Abstract
This review discusses in detail robotic tubal reanastomosis as one of the classic reproductive surgery procedures. Other applications of robotics in adnexal surgery are also reviewed, including adult and pediatric adnexectomy, resection of endometriosis, benign ovarian mass resection, early ovarian cancer resection and staging, ovarian transposition, and treatment of ovarian remnant syndrome and ovarian vein syndrome.
Key words: Robotic surgery, Adnexal surgery, Tubal reanastomosis
Robotic tubal reanastomosis marked the beginning of the era of robotic surgery in gynecology. In 1999, two studies conducted on porcine animal models concluded that a robotic approach to tubal surgery is a safe and feasible technique, with 100% immediate and 67% 4-week patency rate.1,2 The first human case was quick to follow, less than a year later; this was followed by a pilot study of 10 patients, all of whom underwent tubal reanastomosis with the ZEUS® Robotic Surgical System (Computer Motion, now Intuitive Surgical, Sunnyvale, CA).3,4 Degueldre and colleagues5 described eight bilateral tubal reanastomosis cases completed with the da Vinci Surgical System (Intuitive Surgical) in 2000.5 Two later studies reported increased operative times associated with robotic surgery compared with laparoscopy, yet supported the feasibility of robotic approach by demonstrating established tubal patency followed by conception after reanastomosis.6,7
The majority of adnexal procedures currently performed with robotic assistance can be attributed to one of the following domains: reproductive surgery, benign gynecology, and gynecologic oncology. This review discusses in detail robotic tubal reanastomosis as one of the classic reproductive surgery procedures. Other applications of robotics in adnexal surgery are also discussed, including adult and pediatric adnexectomy, benign ovarian tumor resection, early ovarian cancer resection and staging, ovarian transposition, and treatment of ovarian remnant syndrome and ovarian vein syndrome.
Robotic Tubal Reanastomosis
In the United States, surgical sterilization is the second most commonly used form of contraception overall, and the most frequently used method among married women and among women aged > 30 years.8,9 Bilateral tubal ligation is the most effective and commonly used method of surgical sterilization.8,9 Common reasons for undergoing tubal ligation include completion of child bearing and medical indications, yet up to 30% of women will later desire more children and regret their decision.10 Predictors for regret include young age, nonwhite race, postpartum procedure, and change in a partner or marital status.10
Recent advances in assisted reproductive technologies (ART) resulted in increasing popularity of in vitro fertilization (IVF).11 Nevertheless, microsurgical reanastomosis remains an appropriate and effective approach to the management of tubal infertility in a carefully selected patient population. The feasibility of ART and tubal reanastomosis has been established, yet no randomized, controlled trials have been conducted to compare the efficacy of these two approaches.12,13
Microscopic tubal reanastomosis is generally recommended for women without a history of reproductive dysfunction who are considering more than one subsequent pregnancy, yet are opposed to the high probability of multiple gestation.14 Age was identified as an important factor for prediction of pregnancy rates and pregnancy outcomes in both IVF15,16 and tubal reversal,17,18 and, therefore, should be considered along with other reproductive parameters as part of preoperative workup.
Surgical Technique
Several approaches to robotic tubal reanastomosis have been described14,19,20; herein we present the technique employed at Brigham and Women’s Hospital (Boston, MA). After induction of general anesthesia, the patient is placed in a dorsal lithotomy position with arms fully adducted and knees in the same plane as the pelvic girdle. A uterine positioning system is used to ensure consistent intrauterine manipulation and chromopertubation. Pneumoperitoneum is created with Veress needle followed by port placement.
Port Placement
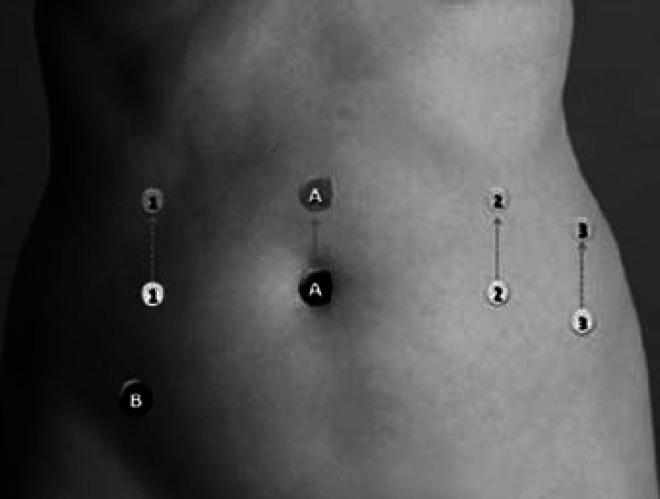
Two port placement protocols are currently employed at our institution.14 The standard protocol involves placement of an 8.5-mm or 12-mm optical trocar at the umbilicus and two 8-mm robotic arm ports (1 and 2) 8 to 10 cm lateral and slightly caudal to the umbilicus (Figure 1). If necessary, the third robotic arm is placed in the left upper quadrant 8 to 10 cm lateral to port 2. Placement of the third robotic arm is associated with increased risk for injury to the deep circumflex vessels and collision of robotic arms,14 and therefore should only be employed as needed (in patients with adhesions or obesity or when attempting the technique for the first time). The assistant port is placed in the right lower quadrant on the vertical line with port 1. The standard protocol allows for positioning of the robot between the patient’s legs, although side docking is our preferred approach due to ease of vaginal access for chromopertubation and uterine manipulation.21
Figure 1.
Standard port placement protocol. Reprinted with permission from Gargiulo AR.14 A, camera port; B, assistant port; 1, 2 and 3, robotic arm ports.
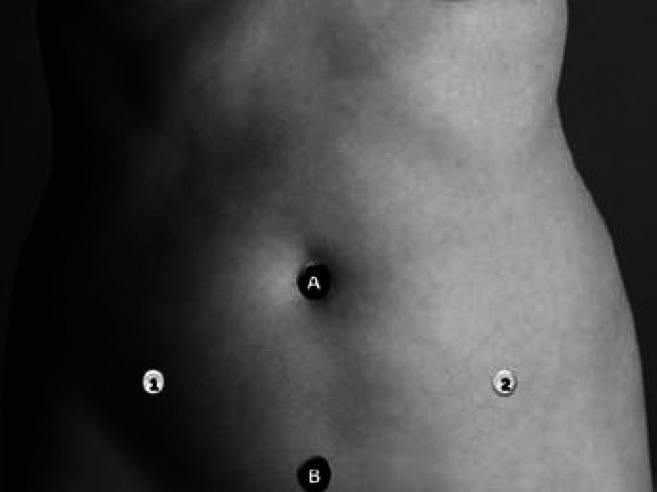
The minimal access protocol allows for improved cosmesis, highly regarded among the young reproductive surgery patient population.22 With this approach, an 8.5-mm or 12-mm optical trocar is placed at the level of umbilicus (Figure 2). Two robotic arms are inserted in the classic laparoscopic location medial to the anterior-superior iliac spines, and the assistant port is suprapubic.14 Placement of a third robotic arm is not possible in this configuration. With minimal access protocol, the robot is positioned at the patient’s left or right knee (side docking) in order to gain access to the suprapubic port.14
Figure 2.
Minimal access protocol for improved cosmetics. Reprinted with permission from Gargiulo AR.14 A, camera port; B, assistant port; 1, 2, robotic arm ports. There is no room for robotic arm port 3.
Preparation for Reanastomosis
Following the docking of the robot, Potts scissors and microsurgical bipolar forceps are used to mobilize the mesosalpinx and amputate the distal and proximal tubal segments. If third robotic arm is available, ProGrasp™ (Intuitive Surgical) robotic forceps are used to improve exposure. To achieve hemostasis, we inject 1 unit of dilute vasopressin into the proximal and distal segments of the mesosalpinx. Use of electrosurgery should also be minimized to avoid damage to the fallopian tube tissues. Following the amputation of the distal and proximal stumps, chromopertubation is performed to assure tubal patency.
Tubal Reanastomosis
In order to best identify the distal tubal lumen and secure the anatomic orientation of the tube during the reanastomosis, we use an endoscopic retrograde cholangiopancreatography catheter as a tubal stent. Other surgeons perform the reanastomosis without tubal stent placement19,20 and achieve the anatomic orientation of the tubal segments by reapproximating mesosalpinx. Two Black Diamond Micro Forceps (EndoWrist®; Intuitive Surgical are utilized for suturing. We place one to two stitches of 6-0 polyglycolic acid suture to reapproximate the mesosalpinx, followed by four interrupted 8-0 polypropylene sutures placed in the 3 o’clock, 6 o’clock, 9 o’clock, and 12 o’clock positions to reapproximate the tube. The suture should just skim the tubal lumen. Others suggest minimizing the number of sutures to two in order to prevent restenosis of the tube.20 The serosa is sometimes reapproximated with interrupted 8-0 polypropylene sutures. At the end of the procedure, chromopertubation is performed to assure tubal patency, and an adhesive barrier is placed.
Postoperative Care and Complications
Patients are taken to the recovery room and observed for several hours. Postoperative pain control is achieved with nonsteroidal anti-inflammatory drugs and narcotic pain medication if needed. Patients are advised to refrain from vigorous activity, including intercourse, until their postoperative visit approximately 14 days later. Conception should not be attempted for at least 4 weeks after the surgery. It is recommended to perform a hysterosalpingogram to confirm the tubal patency a minimum of 8 weeks after surgery.
Following reanastomosis, the risk for ectopic pregnancy is increased up to 10-fold compared with the general population.23–25 For this reason, all patients should undergo a pregnancy test immediately following the first day of a missed menstrual period and be well aware of the first signs and symptoms associated with ectopic pregnancy. If a positive pregnancy test result is obtained, an early pregnancy evaluation protocol applies, similar to what is done for patients who become pregnant following assisted reproduction.
Outcomes
In a retrospective cohort study of 97 patients undergoing robotic tubal reanastomosis, Caillet and colleagues26 reported a pregnancy rate of 71% at 2-year follow-up. The highest pregnancy rate of 91% was observed in patients aged < 35 years, and the lowest pregnancy rate of 33% in those aged > 43 years. Pregnancy rates among women aged 36 to 39 years was 75%; in those aged 40 to 42 years the rate was 50%. The pregnancy rates following robotic tubal reanastomosis compare well and exceed those achieved with ART15,16 when stratified for age.
Surgical outcomes following robotic tubal reanastomosis were reported in a case-controlled study of 67 patients by Rodgers and coworkers27 and a prospective cohort study of 28 patients by Dharia Patel and coworkers.20 Both studies reported prolonged surgical times and increased cost for the robotic versus classic open microsurgical approach to tubal reanastomosis. Hospitalization times and pregnancy and ectopic pregnancy rates were on par between the two groups.27 Dharia Patel and colleagues20 reported shorter hospitalization times and decreased time to recovery in the robotic surgery group. The cost per delivery was similar between the two approaches. Overall, robotic tubal reanastomosis is a safe and feasible technique with pregnancy rates on par with those achieved followingg ART.
Robotic Adnexectomy for Benign Pathology
In 2008, Nezhat and colleagues28 presented retrospective data on 136 robotic-assisted gynecologic surgeries performed in 87 women over a period of 20 months; 53% of performed procedures involved adnexal structures, such as oophorectomy with or without hysterectomy, ovarian cystectomy, ovarian drilling, salpingectomy, adheolysis, and Moskowitz procedure. The mean operative time duration for oophorectomy was 117 minutes. No major complications were reported for adnexal procedures. One patient had an intestinal hernia at the site of the 8-mm robotic arm port. Other comments were related to the bulkiness of robotic equipment, inability to use tools commonly available during laparoscopic procedures (stapler, Babcock clamps, suction-irrigator), lack of tactile feedback, inability to move the surgical table, and additional time associated with assembly and disassembly of robotic equipment. Advantages attributed to robotic endoscopic surgery included fine manipulations in a limited field of vision required for procedures such as tubal surgery, ureteral dissections, and cystectomies.
Another study comparing the outcomes of robotic versus laparoscopic adnexectomy was published by Magrina and associates in 2009.29 A total of 85 patients undergoing a robotic procedure were compared with 91 patients treated with laparoscopy. The study concluded that the advantage of robotic adnexectomy may be achieved in a subset of patients with a body mass index (BMI) > 30, as this group experienced reduced blood loss when compared with those who underwent a laparoscopic approach. Other outcomes were not significantly different across the two groups.
Several studies demonstrated that a laparoscopic approach to dermoid cyst excision has a higher risk of cystic contents spillage compared with laparotomy.30,31 Spillage of cystic contents can cause chemical peritonitis, a rare but potentially serious complication.32 At our institution, application of a robotic approach to dermoid cyst excision has allowed us to minimize the risk for spillage and preserve the benefits of the minimally invasive approach. This is achieved by performance of the entire cyst stripping procedure within the endoscopic bag.
Robotic Adnexal Surgery in Malignant Pathology
One in 72 women will develop epithelial ovarian cancer during her lifetime.33 The overall 5-year survival rate is 46%, but can be as high as 94% if the disease is recognized early.33 Unfortunately, only 15% of women are diagnosed during the early stages of disease, and this is the subgroup in whom robotic surgery can be utilized for treatment or staging. Extensive exploratory dissection of the abdomen, pelvis, and bowel is commonly required with more advanced stages of disease, which can make the minimally invasive approach very difficult. The most comprehensive case-controlled study by Magrina and colleagues34 compared 25 robotic cases of epithelial cancer staging with 27 laparoscopic and 119 open surgery cases. All patients were operated on during the same period and the groups were matched by age, BMI, type, and number of procedures done. Compared with open surgery, the robotic and laparoscopic approaches were associated with decreased blood loss and decreased hospital stay. In addition, in patients with type II ovarian debulking, both robotic and laparoscopic approaches were associated with a significantly decreased postoperative complication rate. No difference in outcomes was reported between robotic and laparoscopic surgery. Survival was also unaffected by surgical approach.
Robotic Adnexal Surgery in Pediatrics
Ovarian pathology is one of the key indications for minimally invasive surgery in neonates.35 According to a recent systematic review of 50 articles describing 1215 patients who weighed < 5 kg, ovarian pathology was responsible for 151 surgeries or 12% of all procedures performed.35 Common indications for intervention included large functional cysts and complex (dermoid/neoplastic) cysts. Therapeutic intervention included cyst aspiration, excision or decapsulation, and ovariectomy.36,37 The mean operative time for ovarian surgery was reported at 54 minutes, with a conversion rate to open surgery of 4%, and a 0% complication rate. A minimally invasive approach (including both laparoscopic and robotic surgery) for the treatment of ovarian pathology in neonates and young infants was deemed safe and feasible.
Another retrospective cohort study of 144 robot-assisted pediatric surgical procedures included two cases of ovarian cyst excision.38 In both cases, no complications were reported and no conversion to open surgery was required. Although no added benefits of a robotic approach were identified, the study confirmed its safety and feasibility in pediatric surgery. Several other case reports described successful application of robotic surgery in pediatric gynecology, including a case of paratubal cystectomy and bilateral salpingo-oophorectomy due to gonadoblastoma.39,40
Robotics Management of Endometriosis
Endometriosis is a chronic inflammatory condition that can involve one or several areas of the pelvis and abdomen, including the adnexal structures. Safe and radical resection of endometriosis has been traditionally considered a challenging operation, even with the enhanced visualization and access provided by laparoscopy. Robotic technology provides exceptional visual feedback, enhanced surgical ergonomics, and advanced instrumentation critical for success of a complex pelvic dissection.41 Three case reports described the application of a robotic approach to the resection of endometriosis of the bladder and rectum, including excision of ovarian cyst and rectosigmoidectomy.42–44 In a retrospective cohort study, Nezhat and coworkers45 compared the outcomes of a laparoscopic versus a robotic approach with the treatment of endometriosis in 78 patients and concluded that the value of robotics lies in the management of advanced and severe cases of stage IV endometriosis, including those with endometriomas, which may result in converting laparotomies to laparoscopies in more advanced cases.45
Other Applications of Robotics in Adnexal Surgery
Ovarian remnant syndrome is a rare condition defined as the finding of histologically confirmed ovarian cortical tissue during surgical exploration in a woman who presents with pain or a pelvic mass and who has had a previous salpingo-oophorectomy.46 It is usually caused by unintentional incomplete dissection during oophorectomy in patients with endometriosis or significant pelvic adhesions. Surgical removal of an ovarian remnant can be challenging and has been associated with the injury of the bowel, bladder, or ureters.47 Two studies compared the outcomes following the surgical treatment of ovarian remnant syndrome via laparotomy, laparoscopy, and robotic approach.46,48 Kho and colleagues46 analyzed 20 patients, 14 of whom were treated laparoscopically. Five patients were treated with robotic assistance, and one had laparoscopy. The mean operative time was 147 minutes, and the mean estimated blood loss was 106 mL. Resolution of symptoms occurred in all but one patient. Precise dissection of adhesions was facilitated by the articulated tips of robotic surgical instruments and the three-dimensional view of the operative field.46 Zapardiel and colleagues48 retrospectively compared 187 laparotomy, 19 laparoscopy, and 17 robotic cases for the treatment of ovarian remnant syndrome. Both the laparoscopic and robotic approaches were associated with decreased blood loss, shorter hospital stay, and similar operative times in comparison with laparotomy. The overall rate of pain regression was 92%, but surprisingly lower (71.4%) in the robotic group. The robotic group also had higher rates of pelvic adhesions and endometriosis.
Additional studies are needed to investigate the outcomes of a robotic approach for the treatment of ovarian remnant syndrome. Although the robotic approach appears to be ideally suited for the meticulous nature of dissection required with this syndrome, clinical data remain contradictory. At the same time, improved surgical outcomes following a robotic approach argue in its favor.
Successful application of robotic approach had been reported for the treatment of ovarian vein syndrome,49 robotic salpingostomy for ectopic pregnancy,50 and ovarian tissue transplantation with subsequent restoration of ovarian function in a patient with non-Hodgkin lymphoma.51 Laparoscopic ovarian transposition is a technically simple and effective minimally invasive technique that is frequently underused, yet it can prevent the loss of fertility and premature menopause in young women undergoing radiation therapy of the pelvis.14 Although oophoropexy is not a technically complex procedure, a robotic approach appears to be an appropriate choice in case of high ovarian displacement.52 In the case presented, the robot was set up similarly to the minimal access protocol described by Gargiulo14 with the difference that a robotic camera was inserted suprapubically as opposed to through the umbilicus.52
Main Points.
Robotic tubal reanastomosis is a safe, practical, and feasible method of fertility restoration in an appropriate patient population with pregnancy outcomes comparable with assisted reproductive technologies and surgical outcomes on par with laparoscopy.
A robotic approach to adnexectomy is a feasible technique and may be associated with improved surgical outcomes (reduced intraoperative blood loss) in a subset of patients with a body mass index > 30.
A robotic approach may be beneficial for the management of advanced stage IV endometriosis and conversion laparotomies to laparoscopies for more advanced cases.
Compared with open surgery, robotic and laparoscopic approaches may be preferable in patients with type II ovarian debulking because of their significantly decreased postoperative complication rate. Survival does not appear to be affected by surgical approach.
The robotic approach to ovarian remnant syndrome management is associated with improved surgical outcomes but a lower rate of pain regression and increased incidence of adhesions and endometriosis compared with the laparoscopic approach.
A robotic approach to cystectomy in the pediatric population may be a safe and feasible procedure with a low rate of complications and conversion to laparotomy.
A robotic approach has been successfully applied in cases of ovarian transposition, ovarian vein syndrome, and salpingostomy for ectopic pregnancy.
References
- 1.Margossian H, Garcia-Ruiz A, Falcone T, et al. Robotically assisted laparoscopic microsurgical uterine horn anastomosis. Fertil Steril. 1998;70:530–534. doi: 10.1016/s0015-0282(98)00196-4. [DOI] [PubMed] [Google Scholar]
- 2.Margossian H, Garcia-Ruiz A, Falcone T, et al. Robotically assisted laparoscopic tubal anastomosis in a porcine model: a pilot study. J Laparoendosc Adv Surg Tech A. 1998;8:69–73. doi: 10.1089/lap.1998.8.69. [DOI] [PubMed] [Google Scholar]
- 3.Falcone T, Goldberg J, Garcia-Ruiz A, et al. Full robotic assistance for laparoscopic tubal anastomosis: a case report. J Laparoendosc Adv Surg Tech A. 1999;9:107–113. doi: 10.1089/lap.1999.9.107. [DOI] [PubMed] [Google Scholar]
- 4.Falcone T, Goldberg JM, Margossian H, Stevens L. Robotic-assisted laparoscopic microsurgical tubal anastomosis: a human pilot study. Fertil Steril. 2000;73:1040–1042. doi: 10.1016/s0015-0282(00)00423-4. [DOI] [PubMed] [Google Scholar]
- 5.Degueldre M, Vandromme J, Huong PT, Cadière GB. Robotically assisted laparoscopic microsurgical tubal reanastomosis: a feasibility study. Fertil Steril. 2000;74:1020–1023. doi: 10.1016/s0015-0282(00)01543-0. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg JM, Falcone T. Laparoscopic microsurgical tubal anastomosis with and without robotic assistance. Hum Reprod. 2003;18:145–147. doi: 10.1093/humrep/deg011. [DOI] [PubMed] [Google Scholar]
- 7.Vlahos NF, Bankowski BJ, King JA, Shiller DA. Laparoscopic tubal reanastomosis using robotics: experience from a teaching institution. J Laparoendosc Adv Surg Tech A. 2007;17:180–185. doi: 10.1089/lap.2006.0035. [DOI] [PubMed] [Google Scholar]
- 8.Mosher WD, Jones J. Use of contraception in the United States: 1982–2008. Vital Health Stat. 2010;23:1–44. [PubMed] [Google Scholar]
- 9.Zite N, Borrero S. Female sterilisation in the United States. Eur J Contracept Reprod Health Care. 2011;16:336–340. doi: 10.3109/13625187.2011.604451. [DOI] [PubMed] [Google Scholar]
- 10.Hillis SD, Marchbanks PA, Tylor LR, Peterson HB. Poststerilization regret: findings from the United States Collaborative Review of Sterilization. Obstet Gynecol. 1999;93:889–895. doi: 10.1016/s0029-7844(98)00539-0. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention, American Society for Reproductive Medicine, Society for Assisted Reproductive Technology, authors. 2009 Assisted Reproductive Technology Success Rates: National Summary and Fertility Clinic Reports. Atlanta, GA: US Department of Health and Human Services; 2011. [Google Scholar]
- 12.Yossry M, Aboulghar M, D’Angelo A, Gillett W. In vitro fertilisation versus tubal reanastomosis (sterilisation reversal) for subfertility after tubal sterilization. Cochrane Database Syst Rev. 2006:CD004144. doi: 10.1002/14651858.CD004144.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomel V. Reversal of tubal sterilization versus IVF in the era of assisted reproductive technology: a clinical dilemma. Reprod Biomed Online. 2007;15:403–407. doi: 10.1016/s1472-6483(10)60365-3. [DOI] [PubMed] [Google Scholar]
- 14.Gargiulo AR. Fertility preservation and the role of robotics. Clin Obstet Gynecol. 2011;54:431–448. doi: 10.1097/GRF.0b013e31822b3b80. [DOI] [PubMed] [Google Scholar]
- 15.Society for Assisted Reproductive Technology; |American Society for Reproductive Medicine, authors. Assisted reproductive technology in the United States: 2001 results generated from the American Society for Reproductive Medicine/Society for Assisted Reproductive Technology registry. Fertil Steril. 2007;87:1253–1266. doi: 10.1016/j.fertnstert.2006.11.056. [DOI] [PubMed] [Google Scholar]
- 16.De Mouzon, Goossens V, Bhattacharya S, et al. European IVF-monitoring (EIM) Consortium, for the European Society of Human Reproduction and Embryology (ESHRE), authors Assisted reproductive technology in Europe, 2006: results generated from European registers by ESHRE. Hum Reprod. 2010;25:1851–1862. doi: 10.1093/humrep/deq124. [DOI] [PubMed] [Google Scholar]
- 17.Dubuisson JB, Swolin K. Laparoscopic tubal anastomosis (the one stitch technique): preliminary results. Hum Reprod. 1995;10:2044–2046. doi: 10.1093/oxfordjournals.humrep.a136232. [DOI] [PubMed] [Google Scholar]
- 18.Hanafi MM. Factors affecting the pregnancy rate after microsurgical reversal of tubal ligation. Fertil Steril. 2003;80:434–440. doi: 10.1016/s0015-0282(03)00661-7. [DOI] [PubMed] [Google Scholar]
- 19.Bedaiwy MA, Barakat EM, Falcone T. Robotic tubal anastomosis: technical aspects. JSLS. 2011;15:10–15. doi: 10.4293/108680810X12924466009041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dharia Patel SP, Steinkampf MP, Whitten SJ, Malizia BA. Robotic tubal anastomosis: surgical technique and cost effectiveness. Fertil Steril. 2008;90:1175–1179. doi: 10.1016/j.fertnstert.2007.07.1392. [DOI] [PubMed] [Google Scholar]
- 21.Einarsson JI, Hibner M, Advincula AP. Side docking: an alternative docking method for gynecologic robotic surgery. Rev Obstet Gynecol. 2011;4:123–125. [PMC free article] [PubMed] [Google Scholar]
- 22.Bush AJ, Morris SN, Millham FH, Isaacson KB. Women’s preferences for minimally invasive incisions. J Minim Invasive Gynecol. 2011;18:640–643. doi: 10.1016/j.jmig.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 23.Gomel V. Microsurgical reversal of female sterilization: a reappraisal. Fertil Steril. 1980:33. doi: 10.1016/s0015-0282(16)44769-2. [DOI] [PubMed] [Google Scholar]
- 24.Hirth R, Zbella E, Sanchez M, Prieto J. Microtubal reanastomosis: success rates as compared to in vitro fertilization. J Reprod Med. 2010;55:161–165. [PubMed] [Google Scholar]
- 25.Schippert C, Soergel P, Staboulidou I, et al. The risk of ectopic pregnancy following tubal reconstructive microsurgery and assisted reproductive technology procedures. Arch Gynecol Obstet. 2012;285:863–871. doi: 10.1007/s00404-011-2092-6. [DOI] [PubMed] [Google Scholar]
- 26.Caillet M, Vandromme J, Rozenberg S, et al. Robotically assisted laparoscopic microsurgical tubal reanastomosis: a retrospective study. Fertil Steril. 2010;94:1844–1847. doi: 10.1016/j.fertnstert.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 27.Rodgers AK, Goldberg JM, Hammel JP, Falcone T. Tubal anastomosis by robotic compared with outpatient minilaparotomy. Obstet Gynecol. 2007;109:1375–1380. doi: 10.1097/01.AOG.0000264591.43544.0f. [DOI] [PubMed] [Google Scholar]
- 28.Nezhat C, Lavie O, Lemyre M, et al. Robot-assisted laparoscopic surgery in gynecology: scientific dream or reality? Fertil Steril. 2009;91:2620–2622. doi: 10.1016/j.fertnstert.2008.03.070. [DOI] [PubMed] [Google Scholar]
- 29.Magrina JF, Espada M, Munoz R, et al. Robotic adnexectomy compared with laparoscopy for adnexal mass. Obstet Gynecol. 2009;114:581–584. doi: 10.1097/AOG.0b013e3181b05d97. [DOI] [PubMed] [Google Scholar]
- 30.Briones-Landa CH, Ayala-Yáñez R, Leroy-López L, et al. Comparison of laparoscopic vs. laparotomy treatment in ovarian teratomas. Ginecol Obstet Mex. 2010;78:527–532. [PubMed] [Google Scholar]
- 31.Kondo W, Bourdel N, Cotte B, et al. Does prevention of intraperitoneal spillage when removing a dermoid cyst prevent granulomatous peritonitis? BJOG. 2010;117:1027–1030. doi: 10.1111/j.1471-0528.2010.02580.x. [DOI] [PubMed] [Google Scholar]
- 32.da Silva BB, dos Santos AR, Lopes-Costa PV, et al. Ovarian dermoid cyst with malignant transformation and rupture of the capsule associated with chemical peritonitis: a case report and literature review. Eur J Gynaecol Oncol. 2009;30:226–228. [PubMed] [Google Scholar]
- 33.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 34.Magrina JF, Zanagnolo V, Noble BN, et al. Robotic approach for ovarian cancer: perioperative and survival results and comparison with laparoscopy and laparotomy. Gynecol Oncol. 2011;121:100–105. doi: 10.1016/j.ygyno.2010.11.045. [DOI] [PubMed] [Google Scholar]
- 35.Sinha CK, Paramalingam S, Patel S, et al. Feasibility of complex minimally invasive surgery in neonates. Pediatr Surg Int. 2009;25:217–221. doi: 10.1007/s00383-008-2318-2. [DOI] [PubMed] [Google Scholar]
- 36.Esposito C, Garipoli V, Di Matteo G, De Pasquale M. Laparoscopic management of ovarian cysts in newborns. Surg Endosc. 1998;12:1152–1154. doi: 10.1007/s004649900804. [DOI] [PubMed] [Google Scholar]
- 37.Dobremez E, Moro A, Bondonny JM, Vergnes P. Laparoscopic treatment of ovarian cyst in the newborn. Surg Endosc. 2003;17:328–332. doi: 10.1007/s00464-001-9099-1. [DOI] [PubMed] [Google Scholar]
- 38.Alqahtani A, Albassam A, Zamakhshary M, et al. Robot-assisted pediatric surgery: how far can we go? World J Surg. 2010;34:975–978. doi: 10.1007/s00268-010-0431-6. [DOI] [PubMed] [Google Scholar]
- 39.Delotte J, Breaud J, Mialon O, et al. A role of robotic-assisted surgery to preserve female fertility? Comments about the first paratubal cystectomy performed with the “Da Vinci S” robotic system in a young girl. Gynecol Obstet Fertil. 2010;38:631–633. doi: 10.1016/j.gyobfe.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 40.Gutt CN, Markus B, Kim ZG, et al. Early experiences of robotic surgery in children. Surg Endosc. 2002;16:1083–1086. doi: 10.1007/s00464-001-9151-1. [DOI] [PubMed] [Google Scholar]
- 41.Gargiulo AR, Nezhat C. Robot-assisted laparoscopy, natural orifice transluminal endoscopy, and single site laparoscopy in reproductive surgery. Semin Reprod Med. 2011;29:155–168. doi: 10.1055/s-0031-1272478. [DOI] [PubMed] [Google Scholar]
- 42.Chammas MF Jr, Kim FJ, Barbarino A, et al. Asymptomatic rectal and bladder endometriosis: a case for robotic-assisted surgery. Can J Urol. 2008;15:4097–4100. [PubMed] [Google Scholar]
- 43.Averbach M, Popoutchi P, Marques OW, et al. Robotic rectosigmoidectomy — pioneer case report in Brazil. Current scene in colorectal robotic surgery. Arq Gastroenterol. 2010;47:116–118. doi: 10.1590/s0004-28032010000100018. [DOI] [PubMed] [Google Scholar]
- 44.Liu C, Perisic D, Samadi D, Nezhat F. Robotic-assisted laparoscopic partial bladder resection for the treatment of infiltrating endometriosis. J Minim Invasive Gynecol. 2008;15:745–748. doi: 10.1016/j.jmig.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 45.Nezhat C, Lewis M, Kotikela S, et al. Robotic versus standard laparoscopy for the treatment of endometriosis. Fertil Steril. 2010;94:2758–2760. doi: 10.1016/j.fertnstert.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 46.Kho RM, Magrina JF, Magtibay PM. Pathologic findings and outcomes of a minimally invasive approach to ovarian remnant syndrome. Fertil Steril. 2007;87:1005–1009. doi: 10.1016/j.fertnstert.2006.12.075. [DOI] [PubMed] [Google Scholar]
- 47.Magtibay PM, Nyholm JL, Hernandez JL, Podratz KC. Ovarian remnant syndrome. Am J Obstet Gynecol. 2005;193:2062–2066. doi: 10.1016/j.ajog.2005.07.067. [DOI] [PubMed] [Google Scholar]
- 48.Zapardiel I, Zanagnolo V, Kho RM, et al. Ovarian remnant syndrome: comparison of laparotomy, laparoscopy and robotic surgery. Acta Obstet Gynecol Scand. 2012;91:965–969. doi: 10.1111/j.1600-0412.2012.01461.x. [DOI] [PubMed] [Google Scholar]
- 49.Badger WJ, De EJ, Kaufman RP. Robotically assisted excision of ovarian vein for intermittent ureteral obstruction. JSLS. 2008;12:166–168. [PMC free article] [PubMed] [Google Scholar]
- 50.Al-Badawi IA, Al-Aker M, Tulandi T. Roboticassisted salpingostomy for ectopic pregnancy. J Obstet Gynaecol Can. 2010;32:627–628. doi: 10.1016/s1701-2163(16)34562-5. [DOI] [PubMed] [Google Scholar]
- 51.Akar ME, Carrillo AJ, Jennell JL, Yalcinkaya TM. Robotic-assisted laparoscopic ovarian tissue transplantation. Fertil Steril. 2011;95:1120. doi: 10.1016/j.fertnstert.2010.09.039. 5–8. [DOI] [PubMed] [Google Scholar]
- 52.Molpus KL, Wedergren JS, Carlson MA. Robotically assisted endoscopic ovarian transposition. JSLS. 2003;7:59–62. [PMC free article] [PubMed] [Google Scholar]