Abstract
Most multicellular organisms can only survive under atmospheric pressure. The reduced pressure of a high vacuum usually leads to rapid dehydration and death. Here we show that a simple surface modification can render multicellular organisms strongly tolerant to high vacuum. Animals that collapsed under high vacuum continued to move following exposure of their natural extracellular surface layer (or that of an artificial coat-like polysorbitan monolaurate) to an electron beam or plasma ionization (i.e., conditions known to enhance polymer formation). Transmission electron microscopic observations revealed the existence of a thin polymerized extra layer on the surface of the animal. The layer acts as a flexible “nano-suit” barrier to the passage of gases and liquids and thus protects the organism. Furthermore, the biocompatible molecule, the component of the nano-suit, was fabricated into a “biomimetic” free-standing membrane. This concept will allow biology-related fields especially to use these membranes for several applications.
Keywords: animal behavior, biophysics, microscopy, nanotechnology, plasma physics
An organism’s surface plays a critical role in maintaining a stable internal physiological environment while also forming a protective barrier against widely varying external environmental conditions. This protection is adequate under most conditions, but there are extremes that most natural surfaces cannot cope with. High vacuum is one such extreme, and its dramatic effects include rapid evaporation of water across an organism’s surface layer, followed by the organism’s collapse and death.
Field-emission scanning electron microscopes (SEMs) are high-vacuum instruments (10−5 to 10−7 Pa) that are used for observing fine surface structures. When the SEM is used for biological materials, the specimens need to be killed, preserved, and stabilized (1). These complex procedures preclude the observation of living organisms and often produce unwanted artifacts. Researchers have tried to modify the SEM design to require lower levels of vacuum to circumvent such problems (2–5). However, all these methods require reduced vacuums (<10−3 Pa) and result in markedly inferior resolution. Observation of living specimens with a high-resolution SEM would be a significant breakthrough. To understand how animals can survive in a high vacuum would facilitate SEM analysis and would be a major advance in an attempt to more fully understand the role of the surface layer in protecting the organism.
In this report we use the high-vacuum conditions (10−5 to 10−7 Pa) in the observation chamber of the SEM as the experimental space and reveal that a simple surface modification by electron beams or plasmas can equip some multicellular organisms with a strong tolerance against the high vacuum. The treatment gives rise to a thin extra layer, which we call a “nano-suit,” that covers the outside of the organism. The nano-suit can be made from endogenous or exogenous materials, with biomimetic exogenous materials capable of being turned into a biocompatible free-standing membrane.
Results
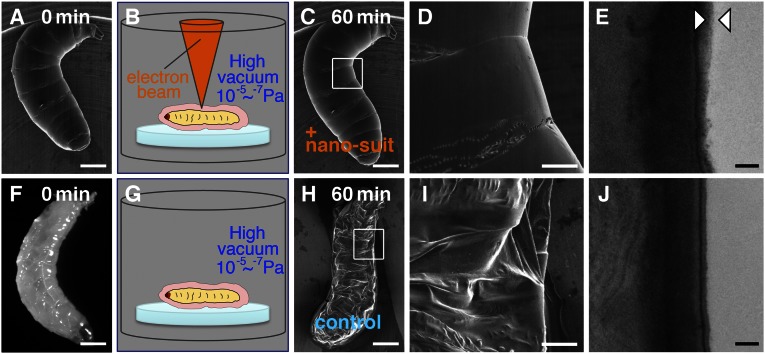
We introduced numerous living organisms belonging to various taxa directly into the SEM to see how long they survived under high vacuum (10−5 to 10−7 Pa). Almost all of the specimens died rapidly and their structural integrity was completely destroyed by the vacuum. Surprisingly, however, we found that Drosophila larvae tolerated the high vacuum well. Although they have a soft cuticle covered by extracellular substances (ECSs) (6), they continued to actively move around for 60 min under the SEM (n = 25) (Movie S1) and subsequently developed normally. No apparent structural changes occurred (Fig. 1 A–C). High-magnification images revealed an intricate wrinkled surface structure (Fig. 1D). When control larvae (Fig. 1F) were placed in the SEM observation chamber for 60 min at an identical vacuum level, but without concurrent electron-beam radiation, subsequent SEM observations revealed they were all dead and structurally badly distorted (Fig. 1 G–I). Transmission electron microscopy (TEM) showed that animals subjected to SEM electron bombardment immediately produce an extra thin layer (∼50- to 100-nm thickness) over their surface (Fig. 1E). No such layer was detected in animals that had been exposed in vacuo for the same time but without electron bombardment (Fig. 1J).
Fig. 1.
(A–D) A living Drosophila larva was exposed to high vacuum with electron-beam irradiation for 60 min. (F and G) Before SEM observation, a different larva (light micrograph in F) was placed in the observation chamber without electron-beam irradiation for 60 min. (H and I) The specimen collapsed completely when subsequently observed by SEM. Each small white square in C and H is shown magnified in D and I, respectively. (E and J) TEM images are shown of vertical sections through the surface of each animal. The layer between the arrowheads in E indicates the limits of the newly formed outer membrane, not present in J. An outer layer covering the animal represents ECSs in B and G. [Scale bars: 0.3 mm (A, C, F, and H), 0.1 mm (D and I), and 0.2 µm (E and J).]
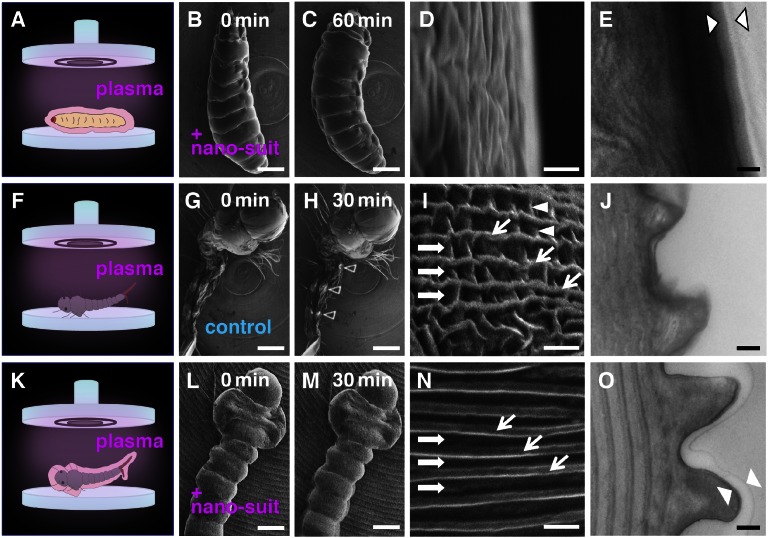
These results led to the hypothesis that electron-beam irradiation (7) enhanced cross-linking within the ECS to form a durable polymer on the surface and that this polymer increased resistance to vacuum conditions. To test this hypothesis, surface polymerization was achieved by a different method: plasma irradiation. The ionized particles of plasma provide the energy necessary to initiate polymer formation, enabling uniform coating of surfaces with solvent-insoluble polymers (8). Although plasma polymerization so far has been used only in creating new inert industrial materials or modification of their surfaces (9), we applied this technique to the ECS of living animals to construct a protective surface barrier referred to as a nano-suit (Fig. S1). Plasma-irradiated specimens left in vacuo for 60 min before SEM observation clearly showed features similar to specimens observed by SEM ab initio (Fig. 1 A–C): They moved continuously, seemingly unharmed (Fig. 2 A–D and Movie S2) without problems of electrical charging (Fig. 2H, arrowheads). An extra thin surface layer was apparent in TEM observations (Fig. 2E). The weight of each larva was measured just before the experiment and 60 min later following exposure to high vacuum. Nonirradiated control larvae and larvae pretreated by plasma radiation showed mean body weight decreases of 56.3 ± 6.0% and 8.7 ± 3.8%, respectively (n = 25), demonstrating the effectiveness of the surface membrane formed by plasma irradiation.
Fig. 2.
(A–C) A living Drosophila larva treated by plasma irradiation for 3 min and observed by SEM for 60 min. (F–H) A living larval mosquito Culex pipiens molestus (which has no natural ECS layer) treated by plasma irradiation for 3 min and observed by SEM for 30 min. (K–M) Images of the larval mosquito, following early electron irradiation, protected by plasma-irradiated Tween 20. (D, I, and N) High-magnification image of the body surface of each animal. Arrowheads in H indicate areas of electrostatic charging. In E, J, and O, TEM images of the surfaces of each animal are shown. Layers between the arrowheads indicate the newly formed nano-suit. An outer layer covering the animal represents an ECS in A or an artificial ECS in K. [Scale bars: 0.3 mm (B, C, G, H, L, and M), 1 µm (D, I, and N), and 0.2 µm (E, J, and O).]
We next examined whether plasma irradiation could be used to protect other organisms. Insects possessing an ECS coat such as the fly maggot Protophormia terraenovae and the Japanese honey bee Apis cerana japonica were successfully protected, but others with no obvious natural ECS were not, pointing to the importance of the ECS. To investigate the chemical properties of the ECS of Drosophila larvae, we carried out FTIR analysis. This revealed that the ECS contained amphiphilic molecules (Fig. 3A). Solutions including amphiphilic molecules were then tested in an attempt to mimic the ECS layer. One of the best results was obtained with a solution of Tween 20, a nontoxic compound commonly used in biological experiments (10). To test the barrier properties of the nano-suit made by this solution, the surfaces of several different animals previously unable to survive SEM exposure were provided exogenous materials by immersing them in 1% Tween 20 solution before electron or plasma irradiation. All tested animals survived, including the flatworm Dugesia japonica, the ant Pristomyrmex punctatus, and the amphipod Talitrus saltator (see Fig. S5). Fig. 2 F–O and Fig. S2 show typical results obtained with larvae of the Asian tiger mosquito, Aedes albopictus, and the northern house mosquito, Culex pipiens molestus. The larvae of these mosquitoes are aquatic and possess a soft-bodied cuticle with no apparent ECS. When live larvae of A. albopictus were observed under the SEM without any additional treatment, they quickly shrank and ceased to move (Fig. S2A). Larvae treated with a 1% Tween 20 solution but not irradiated in the SEM showed the same collapsed structure when observed 30 min later. However, larvae covered with 1% Tween 20 and observed by SEM ab initio retained their morphology and exhibited active movements for 30 min (Fig. S2 B and C and Movie S3). After rearing the latter for 3–4 d, ca. 80% pupated and developed successfully into adult mosquitoes (n = 25) (Fig. S3). Larvae of C. pipiens molestus irradiated by plasma alone and observed under SEM without any additional treatment (untreated specimens) quickly shrank and ceased to move (Fig. 2 F and G), with electrostatic charging beginning after about 30 min (Fig. 2H). However, larvae briefly immersed in 1% Tween 20 solution before plasma irradiation formed a nano-suit (treated specimens), showed rapid movements during 30 ± 10 min of SEM observation, and suffered no observable morphological change (Fig. 2 K–M). Untreated and treated specimens showed decreases in weight of 70.3 ± 4.6% and 11.3 ± 2.7%, respectively, after 30 min in vacuo (n = 25). These results strongly suggest that the artificial ECS played a significant role as an extra barrier ameliorating the effects of high vacuum.
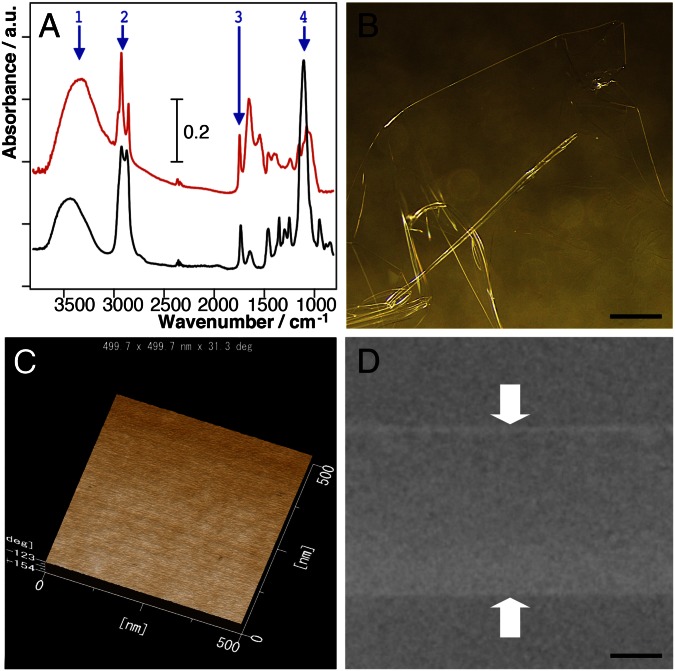
Fig. 3.
(A) Investigation of natural and synthetic membranes with properties affording protection of living tissues against the effects of high vacuum. FTIR spectra indicate that both the ECS of Drosophila larvae (red line) and Tween 20 (black line) possess similar functional groups [1, υO–H (hydroxyl); 2, υC–H (alkyl); 3, υC = O (carboxyl); and 4, υC–O (ether or hydroxyl)]. Long alkyl chains are hydrophobic and hydroxyl, carboxyl, and ether groups are hydrophilic. (B–D) Newly synthesized Tween 20 membrane following plasma polymerization: the surface as observed by light microscopy (B), by AFM (C), and in vertical section by TEM (D). [Scale bars: 5 mm (B) and 2 µm (D).]
In addition, we found that the fine structure of the surviving larvae was completely different from that of untreated specimens and traditionally prepared specimens (Fig. S4). In the present study, each segment of the living animal seemed perfectly preserved and complete (Fig. 2 L and M), with ample body fluid (haemolymph) apparently retained inside. Following traditional preparation, shrinkage of the body was inevitable (Fig. S4A) and distinct furrows and alternating ridges (Fig. S4B, arrows) were identical to those of untreated specimens (Fig. 2I, arrows). There were many wrinkles in the furrows in the two control treatments (Fig. 2I and Fig. S4B, arrowheads), but such wrinkles were completely absent in the treated specimen (Fig. 2N, Fig. S4D, and Movie S4). TEM images clearly showed that the surface of the treated specimen was covered with a nano-suit of ∼50- to 100-nm thickness (Fig. 2O). Although high-magnification imaging (∼×200,000) of treated specimens was possible only when the living animals remained motionless during scanning, the neatly ordered structures covered by the nano-suit suggest that the natural surface structure of the animal is conserved and strongly supports our notion that the nano-suit can preserve the “real-life appearance” down to microscopic details (Fig. S5).
Finally, we examined whether the component of the nano-suit can be fabricated as a biocompatible membrane (11). A diluted solution of Tween 20 was spread by spin casting on a glass substrate, directly irradiated by plasma, and floated on a water surface. This method differs from earlier ones that involved vapor deposition (Discussion). The fabricated free-standing membrane (Fig. 3B) was insoluble in both water and ethanol, clearly showing the effects of plasma polymerization (8). The surface of this plasma-treated membrane was uniformly smooth, as observed by atomic force microscopy (AFM) (Fig. 3C). TEM observations revealed a membrane thickness of ∼5 µm (Fig. 3D).
Discussion
Extreme environments include physical and geochemical extremes (e.g., temperature, radiation, pressure, desiccation, salinity, pH, or oxygen radicals). Water limitation is one of the harshest stressors, because water possesses many unique properties and plays an irreplaceable role in an organism. The greatly reduced pressure of high vacuum leads to dehydration. Until now, it has been believed that most multicellular organisms are only able to thrive under atmospheric or higher-than-atmospheric, viz. deep-sea, pressures. The present study has shown that when Drosophila larvae were simply placed in the SEM sample chamber at a high vacuum level (10−5 to 10−7 Pa) without any exposure to the electron beam their structural features became badly disrupted and they died rapidly (Fig. 1H). However, after exposure to an electron beam or plasma irradiation, which induced the formation of a nano-suit, the animals continued to show spontaneous movements despite long exposures in high vacuo (Figs. 1C and 2C) and went on to develop into normal adults. The newly formed thin layer created by irradiation seems to cover the whole body as an external nano-suit, (Figs. 1E and 2E), which acts as a flexible barrier to the passage of gases and liquids and protects the organism under high vacuum.
Following this success, we examined whether plasma irradiation could be used routinely to protect other organisms against high vacuum. Because organisms with no obvious ECS could not be protected, we looked for amphiphilic molecules that could mimic natural ECS. We discovered that a simple Tween 20 solution (Fig. 3A) could be used to protect multicellular organisms from high vacuum. Mosquito larvae that survived in vacuo wearing an artificially applied nano-suit represent one report of artificial enhancement to the surface layer (Fig. 2O). From the biomimetic point of view, our findings were successful in protecting the animal from the deleterious effects of high vacuum. However, further study will be needed for other kinds of organisms, including single cells and tissues, using different chemicals or combinations. Multicellular organisms originally possess their own ECSs (e.g., biofilms of microbes, ECS of insect larva, and surfactants of the vertebrate lung). Therefore, future studies to investigate substances in the ECS would need to examine the effective compounds of many animals.
Plasma is a state of matter similar to gas but in which a large proportion of the particles is ionized. Such ionized particles can provide the energy necessary to initiate polymer formation, enabling uniform coating of surfaces with solvent-insoluble polymers (8). Although plasma polymerization has so far been used only in creating new inert industrial materials or modifications of their surfaces (7, 8), we showed here that it can be applied to the surface of living animals to construct a protective surface barrier. We further demonstrated that the amphiphilic molecule in solution, the component of the nano-suit, can be fabricated as a biocompatible membrane by only a one-step synthesis (Fig. 3). Recently, the concept of biocompatibility as applied to functional membranes has gained general relevance. Biocompatible membranes have been exploited extensively not only in engineering but also in clinical applications. Readily producing free-standing biocompatible membranes, however, has remained a complex and challenging procedure (11). The nano-suit described in the present experiments was formed without chemical vapor depositions (12) and thus was completely different from the methods used earlier (8, 9). It should make the use of biocompatible membranes with high hermetic properties easier in a variety of biology-related fields especially.
In the present study, after modification of materials on the surface of organisms by exposure to electron-beam (7) or plasma ionization (9), the treated animals showed spontaneous movements in vacuo. Based on these findings, it seems that, with assistance, multicellular organisms possess the potential to tolerate high-vacuum environments. It has been known that under low-vacuum conditions (10 to 10−2 Pa) some organisms can be observed by SEM without any pretreatments (13, 14), and although outer space is the most demanding high-vacuum environment (15), it has been known that cryptobiotic organisms, such as tardigrades (16) and larvae of Polypedilum vanderplanki (17), are able to survive there. However, these animals require gradual desiccation to reach their cryptobiotic state (18) and suffer immediate collapse and death when suddenly exposed to the SEM vacuum (Fig. S6 B and C). Once we provided these animals with the nano-suit, their natural appearance remained intact and they were able to survive (Fig. S6 D–F). When considering the conditions necessary to produce plasma-treated membranes, it is interesting to note that energetic electrons and plasmas are known to occur commonly throughout the universe, including on planet Earth, where flows of electrons producing plasma have occurred under the same conditions as evolving life forms. Ever since Darwin (19), many hypotheses for the early evolution of life have been proposed, including the Panspermia hypothesis (20, 21), which advocates that simple life forms can travel widely throughout the universe. Prokaryotic extremophiles (15) are among possible candidates for such extraterrestrial travel, but if free electrons and plasma together with ECS are able to construct a protective shield, many organisms have the potential to attain this ability. Association of life with a nano-suit now expands our concept of the conditions under which life can survive. This may be the start of a new era of improved understanding not only of surface biology but of a range of other scientific areas as well.
Materials and Methods
Animals.
Third-instar larvae (ca. 3 mm in body length) of the fruit fly Drosophila melanogaster (Oregon-R variety) were used. These larvae possess a soft cuticle covered by ECSs (6). To exclude any effects of culture medium, the larvae were washed at 24 ± 1 °C with distilled water several times before the experiment.
Fourth-instar larvae of the mosquito A. albopictus (ca. 5 mm in body length) and fourth-instar larvae of the mosquito C. pipiens molestus (ca. 6 mm in body length), collected from puddles and maintained in the same water in which they were found, were also used. These larvae have a soft cuticle not covered by ECS. To exclude any effects of the water, they were transferred to distilled water at 24 ± 1 °C for 2 d before the experiment, with distilled water changes every 12 h. The larvae were rinsed thoroughly in distilled water 1 h before experiments began.
The initial weights of single Drosophila and Culex larvae were about 1.2 mg and 2.2 mg, respectively. Five larvae were measured for each trial, and five trials (n = 25) were performed for each species under each condition.
Plasma Polymerization.
The metal emitter from a standard ion-sputtering device (JFC-1100; JEOL) was removed, so that the plasma ions produced within the chamber were based on the remaining rarified air-derived gas molecules. Specimens were irradiated with plasma inside this device for 3 min at a vacuum level of ca. 1.0 Pa and 1.0 kV DC (8.0 mA) at room temperature (Fig. S1).
The amphiphilic compound polysorbitan monolaurate (Tween 20; Wako Pure Chemical Industries) was used to mimic ECS. Animals were dipped into 1% (vol/vol) Tween 20 solution dissolved in distilled water for 1 min, blotted briefly on a dry filter paper to remove excess solution, and then irradiated by plasma to construct a plasma-treated Tween 20 film.
To make a freestanding film for comparison with ECS on animal surfaces, 50% (vol/vol) Tween 20 dissolved in 100% ethanol was spread over a glass plate at 3,000 rpm for 5 s using a spin-coater (SC8001; Aiden) and polymerized in the modified ion coater. The treated glass plate was immersed into a well filled with distilled water to separate the film from the glass plate. The thickness of the film could be controlled both by the duration of irradiation time and the concentration of the solution.
Sample Preparation for the Field-Emission SEM to Observe Living Animals.
The sample was introduced into the SEM directly without conducting any traditional treatments such as chemical fixations, dehydration, or ultrathin coating of electrically conducting materials.
Preparation for Standard Scanning and TEM.
For standard SEM observation, animals were prefixed with 4% (vol/vol) glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) and postfixed in 1% OsO4 in the same buffer. The specimens were then dehydrated, freeze-dried (JFD300; JEOL), and ultra-thin-coated with OsO4 (PMC-5000; Meiwa). For TEM to observe the fine structure of the surfaces, samples were prefixed in 2% glutaraldehyde and 2% paraformaldehyde in 0.1 M cacodylate buffer (pH 7.4) and then postfixed in 1% OsO4 in the same buffer. Dehydrated specimens were embedded in an Epon-Araldite mixture. Ultra-thin vertical sections (∼70 nm) of the surface were stained with 2% uranyl acetate followed by 0.4% lead citrate for 5 min each.
Microscopy.
Field-emission scanning electron microscopy was performed using a Hitachi S-4800 at an acceleration voltage of 5.0 kV. The vacuum level of the observation chamber was 10−5 to 10−7 Pa. The detector for secondary electron was a mixture of signal with upper and lower detectors. Other details of conditions were as follows: working distance, 8 mm; aperture size, φ 100 µm; scan speed, each beam is 10–15 frames/s. To record the dynamic movements of animals, imaging data from the SEM were directly transferred to a high-band digital-formatted video recorder (DVR-DT95; Pioneer). To keep the moving animals from falling from the observation stub, the center of the stub was scraped off as a rounder shape (Fig. 1 A and C and Movies S1–S4). TEM observations were carried out using a JEM-1220 (JEOL) at an acceleration voltage of 120 kV.
Measurement of Surface Thickness on the Free-Standing Plasma-Irradiated Film.
The surface ultrastructure of the film was measured by AFM using a JSPM 5200 scanning probe microscope (JEOL) under atmospheric pressure at room temperature (25 °C). The AFM was used in AC mode at a typical scanning rate of 2.0 Hz and a scanning size of 2,500 × 2,500 nm. The thickness of the structure was analyzed with the WinSPM System computer program provided by the manufacturer (JEOL).
FTIR.
FTIR spectra were measured using a Spectrum 2000 (Perkin-Elmer) scanner to analyze the molecular features of Drosophila larval ECS and Tween 20. Several Drosophila larvae were placed in an agate mortar to adsorb their ECS onto the surface of the mortar. The collected ECS of the Drosophila larvae and of Tween 20 were measured by means of the common KBr pellet method.
Supplementary Material
Acknowledgments
We thank Research Supervisor Yasuhiro Horiike (Fellow Emeritus, National Institute for Materials Science) for allowing us to carry out research with live specimens in a field-emission scanning electron microscope. We acknowledge funding from the Core Research for Evolutional Science and Technology of the Japan Science and Technology Agency.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1221341110/-/DCSupplemental.
References
- 1.Suzuki E. High-resolution scanning electron microscopy of immunogold-labelled cells by the use of thin plasma coating of osmium. J Microsc. 2002;208(Pt 3):153–157. doi: 10.1046/j.1365-2818.2002.01082.x. [DOI] [PubMed] [Google Scholar]
- 2.Danilatos GD. Review and outline of environmental SEM at present. J Microsc. 1991;162:391–402. [Google Scholar]
- 3.Mohan A, Khanna N, Hwu J, Joy DC. Secondary electron imaging in the variable pressure scanning electron microscope. J Scan Microscopy. 1998;20(6):436–441. [Google Scholar]
- 4.Symondson WOC, Williams IB. Low-vacuum electron microscopy of carabid chemoreceptors: A new tool for the identification of live and valuable museum specimens. Entomol Exp Appl. 2003;85(1):75–82. [Google Scholar]
- 5.Stokes DJ. 2003. Investigating biological ultrastructure using environmental scanning electron microscopy (ESEM). Science, Technology and Education of Microscopy: An Overview, ed Méndez-Vilas A (Formatex, Badajoz, Spain), pp 564–570.
- 6.Chambers L, et al. Degradation of extracellular matrix components by defined proteinases from the greenbottle larva Lucilia sericata used for the clinical debridement of non-healing wounds. Br J Dermatol. 2003;148(1):14–23. doi: 10.1046/j.1365-2133.2003.04935.x. [DOI] [PubMed] [Google Scholar]
- 7.Sun KH. 1954. Effects of atomic radiation on high polymers. Modern Plastics 32(1):141–238.
- 8.Yasuda H. Glow discharge polymerization. J Membr Sci. 1981;16(1):199–293. [Google Scholar]
- 9.Friedrich J. Mechanisms of plasma polymerization – Reviewed from a chemical point of view. Plasma Process Polym. 2011;8:783–802. [Google Scholar]
- 10.FAO Toxicological evaluation of some food additives including anticaking agents, antimicrobials, antioxidants, emulsifiers and thickening agents. FAO Nutr Meet Rep Ser. 1974;53A(53A):1–520. [PubMed] [Google Scholar]
- 11.Naito Y, Shin’oka T, Hibino N, Matsumura G, Kurosawa H. A novel method to reduce pericardial adhesion: A combination technique with hyaluronic acid biocompatible membrane. J Thorac Cardiovasc Surg. 2008;135(4):850–856. doi: 10.1016/j.jtcvs.2007.10.062. [DOI] [PubMed] [Google Scholar]
- 12.Peng X, Jin J, Ericsson EM, Ichinose I. General method for ultrathin free-standing films of nanofibrous composite materials. J Am Chem Soc. 2007;129(27):8625–8633. doi: 10.1021/ja0718974. [DOI] [PubMed] [Google Scholar]
- 13.Pease RF, Hayes TL, Camp AS, Amer NM. Electron microscopy of living insects. Science. 1966;154(3753):1185–1186. doi: 10.1126/science.154.3753.1185. [DOI] [PubMed] [Google Scholar]
- 14.Heslop-Harrison Y. Scanning electron microscopy of fresh leaves of pinguicula. Science. 1970;167(3915):172–174. doi: 10.1126/science.167.3915.172. [DOI] [PubMed] [Google Scholar]
- 15.Rothschild LJ, Mancinelli RL. Life in extreme environments. Nature. 2001;409(6823):1092–1101. doi: 10.1038/35059215. [DOI] [PubMed] [Google Scholar]
- 16.Jönsson KI, Rabbow E, Schill RO, Harms-Ringdahl M, Rettberg P. Tardigrades survive exposure to space in low Earth orbit. Curr Biol. 2008;18(17):R729–R731. doi: 10.1016/j.cub.2008.06.048. [DOI] [PubMed] [Google Scholar]
- 17.Okuda T, Gusev O. “Life without water”: The sleeping chironomid and other anhydrobiotic invertebrates and their utilization in astrobiology. Life on Earth and Other Planetary Bodies. 2012;24:121–138. [Google Scholar]
- 18.Guidetti R, Altiero T, Rebecchi L. On dormancy strategies in tardigrades. J Insect Physiol. 2011;57(5):567–576. doi: 10.1016/j.jinsphys.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Darwin C. On the Origin of Species by Means of Natural Selection. London: Murray; 1859. [Google Scholar]
- 20.Arrhenius S. Die Verbreitung des Lebens im Weltenraum. Umschau. 1903;7:481–485. [Google Scholar]
- 21.Hoyle F, Wickramasinghe NC. Lifecloud: The Origin of Life in the Universe. London: J. M. Dent; 1978. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.