the molecular and cellular mechanisms by which stem cells produce specific and diverse types of progeny.
Keywords: polar cell, polar stalk precursor, germarium
Abstract
Asymmetric division of stem cells results in both self-renewal and differentiation of daughters. Understanding the molecules and mechanisms that govern differentiation of specific cell types from adult tissue stem cells is a major challenge in developmental biology and regenerative medicine. Drosophila follicle stem cells (FSCs) represent an excellent model system to study adult stem cell behavior; however, the earliest stages of follicle cell differentiation remain largely mysterious. Here we identify Castor (Cas) as a nuclear protein that is expressed in FSCs and early follicle cell precursors and then is restricted to differentiated polar and stalk cells once egg chambers form. Cas is required for FSC maintenance and polar and stalk cell fate specification. Eyes absent (Eya) is excluded from polar and stalk cells and represses their fate by inhibiting Cas expression. Hedgehog signaling is essential to repress Eya to allow Cas expression in polar and stalk cells. Finally, we show that the complementary patterns of Cas and Eya reveal the gradual differentiation of polar and stalk precursor cells at the earliest stages of their development. Our studies provide a marker for cell fates in this model and insight into the molecular and cellular mechanisms by which FSC progeny diverge into distinct fates.
A defining characteristic of stem cells is their capacity to divide asymmetrically so that one daughter self-renews and the other differentiates. Uncovering the mechanisms governing self-renewal and differentiation is a major goal for which Drosophila oogenesis provides an excellent model (1). The Drosophila ovary is composed of 16–20 strings of egg chambers, called “ovarioles” (Fig. 1 A and B). Each egg chamber contains 16 germline cells surrounded by a monolayer of epithelial follicle cells. Egg chambers are linked by stalks, which are chains of five to eight follicle cells. A pair of special cells known as “polar cells” forms at each end of each egg chamber and function as signaling centers (2–5). Loss of polar cells causes egg-chamber fusion so that a single chamber contains two or more germline clusters (2, 6–9).
Fig. 1.
Castor expression in polar, stalk, and stem cells and its requirement in FSC maintenance. (A) A schematic drawing of a Drosophila ovariole and a magnified germarium showing regions 1–3. (B–K) Confocal micrographs of Cas expression in wild-type ovarioles. (B and C) 3D reconstructions show Cas (green) expressed in stalk cells (open arrows) and polar cells (arrows), which are marked by β-galactosidase (red), expressed with the polar-cell–specific driver updGAL4. (D and E) Cas (green) is expressed in the polar cells (arrow, marked by enriched FasIII staining in red), stalk cells (open arrow), and follicle precursor cells in the germarium. (F–H) FSCs (one of which is indicated by an arrowhead) express Cas and are located at the border of regions 2a and 2b, anterior to the FasIII expression domain (red). (I–K) Cas (red) is expressed in the most anterior cells of FSC clones, which are marked by GFP (green). In B and I, DAPI (blue) labels nuclei. In D, F, and G, VASA (blue) marks the germline cells. (L) The percentage of germaria containing follicle clones for the control (blue), casΔ1 (red), and rescue of casΔ1 (green) as a function of time after clone induction. In the control, the percentage decreases over time because of natural stem cell turnover. A greater percentage of mutant stem cells are lost. The total number of germaria analyzed is shown above each bar. Data are shown as mean ± SD. (Scale bars: 20 μm in B and C; 10 μm in D–K.)
Polar cells, like other follicle cells, arise from follicle stem cells (FSCs) located near the middle of the structure known as the “germarium” at the border between regions 2a and 2b (Fig. 1A) (10–13). When germline stem cells divide at the anterior tip of the germarium (Fig. 1A), escort cells cover the differentiating daughter, which undergoes precisely four rounds of division with incomplete cytokinesis, producing a 16-cell cyst (14, 15). At the junction of regions 2a and 2b, the cysts are released from the escort cells and encounter FSCs; a layer of follicle cells then envelops, or packages, the germline cells. Newly packaged cysts exit the posterior end of the germarium in a process called “egg-chamber budding.” Each egg chamber possesses a pair of polar cells at each end and is separated from adjacent egg chambers by stalk cells (16).
Unlike other follicle cells, which continue to divide until stage 6, polar and stalk cells cease mitotic division soon after cell-fate specification. Specific expression of polar cell markers is evident first in stage 1 egg chambers (also known as region 3 of the germarium) (6, 9). Notch and JAK/STAT signals orchestrate polar and stalk cell fates and are required to prevent egg-chamber fusions. Delta (Dl), the ligand for Notch, is expressed on germline cell surfaces and is required to specify polar cells. Then polar cells secrete Unpaired, which activates JAK/STAT and specifies stalk cells (5, 6, 9, 17–21). The existence of polar/stalk precursors has been suggested (22, 23), but recent evidence refutes the notion that there are lineage-restricted precursors of polar and stalk cells (19). Because of the lack of suitable markers within the germarium, where, when, or how polar, stalk, and main-body follicle cell fates first separate remains largely unknown. In fact this determination is a general challenge for studying tissue stem cell biology.
One useful marker is the protein Eyes absent (Eya), which is a repressor of polar cell fate (24). With the exception of differentiated polar and stalk cells, most follicle cells express Eya. Loss-of-function of eya causes ectopic polar cells, and forced expression of Eya in polar and stalk cells leads to fused egg chambers. Loss of Eya is one of the earliest markers for polar cells identified thus far. It has been proposed that the FSCs might divide to produce a daughter that turns on Eya and a daughter that turns off Eya (24, 25), although direct evidence for these changes in gene expression has been lacking. The progeny of the Eya− cell were proposed to go on to form the polar cells and stalk cells and the progeny of the Eya+ cell go on to form the rest of the follicular epithelium. However, how the absence of Eya leads to polar and stalk cell fate is unclear.
Eya loss also results in hyperactivity of Hedgehog (Hh) signaling (24). Hh plays many roles in embryonic development, adult homeostasis, birth defects, and cancer (26). In Drosophila oogenesis, Hh is expressed in cap cells and the terminal filament (Fig. 1A) and serves as a long-range signal to maintain FSCs and promote follicle cell proliferation. Loss of Hh results in stem cell loss, and hyperactivity induces extra FSCs (27, 28). Excess Hh causes follicle cell hyperproliferation and, curiously, ectopic polar cells (27–32). Conversely, reduction in the activity of Hh signaling causes fused egg chambers.
Here, we report that the gene castor (cas) is expressed in FSCs, early follicle precursors, and in polar and stalk cells. An evolutionarily conserved gene, cas encodes a zinc finger protein required for cell-fate specification and cell differentiation (33–37). Cas and Eya exhibit complementary patterns of expression in egg chambers so that Cas is restricted to polar and stalk cells from which Eya is excluded. Mutation of cas leads to loss of FSCs and fused egg chambers, indicating that cas is required for FSC maintenance and polar/stalk cell fate. Furthermore Eya inhibits Cas expression, and this inhibition explains why suppression of Eya is necessary for polar and stalk cell specification. In the germarium, the combination of Eya and Cas staining reveals the earliest steps in follicle cell differentiation. Thus, Cas is an important regulator of FSCs and of the cell-fate diversification of their progeny.
Results
Cas Is Expressed in FSCs as Well as Polar and Stalk Cells.
In a screen for genes that affect ovarian development (38), we identified cas, a gene known to determine Drosophila neural precursor cell fate (33, 34). Antibody staining revealed that Cas is expressed in follicle cells of the germarium, including the region where the FSCs reside, and later is strikingly restricted to polar and stalk cells (Fig. 1 B and C).
We tested whether Cas is expressed in FSCs and is required for their function. FSCs cannot be identified unambiguously because of the lack of specific molecular markers (11, 16, 19, 32, 39–41). However, indirect methods can be used. A simple approach is to stain with an antibody against Fasciclin III (FasIII); FSCs are the cells immediately anterior to FasIII+ cells (32, 39, 40). Double labeling revealed that Cas is expressed in cells anterior to the FasIII expression (Fig. 1 D and E). One caveat, however, is that some early FSC daughters also express very little FasIII (19). A higher-magnification confocal micrograph further showed that Cas is expressed at the border of regions 2a and 2b (Fig. 1 F–H).
A more definitive method for identifying stem cells is lineage tracing (16). If mitotic recombination is induced and the recombination takes place in the FSC, then marked cells will persist for a long period (Fig. S1A). If a cell other than a stem cell is marked, it will differentiate, contribute to an egg chamber, and eventually leave no marked cell in the germarium (Fig. S1B). Therefore the most-anterior cell of a long-lived clone is an FSC. It takes 3–4 d for follicle cells to differentiate and leave the germarium, so marked cells that remain in the germarium for more than 4 d after clone induction (ACI) are considered FSC clones. In lineage-tracing experiments, mosaic analysis with a repressible cell marker (MARCM) was used to label FSC clones with GFP (42). Fourteen days after induction, the most-anterior cells in the FSC clones expressed Cas (Fig. 1 I–K), albeit at lower levels than differentiating cells.
Cas Is Required for FSC Maintenance.
Because Cas is expressed in the FSC, we tested whether it functions in FSC maintenance. Homozygous cas mutants are lethal (33, 34), so the null allele casΔ1 was recombined with flippase recombination target at 82B (FRT82B) for clonal analysis (43). After heat-shock treatment to induce mitotic clones, flies were kept at 25 °C for 3–21 d before dissection. At day 3 ACI, cas-mutant and control germaria contained similar percentages of clones (∼80%; Fig. 1L). At 7, 14, and 21 d ACI the percentage of germaria containing clones decreased in wild type, reflecting the normal loss of stem cells that occurs over time (32, 39, 40). We observed an even greater loss of cas-mutant stem cells. At 21 d ACI 38% of control germaria contained marked FSC clones, whereas only 20% contained FSC clones homozygous for casΔ1 at 3 wk ACI, a phenotype that was rescued to wild type by including UAS-cas at 25 °C (Fig. 1L). Thus, Cas promotes FSC maintenance.
Cas Is Required for Polar and Stalk Cell Fates.
After egg-chamber budding, Cas expression is restricted to polar and stalk cells and is precisely complementary to expression of the polar cell-fate repressor Eya (Fig. 2 A and B). To test whether Cas is required for polar and stalk cell fates, we used MARCM analysis. In contrast to controls (Fig. 2 C–E), cas-mutant clones (Fig. 2 F–H) caused loss of stalks resulting in fused egg chambers that contained multiple germ cell clusters. Polar cells also were lost in casΔ1 mosaic ovarioles. In contrast to egg chambers containing control clones (Fig. 2 C–E), which contain two FasIII+ polar cells at each end, no FasIII staining was observed when casΔ1-mutant clones spanned the region between two cysts (Fig. 2 F–H). Fused egg chambers also were observed frequently in RNAi experiments. Cas expression was knocked down using c306GAL4 (Fig. S2 A–D) to drive one (Fig. S2 E–H) or two (Fig. S2 I–L) copies of UAS-dsRNA in early follicle cells as well as in polar and stalk cells. Both Cas (Fig. S2 F and J) and FasIII (Fig. S2 G and K) antibody staining were greatly reduced, and egg-chamber fusions were frequent when two copies of the UAS-dsRNA transgene were present, particularly in casΔ1/+ heterozygotes (Fig. S2 I–L). These phenotypes were rescued by re-expression of Cas. The percentage of ovarioles containing fused egg chambers decreased from 60 to 17% upon re-expression of Cas. Therefore, Cas is required for polar and stalk cell fate.
Fig. 2.
Complementary expression patterns and functions of Cas and Eya. (A and B) Confocal micrographs of ovarioles stained for Cas (green) and Eya (red). DAPI (blue) labels nuclei. Eya is expressed in main-body follicle cells of egg chambers but not in polar or stalk cells marked by Cas. (C–H) Loss of Cas causes fused egg chambers. (C–E) In control follicle cell clones marked by GFP, Cas (red) labels polar and stalk cells. FasIII (white) stains polar cells and immature follicle cells. (F–H) Ovarioles containing casΔ1 follicle cell clones, marked by GFP, contain fused egg chambers. Polar and stalk cells are absent between three germline clusters (F), as is FasIII (G) and Cas staining (H). D, E, G, and H show higher magnifications of the boxed regions shown in C and F, respectively. (I–L) Loss of Eya leads to ectopic Cas expression. Homozygous mutant clones are GFP− and are outlined by dashed lines. Ovarioles are stained for Cas (red) and DAPI (blue). eya54C2-mutant clones (J–L) ectopically expressed Cas in many follicle cells (arrows) and caused long, abnormal stalks (arrowheads) in 24 of 24 ovarioles that contained clones between egg chambers when analyzed 7 d ACI. Clones confined to the main body epithelium (n = 78) transformed into ectopic polar cells as previously described (24), and all such cells expressed Cas (Fig. S2 M–P). Control clones appear normal (J). (M–O) Normal Eya expression following overexpression of Cas with c306GAL4. (P–R) Loss of polar and stalk cells and egg-chamber fusions (arrowheads) caused by ectopic expression of Eya in polar and stalk cells using c306GAL4. Germline cysts are outlined in white. (S–U) Coexpression of Cas partially suppresses the fused egg chamber phenotype caused by ectopic Eya expression (see also Table 1). DAPI (blue), Cas (green), and Eya (red) staining are shown in M–U. (V–X) Follicle cells expressing Cas from two UAS-cas transgenes using the FLP-OUT system (GFP+, green) exhibit normal Eya expression (red). DAPI labels all nuclei in blue. Flies carrying genotype P[hsp70-flp]; UAS-cas/AyGAL4, UAS-gfp; UAS-cas, were heat-shocked at 37 °C for 1 h and then were incubated at 25 °C for 2 d before dissection. (Scale bars: 20 μm.)
Eya Negatively Regulates Cas to Promote Main-Body Follicle Fate.
Because Eya and Cas are expressed in complementary patterns, we tested whether Eya regulates Cas expression and vice versa. Loss of Eya resulted in the expression of Cas in main-body follicle cells (Fig. S2 M–P), from which Cas protein normally is absent (Fig. 2I). Mutant cells lacking Eya also could form long, abnormally shaped multicellular arrangements that expressed Cas (Fig. 2 J–L and Fig. S2 M–P). Many of these cells expressed the stalk cell marker Zfh1 (Fig. S3), and others expressed the polar cell marker A101 (Fig. S3). Therefore, loss of eya was sufficient to result in polar-like and stalk-like cell fates. Conversely, ectopic Eya expression caused the loss of polar and stalk fates (24). In wild-type egg chambers (Fig. 2 A–C) and those in which mCD8-GFP was expressed using c306GAL4 (Fig. S2 A–D), normal polar and stalk cells formed. Ovarioles expressing Cas alone with the c306GAL4 driver also appeared normal (Fig. 2 M–O). In contrast, expression of Eya using c306GAL4 caused loss of polar and stalk cells (Fig. 2 P–R and Fig. S4). In more than 60% of c306GAL4;UAS-eya ovarioles, egg chambers completely failed to bud (Fig. S4 J and K). In the remaining ovarioles we observed fused egg chambers containing neither polar cells nor stalk cells between the germline cysts (Fig. 2 P–R and Fig. S4). Coexpression of Cas with Eya suppressed these phenotypes (Fig. 2 S–U and Fig. S4).
Eya expression was de-repressed in cas-mutant cells (Fig. S2 U–X), indicating mutual repression; however, ectopic expression of Cas was insufficient to suppress Eya or to induce ectopic polar or stalk cells (Fig. 2 V–X). We conclude that Eya represses polar and stalk cell fates at least in part by suppressing Cas expression, which is required but not sufficient for their specification.
Cas Expression and Polar/Stalk Specification Require Hh Signaling.
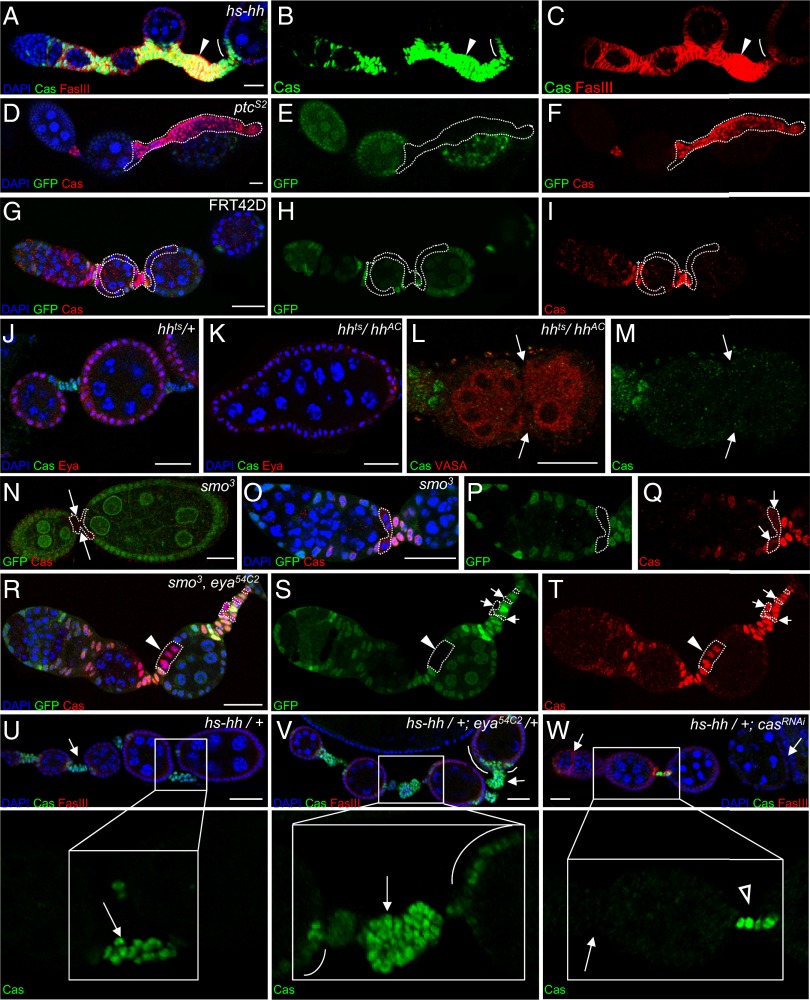
Eya and Hh exhibit mutual repression in the fly ovary (24), so we tested the effect of Hh on Cas using heat-shock–inducible Hh (hs-hh). Ovaries were analyzed following heat shock twice each day for 3 d and incubation at 25 °C for 2 d before dissection. As expected (23, 29, 30), we observed extra polar cells and gigantic stalks; these also showed Cas expression (arrowhead in Fig. 3 A–C). Clones mutant for patched (ptc), a negative regulator of Hh signaling, also caused gigantic stalks and ectopic expression of Cas expression (Fig. 3 D–F), although not every ptc−-mutant cell expressed Cas.
Fig. 3.
Hh-mediated somatic cell proliferation and polar/stalk cell fate specification require Cas. (A–F) Hyperactivity of Hh signaling ectopically induces Cas. (A–C) Heat shock of Hh induced enlarged stalks that express Cas (green) and FasIII (red) (arrowhead). (D–F) ptcS2-mutant clones that are GFP− (marked by dashed lines) also cause massive stalks and extra Cas+ cells (red). (G–I) No such phenotype was observed in control clones. (J–M) Loss of Hh activity causes fused egg chambers. (J) hhts/+ control ovarioles are stained for Cas (green) and Eya (red). Each egg chamber has 16 germ cells [large DAPI-stained nuclei (blue)], six of which appear in this focal plane. (K) An hhts/hhAC fused egg chamber contains 32 germ cell nuclei, 14 of which appear in this focal plane. (L and M) Two germline clusters marked by VASA (red) are encapsulated in one hhts/hhAC fused egg chamber lacking polar or stalk cells labeled by Cas (green). (N–Q) smo3-mutant (GFP−) intercyst cells (outlined in white) show reduced Cas (red). (R–T) smo3, eya54C2 double-mutant clones (GFP−; white outline) exhibit ectopic expression of Cas (red) in the main-body follicle cells (arrowhead) and enlarged stalks (arrows). (U–W) Ovarioles stained for Cas (green) to show stalk cells (arrows) and FasIII (red). (V) Enhancement of the hs-hh phenotype by mutation of one eya allele. Stalks were enlarged further compared with hs-hh alone (arrows), and extra Cas-expressing cells were present near the poles (white arcs). (W) Expression of a Cas RNAi line using hs-GAL4 suppressed the hs-hh phenotype. Stalks reverted to normal (open arrowhead) or to even smaller-than-normal (arrows) size. Boxed regions are shown at higher magnification below. (Scale bars: 20 μm.)
Furthermore, we tested whether polar and stalk cell formation required Hh. Temperature-sensitive hh (hhts)/hhAC flies were grown and kept at room temperature and then were shifted to 29 °C for 7 d to induce fused egg chambers (44). In contrast to the hhts/+ control (Fig. 3J), hhts/hhAC egg chambers contained 32 nuclei in two Vasa clusters with no visible polar or stalk cell or Cas expression (Fig. 3 K–M). These data demonstrated that Hh is required for Cas-dependent polar and stalk cell formation. To prevent the global effects of hhts, we further analyzed mutant clone phenotypes for smoothened (smo), a positive mediator of Hh. In smo3-mutant clones, which were marked by the absence of GFP, Cas staining was undetectable, and the stalk was missing (arrows, Fig. 3N). Cas expression also was undetectable in a small smo3 clone generated at the posterior part of late region 3, in presumptive polar/stalk precursors (Fig. 3 O–Q). Hence, Hh signaling has an active role in specifying polar and stalk cell fates in addition to its function in promoting follicle cell proliferation (28, 29).
Hh Antagonizes Eya to Promote Polar and Stalk Cell Fate.
Hh and Eya exhibit mutual repression, and the absence of Eya converts main-body follicle cells to polar cells (24). This effect explains how ectopic Hh signaling can induce ectopic polar cells. However, spatially patterned Hh activity is not required for specification of polar cells (27, 28). To clarify the relationship between Hh, Eya, and Cas further, we carried out genetic epistasis analysis. In hh, eya double-mutant cells, Cas expression was induced ectopically in the main-body follicle domain (arrowhead, Fig. 3 R–T), and long, multirow stalks were observed (arrows, Fig. 3 R–T). Thus, the double mutant phenocopied eya single mutants rather than hh, placing eya downstream of hh in a common pathway. We infer that Hh promotes cas expression by suppressing eya. This model predicts that reducing the concentration of Eya might enhance the Hh gain-of-function phenotype. To test this hypothesis, we removed one copy of eya in the presence of a single copy of the hs-hh transgene and found that the long stalk phenotype was greatly enhanced. Stalks became even longer and contained more cells as compared with the phenotype caused by one copy of hs-hh alone (arrows, Fig. 3 U and V). There also were extra Cas+ cells close to the poles (arcs, Fig. 3V), similar to the phenotype we observed in the presence of two copies of hs-hh (arcs, Fig. 3 A–C). In contrast, knocking down the expression level of Cas in the presence of one copy of hs-hh suppressed the phenotype, reducing the stalk cell number to normal or to even less than wild type (Fig. 3W). In addition, many egg chambers fused together (arrows, Fig. 3W), placing Cas downstream of Hh. This result further confirms that Cas is essential for polar/stalk cell formation and that without it even increasing Hh signaling activity cannot induce long, thick stalks. Overexpression of Eya also suppressed the ectopic polar cells and excess stalk cells caused by hs-hh (Table 1). For example, in the presence of two copies of the hs-hh transgene, 34% of egg chambers contained ectopic polar cells; this percentage was reduced to 3% when Eya was coexpressed. Taken together, these findings support the idea that Hh signaling positively affects Cas expression and polar and stalk cell fates by suppressing Eya.
Table 1.
Epistatic relationship between hedgehog and eya in follicle cell-fate specification
| Genotype | n | Stalk cell number |
Extra Cas+ main-body follicle cells (%) | n | ||
| <5 (%) | 5–9 (%) | >9 (%) | ||||
| UAS-eya/+ | 93 | 14 | 82 | 4.3 | 0 | 181 |
| UAS-eya/hs-Gal4 | 106 | 97 | 2.8 | 0 | 0 | 146 |
| hs-hh/+;UAS-eya/+ | 51 | 7.8 | 86 | 5.9 | 0 | 112 |
| hs-hh/+;UAS-eya/hs-Gal4 | 79 | 84 | 11 | 5.1 | 0 | 121 |
| hs-hh/hs-hh;UAS-eya/+ | 60 | 0 | 6.7 | 93 | 34 | 105 |
| hs-hh/hs-hh;UAS-eya/hs-Gal4 | 118 | 3.4 | 17 | 80 | 3.2 | 189 |
Because Cas labels polar and stalk cells from very early in oogenesis and is required for their development, we investigated where and when polar and stalk cell fates are first specified. In the germarium, staining with either Eya or Cas alone is not particularly informative, because each exhibits some staining, although more weakly than at later stages (Fig. 4 A and B). However, double labeling reveals that cells located between germline cysts in region 2b have stronger Cas expression and lower Eya expression (arrow, Fig. 4 C and D), whereas the presumptive main-body follicles have more prominent Eya than Cas (arcs, Fig. 4 C–E). Higher magnification of successive stages of germarium development further shows the development of precursor cells between two cysts (Fig. 4 F–J). As follicle cells divide and the cyst grows, so does the population of intercyst cells, which progressively lose Eya expression (brackets, Fig. 4 F–J). At the same time, the cells that are not close to the border between two cysts progressively lose Cas staining (arcs, Fig. 4 F–J). Eventually, a boundary of complementary expression is established upon egg-chamber budding, and Eya expression is undetectable in mature stalks composed of a single row of cells (Fig. 4J). Over time, the number of Eya+ and Cas− cells in each egg chamber increases. This result supports previous findings that polar and stalk cells stop dividing soon after cell-fate specification, whereas main-body follicle cells continue to divide until stage 6 (16, 23, 45). Based on the dynamic expression patterns and the loss- and gain-of-function phenotypes observed in Eya and Cas, we conclude that the distinction between polar and stalk cell and main-body cell fates depends on the ratio of Cas and Eya.
Fig. 4.
Follicle cell fate specification by Hh, Eya, and Cas. (A–J) The combination of Cas (green) and EYA (red) expression. FSCs and the early progeny (arrowheads in C–E) express both proteins. Cas expression increases in the region between two cysts (arrows) but gradually is down-regulated in presumptive main-body follicle cells in region 3 (arc) where Eya expression (red) is up-regulated. (F–J) High magnification of germaria showing successive stages of follicle cell fate specification. VASA staining of the germline is shown in blue. Presumptive polar/stalk precursor cells at the border between two cysts are indicated by brackets, and the presumptive main-body follicle precursors are indicated by arcs. C–J are confocal micrographs. A and B are 3D reconstructions of micrographs taken from the ovariole shown in Fig. 2 A and B. (Scale bars: 20 μm.) (K–M) Schematic diagram illustrating the progression of follicle precursor differentiation. Green and red represent cells expressing high levels of Cas and Eya, respectively. Yellow indicates cells expressing similar levels of Cas and Eya. Orange represents high Eya expression with a residual low level of Cas. (K) The FSC (dark green) gives rise to progeny that continue to divide and gradually differentiate into polar/stalk precursors (bracket) at the border between regions 2b and 3. Meanwhile, the presumptive main-body follicles (arcs) gradually lose Cas protein. (L) The polar/stalk precursors (bracket) separate the cysts. (M) Some of the polar/stalk precursors (bracket) differentiate into polar cells (asterisks). Polar cells secrete Unpaired to induce the neighboring precursor cells to form the stalk. Finally, after egg-chamber budding, only polar and stalk cells (green) express Cas, and Eya expression is restricted to the main-body follicle cells (red). (N) Positive and negative signals controlling the indicated stages of normal follicle cell development.
We also took advantage of these two early cell-fate markers to test whether there is lineage restriction for polar and stalk cells versus main-body follicle cells in the germarium. In agreement with previous studies that relied on later markers of cell fates (19), we found some clones derived from a single cell that included a mixture of polar/stalk and main-body cells even in the germarium. Therefore, there was no strict lineage restriction for the polar/stalk cell fate (Fig. S5).
Relationship Among Notch, JAK/STAT, and Cas in Polar/Stalk Cell Fate Specification.
Even after egg chambers bud, polar and stalk cell fates are reversible and are regulated by the balance of Notch and JAK/STAT activity (6, 18). We took advantage of the early expression of Cas and Eya to examine the effect of Notch (Fig. S6) and JAK/STAT (Fig. S7) on cell fates at the earliest stages of follicle-cell differentiation. Because neither Notch (Fig. S6 A–C) nor stat (Fig. S7 A–C) loss of function affected the pattern of Cas or Eya expression in the germarium, we conclude that these signals do not affect the earliest follicle cell-fate distinctions. However, as egg-chamber development progresses, Notch or stat-mutant clones at the pole ends of egg chambers lead to egg-chamber fusions, consistent with previously published results, and there is a concomitant loss of Cas (Figs. S6 D–F and S7 A–C). Some stat-mutant cells express both Cas and Eya in stage 7 or 8 egg chambers (Fig. S7 D–F), indicating that JAK/STAT signaling is required for the maintenance of cell fates after egg-chamber budding but not for the initial specification of the polar/stalk precursor pool. Further support for the conclusion that Cas functions independently of Notch is that ectopic Cas and constitutively active Notch together caused many extra FasIII+ cells to form, whereas, under the conditions of this experiment, neither one alone was sufficient (Fig. S8; see Materials and Methods for details).
Discussion
Identifying genes required for stem-cell maintenance and elucidating the precise series of molecular steps that govern when and where specific differentiated cell types form from stem cells is critical for regenerative medicine and for understanding normal organogenesis. The Drosophila ovary is an excellent model because of the presence of germline and somatic stem cells and continuous production of differentiated progeny throughout adult life. However, the lack of markers has made it particularly difficult to study FSCs and their immediate progeny in the germarium (2, 4–6, 19). The work reported here shows that Cas is critically required for early development of follicle cells. Cas is required for FSC maintenance, and because we found no evidence of increased cell death in ovarioles containing cas-mutant clones, it is likely that Cas promotes FSC maintenance by preventing their differentiation, possibly by keeping Eya levels low. In addition, because the most anterior Cas+ cells are the FSCs, Cas can facilitate the identification of FSCs even without lineage tracing. Moreover, the combination of Cas and Eya staining reveals early distinctions in gene-expression patterns in the germarium, because cells gradually acquire polar/stalk or main-body precursor fates. Together with Hh signaling, Cas and Eya form a critical and early-acting network (Fig. 4 K–N).
Complementary Expression Patterns and Functions of Cas and Eya Orchestrate Follicle Cell Fates.
Cas is expressed in a striking pattern. In mature egg chambers it is restricted to polar and stalk cells from which Eya is excluded, suggesting complementary functions for these two genes. In the germarium, staining with either marker alone is difficult to interpret. However, when they are combined, the two markers reveal a pattern. Cas and Eya are both expressed at low levels in FSCs, and as their progeny differentiate, cells either express more and more Cas and lose Eya, or vice versa. The cells with higher Cas presumably differentiate into polar or stalk cells, which ultimately express only Cas; the cells in which Eya expression persists and Cas disappears differentiate into the main-body follicle cells.
The complementary expression patterns accurately reflect complementary functions of Eya and Cas in egg-chamber development. Eya is a potent repressor of polar and stalk cell fate, whereas Cas is required for polar and stalk cells. Loss of Eya results in ectopic Cas expression, and ectopic expression of Eya in polar and stalk cells is sufficient to suppress Cas and cause fused egg chambers. Mutual repression between Cas and Eya would provide a simple mechanism for sorting of the two expression domains. However, Cas by itself is not sufficient to suppress Eya or induce polar or stalk cell fate. This finding is intriguing, because it suggests that when the balance tips so that a cell expresses more Eya than Cas, the cell-fate decision is irreversible, and main body cell fate is sealed. However, when the balance tips in favor of Cas expression, cell fate remains labile, and the polar/stalk precursor fate can revert to the main-body fate, providing a mechanism for reducing the numbers of polar and stalk cells if too many precursors form.
Early Cell-Fate Distinctions.
Proposed mechanisms by which polar and stalk cell fates become distinguished from the main-body follicle cells have been contradictory and controversial. One study suggested that there is a polar/stalk lineage (22, 23). Others have suggested no lineage restriction (19). One challenge has been the lack of markers to follow cell fates at the earliest stages and therefore the need to make inferences about early events based on analysis of late stages. Using the early expression of Cas and Eya to analyze early stages of follicle cell differentiation, we found that both Cas and Eya are expressed in FSCs as well as the early progeny in region 2b, but the balance of expression begins to break as cells progress beyond this stage and a pool of polar and stalk precursor cells becomes discernible (Fig. 4 F–J). These observations can help explain previously contradictory results. Because the polar/stalk precursors separate from the other follicle precursors at the posterior of region 2b, and further divisions take place, our results provide support for a polar/stalk precursor population and even some support for a polar/stalk lineage. However, we find that the polar/stalk precursor cell fates are still labile and depend on intercellular signaling and cell position to be maintained; hence the lineage restriction is not strict (19). This model also can explain what becomes of polar/stalk precursor cells that are produced in excess of what is needed for polar and stalk development: They change to main-body fate. Consistent with this model, there are no strict follicle cell lineage restrictions.
Hh Regulates Cas, Eya, and Cell Identity.
One additional component in the genetic circuitry that segregates polar and stalk cells from the rest of the epithelial follicle cells is Hh. Eya and Hh mutually inhibit one another (24) so that eya-mutant cells exhibit elevated Hh pathway activity, and mutation of negative regulators of the Hh pathway, such as costal2 (cos2) or patched (ptc), result in ectopic, Eya− polar cells. Mutations in hh or fused, which is a positive component of the Hh signal-transduction pathway, result in fused egg chambers, like cas mutations. However, Hh signaling in the ovary is complex, and some aspects of it do not fit the canonical model. For example, fused is not required for FSC maintenance or follicle cell proliferation even though Hh is required (45). Mutation of Cubitus interruptus (Ci), the major downstream effector in conventional hh signaling, does not affect egg-chamber patterning (24, 28) or Cas expression (Fig. S9). Therefore, the effect of Hh on Cas-dependent polar/stalk precursor cell fate must be mediated by an effector other than Ci.
Hh signaling promotes follicle cell proliferation (28, 29). Because of the lack of specific prefollicle markers to monitor cell-fate transitions in the germarium, the prevailing view has been that the fused egg chamber phenotype caused by hhts is primarily the result of insufficient numbers of follicle cells rather than defects in polar or stalk cell fate specification. However, we found that blocking Hh activity repressed Cas expression, leading to dramatic effects on egg-chamber patterning. Molecular epistasis analysis indicated that Hh signaling antagonizes Eya to maintain Cas expression and hence potentiate polar/stalk cell fate. Therefore, the ratio of Hh pathway activity to Eya expression in follicle cell precursors may be the critical determinant of which fate they adopt. However, Eya expression is not restored completely in smo3-mutant cells, including main-body cells. So, although high-level Hh signaling suppresses Eya in this tissue, some Smo function also is required to maintain Eya expression.
Hyperactivity of Hh signaling does not transform every main-body follicle to polar/stalk cell fate (23, 24, 29). Moreover, deleting protein kinase A and disabling Hh at the same time results in normal ovariole development, suggesting that although Hh signaling is important, no spatial pattern of the signal is required (28). So the question of how the alternating pattern of Cas and Eya expression arises in region 2b remains. Lateral inhibition between polar/stalk precursors and main-body precursors could be an appealing notion; however, Notch signaling, which is the best-characterized mediator of lateral inhibition, is not required for the earliest cell-fate decision. Another intriguing possibility is that temporal oscillations in Hh signaling, rather than a spatial pattern, could contribute to alternating polar/stalk and main-body cell fates. In contrast to embryonic and larval pattern formation, which occurs in a single linear sequence, oogenesis is an ongoing process requiring continuous, repeated patterning as new egg chambers form. Therefore, in principle, temporally patterned signals could generate alternating cell fates. Alternatively, the balance between Eya and Cas may be broken stochastically (46). Whatever the mechanism, it appears that roughly equal numbers of polar/stalk and main-body precursors are specified initially; then at least two mechanisms dramatically change the number of cells in the two lineages. First, polar and stalk cells stop dividing earlier. Second, excess polar/stalk precursors can change their fate to main body cells.
Before the current work, Cas had been identified and studied for its role in determining neural cell fate. Drosophila neuroblasts (NBs) are not actually self-renewing stem cells. Rather, they are progenitors that produce a defined set of progeny in a specific temporal sequence. Each NB divides asymmetrically to produce a ganglion mother cell (GMC), which goes on to produce a pair of neurons. Each NB division produces a GMC with a distinct fate, which depends on the sequential expression of a series of transcription factors, ending with Cas. In contrast, FSC divisions are truly self-renewing and iterative: They keep producing polar/stalk and main body precursors division after division. Because of these differences, it is not surprising that the upstream regulation of Cas differs in NBs and follicle cells. The transcription factors that act earlier in the NB temporal sequence, Kruppel, Hunchback, and POU domain protein 2 (Pdm2) are not expressed detectably in follicle cells. Instead Hh, a known stem cell factor, regulates Cas expression directly or indirectly.
The progeny of NBs are GMCs, which produce two neurons each. The progeny of FSCs are polar/stalk (or main body) precursors, which produce a more variable number of progeny, the fates of which are determined subsequently by their ratio of Eya to Hh/Cas. So is there anything in common between the role of Cas in the nervous system and the ovary? The function of Cas in the NB is to repress Pdm so that the NB can produce interneurons instead of RP5 motor neurons. Similarly, Cas in polar and stalk cells is required to repress Eya and the main body cell fate. Furthermore, in the nervous system Cas is required to inhibit the third fate, thereby allowing the fourth fate. Ectopic expression of Cas is not sufficient to convert the first or second fate into the fourth fate. Similarly Cas in follicle cells is necessary but not sufficient to specify polar and stalk cell fates.
Overall this work provides insight into the cellular and molecular mechanisms governing maintenance of an adult tissue stem cell and the earliest steps of cell-fate diversification of the progeny. This work also provides useful markers for future studies. Inappropriate sonic hedgehog activation is observed in human familial and sporadic basal-cell carcinomas and in prostate and breast cancers. Hh pathway inhibitors hold promise for treatment of these conditions (47), although the requirement for hh signaling in normal tissues could result in severe side effects of such drugs. Therefore, identification of tissue-specific effectors of Hh may represent even better drug targets (48). It will be of interest to determine whether the human homolog of Cas also proves to function as a tissue-specific downstream target of Hh.
Materials and Methods
Drosophila Genetics.
The following Drosophila strains were used in this study: w1118 was used as wild-type control. Mutant clones were generated by mitotic recombination using the FLP/FRT system as follows. Adult female flies were heat shocked twice daily for 1 h (about 8 h apart) at 37 °C for 3 d and then were kept at 25 °C for 3–21days. Flies were shifted to 29 °C and fed with wet yeast for overnight fattening before dissection. The following alleles were used: eya54C2, FRT40A; smo3, FRT40A (from Jin Jiang, University of Texas Southwestern Medical Center) and FRT42D, ptcS2, and y, w, hsp70-FLP; FRT42 P[ci1] hsp70-GFP/FRT42; ci94/ci94 (from Henry Y. Sun, Academia Sinica, Taiwan). casΔ1 (from Ward F. Odenwald, National Institutes of Health) was recombined with FRT82B (from Bloomington Drosophila Stock Center) for clonal analysis. For MARCM, hs-FLP, UAS-srcEGFP; actin-GAL4, UAS-EGFP; FRT82B, tub-GAL80 (from Ting Xie, the Stowers Institute for Medical Research) was used to mark clones for rescue analysis directly with UAS-cas (from Ward F. Odenwald). To enhance GFP expression in marked clones, flies with induced clones were incubated at 29 °C for 24 h before dissection. The genotypes for rescue experiments were hs-FLP, UAS-srcEGFP; UAS-cas/actin-GAL4, UAS-EGFP; FRT82B, casΔ1/FRT82B, tub-GAL80. For ectopic polar cell induction, flies carrying UAS-Nintra (from Kenneth D. Irvine, Rutgers University), UAS-cas, or both were cultured below 20 °C and then shifted to 31 °C for 3 d before dissection, to inactivate the GAL80 repressor. For FLP-OUT experiments female flies were heat shocked at 37 °C for 1 h and then were incubated at 25 °C for 2 d before dissection. statts/stat1681 transheterozygotes were transferred from 25 °C to 29 °C for 5 d before dissection.
To knock down castor expression, UAS-casRNAi2928 and UAS-casRNAi100305 were obtained from ViennaDrosophila RNAi Center. c306/+;;casΔ1, UAS-casRNAi2928/+, and c306/+; UAS-casRNAi100305/+; casΔ1, UAS-casRNAi2928/+ were grown at 18 °C and 2 d after enclosure were shifted to 29 °C for 4 d before dissection.
UAS-eya2 (from Nancy Bonini, University of Pennsylvania), UAS-cas, or both were expressed using c306GAL4. To avoid the lethality caused by Eya or Cas, the temperature-sensitive (ts) transcriptional repressor GAL80ts (Bloomington Drosophila Stock Center) was used, and flies were cultured below 20 °C. After eclosion, adult flies were shifted to 31 °C for 36 h to inactivate GAL80 and allow GAL4 activation. Then the ovaries were dissected, stained with antibody, and examined.
hs-hh flies with indicated genotypes were heat shocked twice daily at 37 °C for 1 h (about 8 h apart) for 3 d and were dissected 2 d later for further immunostaining. UAS-eya on the second chromosome was obtained from Ilaria Rebay (University of Chicago). hhts/hhAC flies (generous gifts from Cheng-Ting Chien, Academia Sinica, Taiwan) were kept at room temperature and then were shifted to 29 °C for 7 d (44).
Immunohistochemistry.
Ovarioles were dissected in S2 cell medium (Sigma) containing 10% (vol/vol) FBS, fixed on ice in PBS with 4% (vol/vol) formaldehyde for 30 min, and then rinsed three times in PBS with 0.3% Triton X-100. The following primary antibodies from the Developmental Studies Hybridoma Bank) were used for immunostaining: mouse anti-Fascicilin III (7G10; 1:25); mouse anti–β-galactosidase (40-1a; 1:25); mouse anti-EYA (10H6; 1:50); mouse anti-Armadillo (N27A1; 1:25); rat anti-dCAD2 (1:10); mouse anti-Notch, intradomain (C17.9C6; 1:1,000); and rat anti-VASA (VASA; 1:10). Rabbit anti-Cas antibody (1:5,000) and rabbit anit-Zfh1 (1:1,000) antibody were generous gifts from Ward F. Odenwald (33) and Ruth Lehmann (Skirball Institute, New York University), respectively. Secondary antibodies that conjugated with Alexa-488, Alex-568, or Alexa-647 were used in 1:400 dilutions (Molecular Probes).
Supplementary Material
Acknowledgments
We thank Ward F. Odenwald, Henry Y. Sun, Ting Xie, Kenneth D. Irvine, Cheng-Ting Chien, Nancy Bonini, Ilaria Rebay, Ruth Lehmann, Jin Jiang, Chris Q. Doe, the Vienna Drosophila RNAi Center, the Bloomington Drosophila Stock Center, and the Developmental Studies Hybridoma Bank for fly stocks or reagents and the Instrument Development Center of the National Cheng Kung University for access to and technical support with the Carl Zeiss LSM 780 laser-scanning microscope. This work was supported by National Institute of General Medical Sciences Grant GM46425 (to D.J.M.) and National Science Council of the Republic of China Grant 100-2311-B-006-008-MY2 (to A.C.-C.J.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1300725110/-/DCSupplemental.
References
- 1.Spradling AC. Developmental genetics of oogenesis. In: Martinez-Arias B, editor. The Development of Drosophila melanogaster. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1993. pp. 1–70. [Google Scholar]
- 2.Grammont M, Irvine KD. Fringe and Notch specify polar cell fate during Drosophila oogenesis. Development. 2001;128(12):2243–2253. doi: 10.1242/dev.128.12.2243. [DOI] [PubMed] [Google Scholar]
- 3.Grammont M, Irvine KD. Organizer activity of the polar cells during Drosophila oogenesis. Development. 2002;129(22):5131–5140. doi: 10.1242/dev.129.22.5131. [DOI] [PubMed] [Google Scholar]
- 4.Vachias C, Couderc JL, Grammont M. A two-step Notch-dependant mechanism controls the selection of the polar cell pair in Drosophila oogenesis. Development. 2010;137(16):2703–2711. doi: 10.1242/dev.052183. [DOI] [PubMed] [Google Scholar]
- 5.Xi R, McGregor JR, Harrison DA. A gradient of JAK pathway activity patterns the anterior-posterior axis of the follicular epithelium. Dev Cell. 2003;4(2):167–177. doi: 10.1016/s1534-5807(02)00412-4. [DOI] [PubMed] [Google Scholar]
- 6.Assa-Kunik E, Torres IL, Schejter ED, Johnston DS, Shilo BZ. Drosophila follicle cells are patterned by multiple levels of Notch signaling and antagonism between the Notch and JAK/STAT pathways. Development. 2007;134(6):1161–1169. doi: 10.1242/dev.02800. [DOI] [PubMed] [Google Scholar]
- 7.López-Schier H, St Johnston D. Delta signaling from the germ line controls the proliferation and differentiation of the somatic follicle cells during Drosophila oogenesis. Genes Dev. 2001;15(11):1393–1405. doi: 10.1101/gad.200901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruohola H, et al. Role of neurogenic genes in establishment of follicle cell fate and oocyte polarity during oogenesis in Drosophila. Cell. 1991;66(3):433–449. doi: 10.1016/0092-8674(81)90008-8. [DOI] [PubMed] [Google Scholar]
- 9.Torres IL, López-Schier H, St Johnston D. A Notch/Delta-dependent relay mechanism establishes anterior-posterior polarity in Drosophila. Dev Cell. 2003;5(4):547–558. doi: 10.1016/s1534-5807(03)00272-7. [DOI] [PubMed] [Google Scholar]
- 10.de Cuevas M, Lilly MA, Spradling AC. Germline cyst formation in Drosophila. Annu Rev Genet. 1997;31:405–428. doi: 10.1146/annurev.genet.31.1.405. [DOI] [PubMed] [Google Scholar]
- 11.Kirilly D, Xie T. The Drosophila ovary: An active stem cell community. Cell Res. 2007;17(1):15–25. doi: 10.1038/sj.cr.7310123. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Han Y, Xi R. Polycomb group genes Psc and Su(z)2 restrict follicle stem cell self-renewal and extrusion by controlling canonical and noncanonical Wnt signaling. Genes Dev. 2010;24(9):933–946. doi: 10.1101/gad.1901510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skora AD, Spradling AC. Epigenetic stability increases extensively during Drosophila follicle stem cell differentiation. Proc Natl Acad Sci USA. 2010;107(16):7389–7394. doi: 10.1073/pnas.1003180107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Decotto E, Spradling AC. The Drosophila ovarian and testis stem cell niches: Similar somatic stem cells and signals. Dev Cell. 2005;9(4):501–510. doi: 10.1016/j.devcel.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Morris LX, Spradling AC. Long-term live imaging provides new insight into stem cell regulation and germline-soma coordination in the Drosophila ovary. Development. 2011;138(11):2207–2215. doi: 10.1242/dev.065508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Margolis J, Spradling A. Identification and behavior of epithelial stem cells in the Drosophila ovary. Development. 1995;121(11):3797–3807. doi: 10.1242/dev.121.11.3797. [DOI] [PubMed] [Google Scholar]
- 17.Baksa K, Parke T, Dobens LL, Dearolf CR. The Drosophila STAT protein, stat92E, regulates follicle cell differentiation during oogenesis. Dev Biol. 2002;243(1):166–175. doi: 10.1006/dbio.2001.0539. [DOI] [PubMed] [Google Scholar]
- 18.McGregor JR, Xi R, Harrison DA. JAK signaling is somatically required for follicle cell differentiation in Drosophila. Development. 2002;129(3):705–717. doi: 10.1242/dev.129.3.705. [DOI] [PubMed] [Google Scholar]
- 19.Nystul T, Spradling A. Regulation of epithelial stem cell replacement and follicle formation in the Drosophila ovary. Genetics. 2010;184(2):503–515. doi: 10.1534/genetics.109.109538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen HJ, et al. The Hippo pathway controls polar cell fate through Notch signaling during Drosophila oogenesis. Dev Biol. 2011;357(2):370–379. doi: 10.1016/j.ydbio.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Shyu LF, Sun J, Chung HM, Huang YC, Deng WM. Notch signaling and developmental cell-cycle arrest in Drosophila polar follicle cells. Mol Biol Cell. 2009;20(24):5064–5073. doi: 10.1091/mbc.E09-01-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larkin MK, Holder K, Yost C, Giniger E, Ruohola-Baker H. Expression of constitutively active Notch arrests follicle cells at a precursor stage during Drosophila oogenesis and disrupts the anterior-posterior axis of the oocyte. Development. 1996;122(11):3639–3650. doi: 10.1242/dev.122.11.3639. [DOI] [PubMed] [Google Scholar]
- 23.Tworoger M, Larkin MK, Bryant Z, Ruohola-Baker H. Mosaic analysis in the drosophila ovary reveals a common hedgehog-inducible precursor stage for stalk and polar cells. Genetics. 1999;151(2):739–748. doi: 10.1093/genetics/151.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bai J, Montell D. Eyes absent, a key repressor of polar cell fate during Drosophila oogenesis. Development. 2002;129(23):5377–5388. doi: 10.1242/dev.00115. [DOI] [PubMed] [Google Scholar]
- 25.Adam JC, Montell DJ. A role for extra macrochaetae downstream of Notch in follicle cell differentiation. Development. 2004;131(23):5971–5980. doi: 10.1242/dev.01442. [DOI] [PubMed] [Google Scholar]
- 26.Jiang J, Hui CC. Hedgehog signaling in development and cancer. Dev Cell. 2008;15(6):801–812. doi: 10.1016/j.devcel.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vied C, Kalderon D. Hedgehog-stimulated stem cells depend on non-canonical activity of the Notch co-activator Mastermind. Development. 2009;136(13):2177–2186. doi: 10.1242/dev.035329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Kalderon D. Regulation of cell proliferation and patterning in Drosophila oogenesis by Hedgehog signaling. Development. 2000;127(10):2165–2176. doi: 10.1242/dev.127.10.2165. [DOI] [PubMed] [Google Scholar]
- 29.Forbes AJ, Lin H, Ingham PW, Spradling AC. hedgehog is required for the proliferation and specification of ovarian somatic cells prior to egg chamber formation in Drosophila. Development. 1996;122(4):1125–1135. doi: 10.1242/dev.122.4.1125. [DOI] [PubMed] [Google Scholar]
- 30.Forbes AJ, Spradling AC, Ingham PW, Lin H. The role of segment polarity genes during early oogenesis in Drosophila. Development. 1996;122(10):3283–3294. doi: 10.1242/dev.122.10.3283. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Montell DJ. Identification of mutations that cause cell migration defects in mosaic clones. Development. 1999;126(9):1869–1878. doi: 10.1242/dev.126.9.1869. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Kalderon D. Hedgehog acts as a somatic stem cell factor in the Drosophila ovary. Nature. 2001;410(6828):599–604. doi: 10.1038/35069099. [DOI] [PubMed] [Google Scholar]
- 33.Mellerick DM, Kassis JA, Zhang SD, Odenwald WF. castor encodes a novel zinc finger protein required for the development of a subset of CNS neurons in Drosophila. Neuron. 1992;9(5):789–803. doi: 10.1016/0896-6273(92)90234-5. [DOI] [PubMed] [Google Scholar]
- 34.Cui X, Doe CQ. ming is expressed in neuroblast sublineages and regulates gene expression in the Drosophila central nervous system. Development. 1992;116(4):943–952. doi: 10.1242/dev.116.4.943. [DOI] [PubMed] [Google Scholar]
- 35.Vacalla CM, Theil T. Cst, a novel mouse gene related to Drosophila Castor, exhibits dynamic expression patterns during neurogenesis and heart development. Mech Dev. 2002;118(1-2):265–268. doi: 10.1016/s0925-4773(02)00282-4. [DOI] [PubMed] [Google Scholar]
- 36.Christine KS, Conlon FL. Vertebrate CASTOR is required for differentiation of cardiac precursor cells at the ventral midline. Dev Cell. 2008;14(4):616–623. doi: 10.1016/j.devcel.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Z, et al. CASZ1, a candidate tumor-suppressor gene, suppresses neuroblastoma tumor growth through reprogramming gene expression. Cell Death Differ. 2011;18(7):1174–1183. doi: 10.1038/cdd.2010.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jang AC, Chang YC, Bai J, Montell D. Border-cell migration requires integration of spatial and temporal signals by the BTB protein Abrupt. Nat Cell Biol. 2009;11(5):569–579. doi: 10.1038/ncb1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song X, Xie T. DE-cadherin-mediated cell adhesion is essential for maintaining somatic stem cells in the Drosophila ovary. Proc Natl Acad Sci USA. 2002;99(23):14813–14818. doi: 10.1073/pnas.232389399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song X, Xie T. Wingless signaling regulates the maintenance of ovarian somatic stem cells in Drosophila. Development. 2003;130(14):3259–3268. doi: 10.1242/dev.00524. [DOI] [PubMed] [Google Scholar]
- 41.Nystul T, Spradling A. An epithelial niche in the Drosophila ovary undergoes long-range stem cell replacement. Cell Stem Cell. 2007;1(3):277–285. doi: 10.1016/j.stem.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 42.Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22(3):451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- 43.Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117(4):1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- 44.Ma C, Zhou Y, Beachy PA, Moses K. The segment polarity gene hedgehog is required for progression of the morphogenetic furrow in the developing Drosophila eye. Cell. 1993;75(5):927–938. doi: 10.1016/0092-8674(93)90536-y. [DOI] [PubMed] [Google Scholar]
- 45.Besse F, Busson D, Pret AM. Fused-dependent Hedgehog signal transduction is required for somatic cell differentiation during Drosophila egg chamber formation. Development. 2002;129(17):4111–4124. doi: 10.1242/dev.129.17.4111. [DOI] [PubMed] [Google Scholar]
- 46.Johnston RJ, Jr, Desplan C. Stochastic mechanisms of cell fate specification that yield random or robust outcomes. Annu Rev Cell Dev Biol. 2010;26:689–719. doi: 10.1146/annurev-cellbio-100109-104113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beachy PA, Hymowitz SG, Lazarus RA, Leahy DJ, Siebold C. Interactions between Hedgehog proteins and their binding partners come into view. Genes Dev. 2010;24(18):2001–2012. doi: 10.1101/gad.1951710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar SK, et al. Targeted inhibition of hedgehog signaling by cyclopamine prodrugs for advanced prostate cancer. Bioorg Med Chem. 2008;16(6):2764–2768. doi: 10.1016/j.bmc.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.