Abstract
Engineering microorganisms to produce biofuels is currently among the most promising strategies in renewable energy. However, harvesting these organisms for extracting biofuels is energy- and cost-intensive, limiting the commercial feasibility of large-scale production. Here, we demonstrate the use of a class of transport proteins of pharmacological interest to circumvent the need to harvest biomass during biofuel production. We show that membrane-embedded transporters, better known to efflux lipids and drugs, can be used to mediate the secretion of intracellularly synthesized model isoprenoid biofuel compounds to the extracellular milieu. Transporter-mediated biofuel secretion sustainably maintained an approximate three- to fivefold boost in biofuel production in our Escherichia coli test system. Because the transporters used in this study belong to the ubiquitous ATP-binding cassette protein family, we propose their use as “plug-and-play” biofuel-secreting systems in a variety of bacteria, cyanobacteria, diatoms, yeast, and algae used for biofuel production. This investigation showcases the potential of expressing desired membrane transport proteins in cell factories to achieve the export or import of substances of economic, environmental, or therapeutic importance.
Keywords: ABC transporters, biofuel milking, green fuel, multidrug efflux
The rising cost of transportation fuel and concerns over the stability of supply have coincided with the rising era of synthetic genomics and metabolic engineering in the last few years. This scenario has led to the development of engineered microorganisms as attractive sources for the production of green fuels (1). The first-generation biofuel, ethanol, derived from crop-fed microbes, has limitations in terms of its energy density, hygroscopic properties, and the use of cultivable land (2). As a result, microbes with more economic nutritional requirements are now being heavily investigated for the production of advanced biofuel molecules, with properties comparable to petroleum-based fuels (2). Several algae, cyanobacteria, yeast, and bacteria have been investigated as potential production hosts (2). Potential biofuel compounds include derivatives of alcohols, fatty acids, polyketides, and isoprenoids (2).
A major problem that hinders successful commercialization of microbiofuel production is the energy and costs incurred while harvesting cells and extracting the fuel, because most biofuel molecules are synthesized and stored inside cells (3). The most common approach taken to abate this problem today includes lytic, metabolic, enzymatic, or electrical degradation of the cell membranes and walls to leak out biofuel molecules (3–5). An alternative approach is to use membrane transport protein pumps that can export/secrete intracellular biofuels to the extracellular milieu (“secretion” is used interchangeably with “export/efflux” in this paper, as the exported molecules are produced within the same cell and collected outside the cell) (5–7).
Analogous to obtaining milk from cows without killing or weakening them, the term “milking” is often used to describe the biofuel secretion strategy (6). Biofuel secretion from healthy living cells is expected to allow the cells to replenish their intracellular biofuel content, resulting in a sustainable system with overall higher yields compared with nonsecreting systems. The increase in yield could possibly allow liquid biofuel molecules to overcome the phase separation threshold to ultimately appear as a layer above the culture medium, enabling easy recovery. Biofuel secretion is also predicted to reverse the cellular toxicity that is associated with the intracellular accumulation of certain types of biofuel molecules.
To our knowledge, the only report concerning the use of membrane transport proteins for achieving biofuel secretion is from Dunlop et al. (8). They expressed transporters belonging to the RND (resistance-nodulation-cell division) family in bacteria and observed an ∼1.5-fold increase in the production of limonene (8). However, a major drawback of this strategy is that RND transporters are large, tripartite protein complexes, spanning two membranes and crossing the periplasmic space; this configuration is exclusively found in gram-negative bacteria and some similar cyanobacteria (9). This tripartite structure limits the translation of RND secretion systems to other economically viable biofuel production hosts such as algae and yeasts.
Unlike RND proteins, transporters belonging to the ATP-binding cassette (ABC) protein family are widely found in all five kingdoms of life (10). They share a conserved structural architecture and specifically import or export a wide variety of molecules and ions across cellular membranes (11). We exclusively deal with exporters in this study. ABC exporters (particularly of the ABCB and ABCG subfamilies) are known to export a diverse range of extremely hydrophobic molecules such as lipids, drugs, and steroids (12); human P-glycoprotein (P-gp/ABCB1) is a well-known example of high pharmacological interest owing to its role in multidrug efflux (13). The functional conservation and diverse substrate poly-specificity among ABC exporters are exemplified by the studies in which a bacterial ABC exporter, LmrA, was found capable of substituting the drug transport function of P-gp in human cells (14). This finding clearly depicts the cross-species translation potential of these transporters.
The broad poly-specificity of ABC transporters in general led us to hypothesize that they could be used to recognize certain biofuel molecules and achieve their secretion out of the cell. There is considerable evidence that a wide range of extremely “greasy” molecules can be transported (12). In plants, for example, transporters from the ABCG subfamily export cuticular waxes (15). In this study, we tested our hypothesis using an isoprenoid-producing Escherichia coli model system. We report the secretion of four different long-chain isoprenoid compounds mediated by different bacterial ABC transporters.
Results
Model Test System.
To test our hypothesis that ABC transporters (we exclusively refer to exporters) can secrete biofuel molecules, we established a simple model test system in E. coli. Two different plasmids were cotransformed into E. coli BL21 (DE3) cells. These plasmids have different origins of replication and antibiotic resistance markers, so that they could be stably maintained together in each cell. The first plasmid was used for the constitutive production of isoprenoids, whereas the second was used for the inducible expression of ABC transporters.
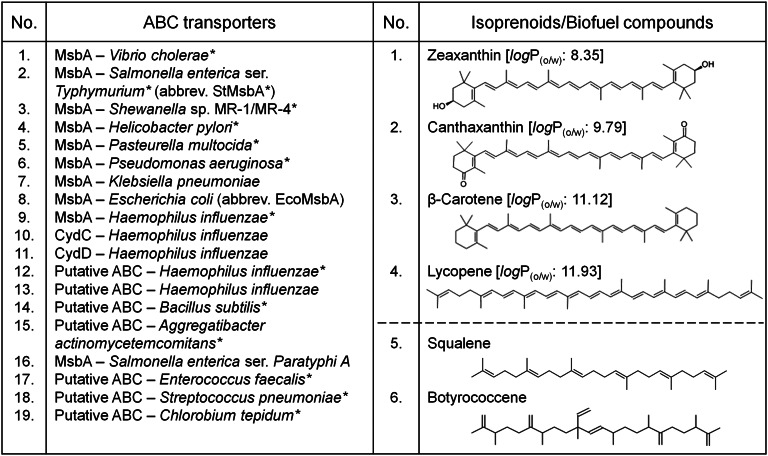
pAC184-based plasmids have been previously engineered to carry operons responsible for the constitutive production of various isoprenoids via the mevalonate pathway (MEV) pathway (16). We used these plasmids for the production of four isoprenoids (17), zeaxanthin, canthaxanthin, β-carotene, and lycopene, in E. coli (Fig. 1). These selected isoprenoids are long-chain C40 carotenoids and were chosen as model biofuel compounds for several reasons: (i) they are readily detectable using colorimetric methods (each of these compounds has a unique characteristic absorbance spectrum in the visible region, allowing easy detection and quantification using absorbance spectrophotometry; Fig. S1); (ii) they lack an indigenous background in noncarotenogenic E. coli; and (iii) they have a similar overall chemical composition to squalene and botryococennes (Fig. 1), which have been validated to be hydrocracked to yield gasoline and other useful products (18).
Fig. 1.
A battery of 19 bacterial ABC transporters were tested for the secretion of four isoprenoid carotenoid molecules that were chosen as model biofuel compounds in this study. *Against transporters indicates mutation(s) in protein sequence, which are detailed in the Table S1. The octanol/water partition coefficients (logP(o/w); from www.chemicalize.org) of the carotenoids show increasing hydrophobicity (decreasing polarity) from zeaxanthin to lycopene. Structural comparisons of the carotenoids with validated biofuel compounds, squalene and botryococcene (18, 30), are shown.
pET19b-derived plasmids are widely used to heterologously express proteins in E. coli in an isopropyl β-D-1-thiogalactopyranoside (IPTG)-inducible manner. We have previously used pET19b to overexpress bacterial ABC transporters for X-ray crystal structure determination (19). We chose to express a panel of 19 different bacterial ABC transporters, many of which are homologs of the ABC exporter, MsbA (Fig. 1; Table S1). MsbA is known to export the lipid A–core moiety of LPS (lipopolysaccharide) from the inner leaflet to the outer leaflet of the inner membrane in E. coli (Fig. 2A) (20). Subsequently, various lipid transfer proteins and transporters participate to move the lipid A–core (assembled into LPS) to the outer membrane during LPS biogenesis (Fig. 2A) (21). MsbA is also known to be capable of exporting a broad range of chemotherapeutic drugs and antibiotics, likely using a mechanism that is similar to lipid A export (22, 23).
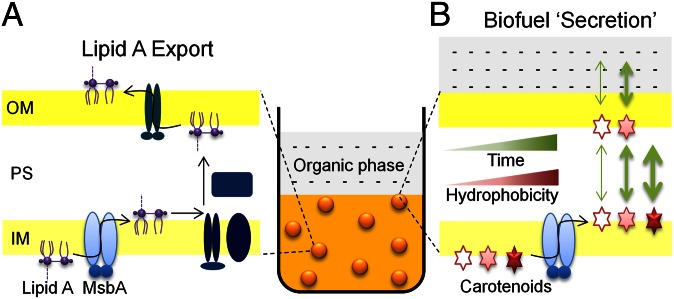
Fig. 2.
Schematic representation of the biofuel secretion system. To collect and detect hydrophobic biofuel compounds secreted from E. coli cells (golden circles), a two-phase culture system was adopted. A biocompatible organic solvent phase was layered over E. coli liquid cultures. (A) Physiologically, the lipid A-core moiety of LPS is exported from the inner leaflet to the outer leaflet of the inner membrane (IM) by the multidrug ABC exporter, MsbA, in E. coli (20). A network of different transporters and lipid transfer proteins (all dark blue symbols) subsequently participate in moving the lipid A-core (assembled into LPS) through the periplasmic space (PS) to the outer membrane (OM), during LPS biogenesis (21). (B) Carotenoids that are C40 isoprenoid compounds (Fig. 1) were selected as model biofuel compounds. The IM localization of biosynthesized carotenoids in E. coli resembles that of lipid A, making them accessible to MsbA for export. In the absence of specific transfer proteins for the “foreign” carotenoids in E. coli, the time taken for carotenoids to partition through the PS and OM into the organic phase increases with an increase in carotenoid hydrophobicity. Eventually, a threshold in hydrophobicity is reached where the partitioning is negligible. In this paper, the process of biofuel export by MsbA across the IM, combined with the partitioning of biofuels into the organic phase, is collectively termed biofuel “secretion.”
Carotenoid-producing E. coli generally accumulate the product carotenoids in the inner membrane, likely in the inner leaflet (24). This localization is probably because the carotenoid biosynthetic pathway enzymes encoded by the pAC184-derived plasmids are membrane-localized (24, 25), and in part, because these carotenoids are virtually insoluble in aqueous environments like the cytoplasm, periplasm, and growth medium. The inner membrane localization of carotenoids is akin to that of lipid A (Fig. 2 A and B), making carotenoids amenable to be exported by MsbA and probably sharing the same alternating access transport mechanism that is conserved among these types of ABC transporters (11, 26). However, with regard to secretion, the aqueous insolubility of carotenoids would prevent them from exiting the membranes in ordinary culture medium.
To circumvent the insolubility issue, the double transformant E. coli liquid cultures were grown with an overlay of decane, which is a biocompatible organic solvent (Fig. 2). The use of such two-phase culture systems is common for collecting hydrophobic secreted products from cells (8, 27, 28). Our hypothesis was that the MsbA-exported carotenoids would partition from the membranes and periplasmic space into the decane phase (Fig. 2B). We also hypothesized that the hydrophobicity of the carotenoid would determine the speed of partitioning into decane (Fig. 2B), thus affecting the time taken to detect the secreted carotenoid. Testing this hypothesis was made possible through our selection of carotenoids that span a range of increasing hydrophobicities (decreasing polarity), from zeaxanthin to lycopene (Fig. 1).
Isoprenoid Secretion from Cells in Culture Medium.
In our carotenoid-producing double transformants, the expression of 3 of our battery of 19 transporters was not detectable on a Western blot; these were excluded from further analysis (Fig. S2). The screens for carotenoid secretion involved three different measurements for each sample: the concentration of secreted carotenoid (in the decane phase; Figs. S3 A and B and S4 A and B), the concentration of produced carotenoid (in cells, extracted with acetone for 15 min at 55 °C; (Figs. S3 C and D and S4 C and D), and cell viability (optical density of the cell culture; Figs. S3E and S4E). A positive hit was defined as a transporter that, when expressed, led to an increase in the secreted amount of carotenoid compared with the nonexpressing (empty vector) control, without reductions in the amount of produced carotenoid and cell viability.
In our screens for zeaxanthin and canthaxanthin secretion, the best hits were Salmonella enterica ser. typhymurium MsbA (containing a substitution I89T, abbreviated as StMsbA*) for zeaxanthin and Escherichia coli MsbA (abbreviated as EcoMsbA) for canthaxanthin (Figs. S3 and S4). Other expressed ortholog transporters showed comparatively poor secretion/negative hits (Figs. S3 and S4), suggesting that zeaxanthin and canthaxanthin secretion by StMsbA* and EcoMsbA, respectively, were unlikely to be a result of secondary effects like lipid A cotransport or the expression of unrelated pumps/proteins. The failure of many transporters in these screens, particularly the conserved MsbA-homologs, suggests that even minor differences in protein sequence have notable effects on biofuel secretion.
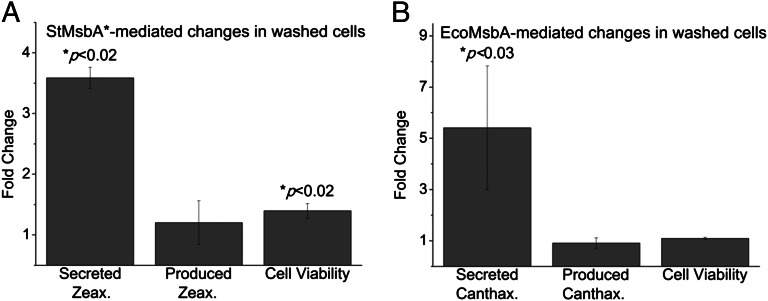
We could detect zeaxanthin secreted by StMsbA* in the decane phase after 24 h of aerobic incubation at 30 °C, and the secreted amounts further increased over the next 48 h (Fig. 3 A and B). By 72 h, there was a highly significant (P < 0.003) ∼2.4-fold increase in secreted zeaxanthin mediated by StMsbA* compared with the nonexpressing control (Fig. 3B). Notably, there were no reductions in produced zeaxanthin amounts per cell (Fig. 3C) or cell viabilities (Fig. S5A), suggesting that the increase in secreted zeaxanthin was not due to cell death. An ∼4.4-fold, significant (P < 0.03) increase in secreted canthaxanthin was observed to be mediated by overexpressed EcoMsbA compared with the control following 96 h of aerobic incubation at 30 °C (Fig. 3 D and E). Again, there were no reductions in the produced canthaxanthin amounts (Fig. 3F) or cell viabilities (Fig. S5B). We confirmed that the expression of our transporters was steady over the 96-h period, and so we believe that the difference in detection times between zeaxanthin (24 h) and canthaxanthin (96 h) is more likely to arise from differences in carotenoid hydrophobicity and partitioning between the membranes, periplasmic space, and decane (discussed later).
Fig. 3.
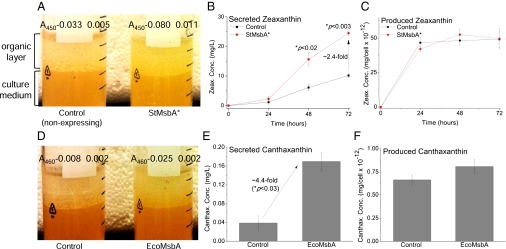
MsbA-mediated isoprenoid secretion from cells in culture medium. (A) Zeaxanthin-producing E. coli BL21(DE3), overexpressing StMsbA* against nonexpressing control, were grown in 10 mL LB medium containing 25 µg/mL chloramphenicol, 50 µg/mL carbenicillin, and 1 mM IPTG, gently overlayed with 5 mL decane, at 30 °C, with aeration at 250 rpm for 72 h, with aliquots of the decane and culture phases taken every 24 h. The image represents the 48-h time point. The absorbencies of the decane phases at the absorbance maxima (λmax) – 450 nm are shown for comparison (n = 3 biological repeats, i.e., performed with independently cultured cells ± SEM). (B) Secreted zeaxanthin concentrations in the decane phase were measured spectrophotometrically using a standard curve (n = 3 biological repeats ± SEM; P from two-tailed, paired Student t tests). (C) Cells from the culture phase were harvested by centrifugation at 16,000 × g for 1 min. Supernatant was removed, and the cell pellets were treated with acetone for 15 min at 55 °C with thorough agitation. The cell debris was collected by a second centrifugation, and the produced zeaxanthin concentration, now in the acetone phase, was analyzed spectrophotometrically. These values were normalized against the bacterial cell number, estimated using absorbance (OD) at 595 nm of 1 = 8 × 1011 cells/L (n = 3 biological repeats ± SEM; p from two-tailed, paired Student t tests). (D–F) Canthaxanthin overproducing E. coli BL21 (DE3) cells, overexpressing E. coli MsbA, or nonoverexpressing controls were grown and subjected to canthaxanthin secretion and production analyses as described in A–C (canthaxanthin λmax − 460 nm). Data obtained at the 96-h time point are shown (n = 4 biological repeats ± SEM; P from two-tailed, paired Student t tests).
The overexpression of most transporters (including EcoMsbA and StMsbA*) typically facilitated E. coli growth, regardless of the produced/secreted carotenoid (Figs. S3E, S4E, and S5). This observation is not surprising considering that MsbA and its homologs are known to support cellular growth through their role in lipid transport (12, 20). Furthermore, we found no correlation between cell viabilities and secreted carotenoid amounts (Figs. S3 and S4; Fig. 4), indicating that the facilitation of growth was not due to carotenoid secretion. The data also suggest that the increase in extracellular zeaxanthin and canthaxanthin was not merely caused by passive diffusion from cells into decane.
Fig. 4.
MsbA-mediated isoprenoid secretion from washed cells in buffer. (A) Zeaxanthin- or (B) canthaxanthin-producing cells containing the pET19b-derived plasmid for StMsbA* or EcoMsbA overexpression or the pET19b empty vector control were grown in 5 mL LB medium containing 25 µg/mL chloramphenicol and 50 µg/mL carbenicillin at 37 °C, with aeration at 200 rpm to OD595 0.5–0.6; 1 mM IPTG was then added, and the cultures were grown for a further 4 h under the same conditions. Cells were then harvested at 3,000 x g, 10 min, and 4 °C. Pellets were washed with ice cold 50 mM KPi buffer, pH 7.0, containing 5 mM MgSO4. After three similar washes, cells were resuspended in 5 mL wash buffer containing 0.5% glucose, gently overlayed with 2 mL decane, and incubated at 30 °C, with aeration at 250 rpm. Secreted and produced zeaxanthin/canthaxanthin amounts and cell viability were measured as described in the legend to Fig. 3. Fold change for the values obtained from transporter-expessing cells compared with control cells are presented, where a fold change of 1 represents identical values (zeaxanthin: n = 4 biological repeats ± SEM; canthaxanthin: n = 5 biological repeats ± SEM; Student t tests as in legend to Fig. 3).
Isoprenoid Secretion from Washed Cells in Buffer.
In our isoprenoid secretion experiments described above, the double transformant E. coli cells were grown in decane-overlayed LB medium over a period of 72–96 h in the presence of the antibiotics chloramphenicol (25 µg/mL) and carbenicillin (50 µg/mL) and the inducer IPTG (1 mM). To support our results from these experiments (Fig. 3), we aimed to demonstrate that zeaxanthin secretion by StMsbA* and canthaxanthin secretion by EcoMsbA were not caused by secondary growth effects such as IPTG-related toxicity or passive diffusion over time. To achieve this, we performed the isoprenoid secretion experiments in washed cells. The double transformant cells (StMsbA*/EcoMsbA-expressing or nonexpressing control) producing zeaxanthin or canthaxanthin were grown aerobically in ordinary single-phase LB medium with the antibiotics and induced at similar optical densities with 1 mM IPTG for 4 h. Cells were then washed with buffer and resuspended in buffer containing glucose, with overlayed decane.
We observed a significant (P < 0.02) ∼3.6-fold increase in secreted zeaxanthin in StMsbA*-expressing cells (Fig. 4A), and a significant (P < 0.03) ∼5.4-fold increase in secreted canthaxanthin in EcoMsbA-expressing cells (Fig. 4B) compared with controls. In these experiments, we did not find any significant changes in produced carotenoid amounts between transporter-expressing and control cells (Fig. 4 A and B). In addition, compared with control, the fold difference in cell viability was insignificant and negligible for EcoMsbA expressors and significant (P < 0.02) but considerably modest (∼1.4-fold increase) for StMsbA* expressors (Fig. 4 A and B). The incubation times required for detecting carotenoids in decane were 48–72 h for zeaxanthin and 72–96 h for canthaxanthin (Fig. 4 A and B), consistent with our previous results using cells in culture medium (Fig. 3).
Taken together, our results from experiments using intact cells in culture medium and intact washed cells in buffer demonstrate that StMsbA* and EcoMsbA can mediate zeaxanthin and canthaxanthin efflux, respectively. Transporter-mediated isoprenoid secretion did not affect cell viability, enabling the isoprenoid-producing cells to efficiently replenish the secreted amounts. Additionally, the kinetic hurdle for isoprenoids to partition into decane from the membranes and periplasm (Fig. 2, green arrows) may have allowed sufficient time for this replenishment to occur (further discussed later). Identical amounts of produced intracellular isoprenoids in secreting and nonsecreting cells naturally led to a higher total yield of isoprenoids in secreting cells, where the increase equates with the fold increase in the secreted amount.
Isoprenoid Hydrophobicity Affects Secretion Detection Times.
Zeaxanthin and canthaxanthin are “foreign” to the heterologous host, E. coli, which is unlikely to harbor specific carotenoid-binding/transfer proteins. As a result, we hypothesized that the partitioning of free carotenoid molecules from the membranes and periplasm into decane should primarily depend on their chemical polarity and hydrophobicity (Figs. 1 and 2). Canthaxanthin (detected in decane after 96 h) is moderately more hydrophobic than zeaxanthin (detected in decane after 24 h; Fig. 1), which according to our hypothesis, should be responsible for the longer incubation time required.
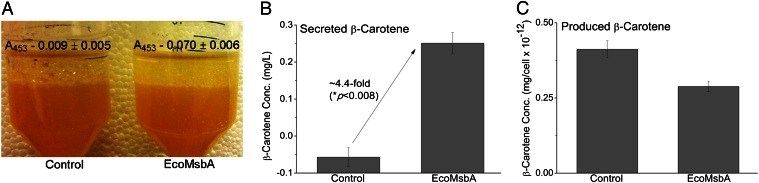
To further test this hypothesis, we attempted to measure the secretion of more hydrophobic carotenoids: β-carotene and lycopene (Fig. 1). From our ATPase measurements (described later), we had an indication that EcoMsbA could interact with β-carotene. In our secretion experiments using intact cells in culture medium, we found a highly significant (P < 0.008) ∼4.4-fold increase in secreted β-carotene from EcoMsbA-expressing cells versus nonexpressing control (Fig. 5 A and B), without any significant reductions in the produced amount (Fig. 5C) and cell viability (Fig. S5C). However, β-carotene took 120–144 h to be secreted, which is 1–2 d longer than it took for canthaxanthin.
Fig. 5.
EcoMsbA secretes β-carotene. The assay was performed, and the results were analyzed and are presented in a manner identical to the description in legend to Fig. 3. (A and B) Secreted β-carotene was quantified (A-absorbance at λmax – 453 nm) in the decane phase after 144-h incubation at 30 °C, with aeration at 250 rpm, and was significantly higher for EcoMsbA-expressing cells compared with nonexpressing control (n = 3 biological repeats ± SEM; Student t test as in the legend to Fig. 3). Control was indistinguishable from background, probably due to the relatively lower expression of genomic MsbA and negligible passive diffusion of β-carotene, but was nevertheless quantified for uniformity. (C) No significant differences (P > 0.11) were observed in the intracellular produced amounts of β-carotene between EcoMsbA-expressing and nonexpressing cells.
Lycopene is one of the most hydrophobic carotenoids and is slightly more hydrophobic than β-carotene (Fig. 1). Yoon et al. (28) reported earlier that very little lycopene could be recovered from E. coli despite culturing in two-phase systems with decane, unless the outer membrane was removed through spheroplast formation. We reproduced this experimental finding by making spheroplasts out of our double transformant E. coli cells (Fig. S6A), using the same and well-established osmolytic method (28, 29). Our transporter screen in spheroplasts revealed three hits for lycopene secretion, out of which StMsbA* resulted in a highly significant (P < 0.004) ∼4.3-fold increase in secreted lycopene (Fig. S6B).
These results strongly support our hypothesis: the time taken for secreted isoprenoids to appear in the decane phase in two-phase E. coli culture systems increases with the increase in hydrophobicity of the isoprenoid. In other words, isoprenoid secretion out of cells is not synchronous with isoprenoid export across the inner membrane; the former is dependent on the partitioning of isoprenoids between the membranes, periplasmic space, and decane, and the latter (transporter-mediated export) is dependent on the transporter’s kinetics per se.
In Vitro Detection of Isoprenoid–ABC Transporter Interactions.
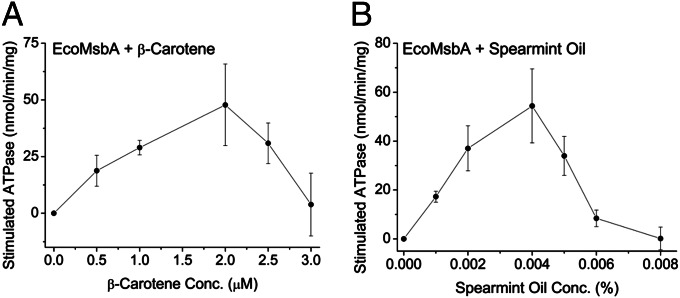
To substantiate our in vivo results on isoprenoid secretion mediated by ABC transporters, we attempted to obtain in vitro evidence of substrate-like interactions between isoprenoid molecules and the ABC transporter MsbA. Many compounds (substrates, inhibitors, or modulators) that interact with ABC transporters modulate (stimulate/inhibit) their ATP hydrolysis (ATPase) activities in a concentration-dependent manner (22). We tested isoprenoid compounds for their ability to modulate the ATPase activity of EcoMsbA.
Organic solvents such as decane, hexane, acetone, ethanol, or methanol readily denature pure detergent-solubilized proteins. Thus, the poor solubility of isoprenoids in aqueous or polar aprotic solvents such as DMSO that are routinely used for ATPase studies presented a problem for this assay. We observed that 1 mM methanol solutions of β-carotene could be diluted 1:20 in water to produce clear working stock solutions of 50 µM β-carotene. Equal volumes of a 1:20 methanol:water solution were used as background controls. The background-subtracted ATPase activity of EcoMsbA revealed a β-carotene concentration-dependent stimulation-inhibition pattern that is characteristic of many known substrates of MsbA and other ABC transporters (Fig. 6A). Furthermore, the concentration range across which stimulation was observed (0.5–2 µM) is consistent with the lower micromolar affinity constants observed for almost all substrates of ABC transporters (Fig. 6A) (22). These data strongly suggest substrate-like interactions between isoprenoids and MsbA and provide excellent complementation to our in vivo data demonstrating isoprenoid secretion by MsbA.
Fig. 6.
In vitro substrate-like interactions between biofuel compounds and MsbA. ATP hydrolysis (ATPase) activity of purified EcoMsbA was measured by the colorimetric detection of Pi using the absorbance of malachite green-ammonium molybdate at 595 nm, as established elsewhere (31). Briefly, detergent-purified MsbA was incubated with substrates on ice for 15 min, before the addition of 2.5 mM ATP, incubation at 30 °C for 5 min, and subsequent addition of the colorimetric reagent. Substrates included (A) β-carotene in methanol-water (see text for main details) or (B) spearmint oil in DMSO at the indicated concentrations controlled against equal volumes of the diluents, methanol-water, or DMSO. Control values were subtracted, and substrate-stimulated ATPase activities of EcoMsbA are presented (n = 4 independent experiments ± SEM for both experiments).
To further ensure that the ATPase modulation observed was not due to methanol present in β-carotene solutions, we resorted to test substrate-like interactions between EcoMsbA and other terpenoid compounds that could be dissolved in routine solvents like DMSO. We observed that essential oils, such as spearmint oil, for example, could be readily mixed with DMSO at 0.1%. The DMSO background-subtracted ATPase activity of EcoMsbA showed a similar stimulation-inhibition pattern as was observed for β-carotene (Fig. 6B), lending further in vitro evidence for substrate-like interactions between oily model biofuel compounds and ABC transporters.
Discussion
Harvesting large quantities of cells and chemically extracting biofuels are major hindrances to successful commercialization of biofuel production. The use of ABC transporters to facilitate the export of specific biofuel molecules from cells, abating the need for harvest and extraction, could enable easy extracellular recovery and sustained intracellular production. To our knowledge, this study is the first demonstration of using ABC transporters to mediate the secretion of isoprenoid biofuel compounds from intact, healthy, biofuel-producing cells. Concurrent with biofuel secretion, cells rapidly replenish their intracellular biofuel reservoirs allowing production to be sustained over a longer period. In our E. coli model test system, the secretion process was sustained for at least 6 d without the need to replenish the growth medium or culture. Thus, for the same quantity of biofuel produced conventionally, we have a dramatic reduction in biomass scale and significant gain in the ease of recovering the biofuel.
The possibility of extending this study for liquid alkanes/alkenes could abate the requirement of a two-phase system necessary to collect the secreted isoprenoids tested in this study. These test carotenoids are solids in their pure state and insoluble in water; hence, the need for decane in our assays (Fig. 2). This strategy, whereas attractive for the commercial production of carotenoids, is, however, not commercially feasible for biofuel production on a large scale. Instead, isoprenoids that are liquids under standard conditions, such as squalene produced by microbes, animals, and plants, and botryococcenes produced by the Botryococcus braunii Race B, may be more attractive (18, 30). Most of these oils have proven to be direct substitutes for crude oil in the production of gasoline (18). If these oils can be secreted in large enough quantities, they could potentially overcome the partition threshold to generate an oil layer above the culture medium that can be easily harvested.
Our model system coupling a biofuel synthetic pathway with an ABC transporter system has the potential to become plug-and-play, which is important for translating to other organisms like cyanobacteria, diatoms, algae, and yeast. The promiscuity of ABC transporters throughout all kingdoms of life significantly increases the probability of their successful heterologous expression in the production host. The poly-specificity of these proteins also provides confidence in obtaining a hit when screening a small number of ABC transporters that may be preselected based on expression in the desired host. Large-scale production of advanced biofuels, which includes harvesting, extraction, and refining, is currently pursued by several biotech companies. We envision that our plug-and-play secretion system would add significant value when applied to these commercial ventures.
Taken in a broader context, this study also highlights possibilities of using heterologously expressed ABC transporters to achieve the import (removal) or export (secretion) of various biomolecules and ions of economic, therapeutic and environmental interest. The X-ray structures of some of the ABC transporters used in this study have already been determined, allowing for the possibility of rational design of the substrate binding site (19). Structural studies of biofuel molecules bound to ABC transporters could facilitate structure-based engineering of the transporter to dramatically increase biofuel specificity and transport rate.
Materials and Methods
E. coli BL21 (DE3; Life Technologies) was used as the production host for all carotenoids and for the expression of transporters. Cells were grown in Luria-Bertani medium with 25 µg/mL chloramphenicol and 50 µg/mL carbenicillin, and 1 mM IPTG was used to induce transporter expression. Two-phase culture systems contained 50% (for cells in medium) and 40% (for cells in buffer) (vol/vol) decane over medium. Extra/intracellular carotenoid concentrations and cell viabilities were measured using absorbance spectrophotometry. ATPase measurements were performed using the colorimetric Pi detection reagent malachite green-ammonium molybdate (31). Further descriptions of methods are available in the figure legends and in SI Materials and Methods.
Supplementary Material
Acknowledgments
pAC184-derived plasmids used for carotenoid production were kind gifts from Francis Cunningham (University of Maryland) and Juergen Polle (City University of New York). Eric Buxton and C. B. Roth are thanked for technical assistance. This work was supported by US Air Force Office of Scientific Research Grant FA9550-10-1-0440.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1301358110/-/DCSupplemental.
References
- 1.Keasling JD. Manufacturing molecules through metabolic engineering. Science. 2010;330(6009):1355–1358. doi: 10.1126/science.1193990. [DOI] [PubMed] [Google Scholar]
- 2.Peralta-Yahya PP, Zhang F, del Cardayre SB, Keasling JD. Microbial engineering for the production of advanced biofuels. Nature. 2012;488(7411):320–328. doi: 10.1038/nature11478. [DOI] [PubMed] [Google Scholar]
- 3.Mercer P, Armenta RE. Developments in oil extraction from microalgae. Eur J Lipid Sci Technol. 2011;113(5):539–547. [Google Scholar]
- 4.Liu X, Fallon S, Sheng J, Curtiss R., 3rd CO2-limitation-inducible Green Recovery of fatty acids from cyanobacterial biomass. Proc Natl Acad Sci USA. 2011;108(17):6905–6908. doi: 10.1073/pnas.1103016108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunlop MJ. Engineering microbes for tolerance to next-generation biofuels. Biotechnol Biofuels. 2011;4(1):32. doi: 10.1186/1754-6834-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramachandra TV, Mahapatra DM, B K, Gordon R. Milking diatoms for sustainable energy: Biochemical engineering versus gasoline-secreting diatom solar panels. Ind Eng Chem Res. 2009;48(19):8769–8788. [Google Scholar]
- 7.Radakovits R, Jinkerson RE, Darzins A, Posewitz MC. Genetic engineering of algae for enhanced biofuel production. Eukaryot Cell. 2010;9(4):486–501. doi: 10.1128/EC.00364-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunlop MJ, et al. Engineering microbial biofuel tolerance and export using efflux pumps. Mol Syst Biol. 2011;7:487. doi: 10.1038/msb.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nikaido H, Takatsuka Y. Mechanisms of RND multidrug efflux pumps. Biochim Biophys Acta. 2009;1794(5):769–781. doi: 10.1016/j.bbapap.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins CF. ABC transporters: From microorganisms to man. Annu Rev Cell Biol. 1992;8(1):67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 11.Rees DC, Johnson E, Lewinson O. ABC transporters: The power to change. Nat Rev Mol Cell Biol. 2009;10(3):218–227. doi: 10.1038/nrm2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pohl A, Devaux PF, Herrmann A. Function of prokaryotic and eukaryotic ABC proteins in lipid transport. Biochim Biophys. Acta. 2005;1733(1):29–52. doi: 10.1016/j.bbalip.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Aller SG, et al. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323(5922):1718–1722. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Veen HW, et al. A bacterial antibiotic-resistance gene that complements the human multidrug-resistance P-glycoprotein gene. Nature. 1998;391(6664):291–295. doi: 10.1038/34669. [DOI] [PubMed] [Google Scholar]
- 15.Pighin JA, et al. Plant cuticular lipid export requires an ABC transporter. Science. 2004;306(5696):702–704. doi: 10.1126/science.1102331. [DOI] [PubMed] [Google Scholar]
- 16.Martin VJJ, Pitera DJ, Withers ST, Newman JD, Keasling JD. Engineering a mevalonate pathway in Escherichia coli for production of terpenoids. Nat Biotechnol. 2003;21(7):796–802. doi: 10.1038/nbt833. [DOI] [PubMed] [Google Scholar]
- 17.Cunningham FX, Jr, Gantt E. A portfolio of plasmids for identification and analysis of carotenoid pathway enzymes: Adonis aestivalis as a case study. Photosynth Res. 2007;92(2):245–259. doi: 10.1007/s11120-007-9210-0. [DOI] [PubMed] [Google Scholar]
- 18.Tracy NI, Crunkleton DW, Price GL. Catalytic cracking of squalene to gasoline-range molecules. Biomass Bioenergy. 2011;35(3):1060–1065. [Google Scholar]
- 19.Ward A, Reyes CL, Yu J, Roth CB, Chang G. Flexibility in the ABC transporter MsbA: Alternating access with a twist. Proc Natl Acad Sci USA. 2007;104(48):19005–19010. doi: 10.1073/pnas.0709388104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doerrler WT, Gibbons HS, Raetz CRH. MsbA-dependent translocation of lipids across the inner membrane of Escherichia coli. J Biol Chem. 2004;279(43):45102–45109. doi: 10.1074/jbc.M408106200. [DOI] [PubMed] [Google Scholar]
- 21.Ma B, Reynolds CM, Raetz CRH. Periplasmic orientation of nascent lipid A in the inner membrane of an Escherichia coli LptA mutant. Proc Natl Acad Sci USA. 2008;105(37):13823–13828. doi: 10.1073/pnas.0807028105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eckford PDW, Sharom FJ. Functional characterization of Escherichia coli MsbA: interaction with nucleotides and substrates. J Biol Chem. 2008;283(19):12840–12850. doi: 10.1074/jbc.M708274200. [DOI] [PubMed] [Google Scholar]
- 23.Reuter G, et al. The ATP binding cassette multidrug transporter LmrA and lipid transporter MsbA have overlapping substrate specificities. J Biol Chem. 2003;278(37):35193–35198. doi: 10.1074/jbc.M306226200. [DOI] [PubMed] [Google Scholar]
- 24.Meckel RA, Kester AS. Extractability of carotenoid pigments from non-photosynthetic bacteria with solvents and detergents: Implications for the location and binding of the pigments. J Gen Microbiol. 1980;120(1):111–116. [Google Scholar]
- 25.Mathews MM, Sistrom WR. Intracellular location of carotenoid pigments and some respiratory enzymes in Sarcina lutea. J Bacteriol. 1959;78(6):778–787. doi: 10.1128/jb.78.6.778-787.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gutmann DAP, Ward A, Urbatsch IL, Chang G, van Veen HW. Understanding polyspecificity of multidrug ABC transporters: Closing in on the gaps in ABCB1. Trends Biochem Sci. 2010;35(1):36–42. doi: 10.1016/j.tibs.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peralta-Yahya PP, et al. Identification and microbial production of a terpene-based advanced biofuel. Nat Commun. 2011;2:483. doi: 10.1038/ncomms1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoon K-W, Doo E-H, Kim S-W, Park J-B. In situ recovery of lycopene during biosynthesis with recombinant Escherichia coli. J Biotechnol. 2008;135(3):291–294. doi: 10.1016/j.jbiotec.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Birdsell DC, Cota-Robles EH. Production and ultrastructure of lysozyme and ethylenediaminetetraacetate-lysozyme spheroplasts of Escherichia coli. J Bacteriol. 1967;93(1):427–437. doi: 10.1128/jb.93.1.427-437.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Metzger P, Largeau C. Botryococcus braunii: A rich source for hydrocarbons and related ether lipids. Appl Microbiol Biotechnol. 2005;66(5):486–496. doi: 10.1007/s00253-004-1779-z. [DOI] [PubMed] [Google Scholar]
- 31.Doshi R, Woebking B, van Veen HW. Dissection of the conformational cycle of the multidrug/lipid A ABC exporter MsbA. Proteins Struct Function Bioinform. 2010;78(14):2867–2872. doi: 10.1002/prot.22813. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.